Abstract
Sex differences in brain structure have been examined extensively but are not completely understood, especially in relation to possible functional correlates. Our two aims in this study were to investigate sex differences in brain structure, and to investigate a possible relation between orbitofrontal cortex subregions and affective individual differences. We used tensor-based morphometry to estimate local brain volume from MPRAGE images in 117 healthy right-handed adults (58 female), age 18–40 years. We entered estimates of local brain volume as the dependent variable in a GLM, controlling for age, intelligence and whole-brain volume. Men had larger left planum temporale. Women had larger ventromedial prefrontal cortex (vmPFC), right lateral orbitofrontal (rlOFC), cerebellum, and bilateral basal ganglia and nearby white matter. vmPFC but not rlOFC volume covaried with self-reported emotion regulation strategies (reappraisal, suppression), expressivity of positive emotions (but not of negative), strength of emotional impulses, and cognitive but not somatic anxiety. vmPFC volume statistically mediated sex differences in emotion suppression. The results confirm prior reports of sex differences in orbitofrontal cortex structure, and are the first to show that normal variation in vmPFC volume is systematically related to emotion regulation and affective individual differences.
Keywords: sex differences, orbitofrontal cortex, emotion regulation, affect, individual differences
INTRODUCTION
Men and women differ in global features of the brain, such as overall volume, as well as aspects of some localized structures. It is an intriguing possibility that differences in structure might support differences in psychological function, reflecting innate or acquired differences in specific skills. Skill acquisition is known to increase gray matter (GM) in some cases, e.g. as suggested for juggling (Draganski et al., 2004). In terms of psychological function, a considerable literature shows that, as groups, men and women differ in emotional intelligence, social cognition and perception, certain facets of linguistic ability, emotional memory, spatial reasoning and even humor (Azim et al., 2005; Bloise and Johnson, 2007; Brackett et al., 2006; Butler et al., 2006; Cahill, 2003; Cahill et al., 2004; Canli et al., 2002; Fischer et al., 2004; Garcia-Falgueras et al., 2006; Gross and John, 2003; Hofer et al., 2006a and b; Hyde and Linn, 1988; Kilpatrick et al., 2006; Kramer et al., 1988; Mackiewicz et al., 2006; Petrides and Furnham, 2000; Schienle et al., 2004; Watson and Kimura, 1991; Williams et al., 2005). Women are also more susceptible to anxiety and depression (Leach et al., 2008; Nolen-Hoeksema, 2001). Though there have been some studies reporting sex differences in functional brain activity while processing emotional stimuli (e.g. Canli et al. 2002; Cahill et al., 2004) or even engaging in emotion regulation (Koch et al., 2007; McRae et al., 2008), there have been relatively few studies relating sex differences in brain structure to differences in psychological function.
As far as we are aware, no studies have sought to relate sex and brain structure to normal affective individual differences. We first sought to replicate previous reports of sex differences in orbitofrontal cortex (OFC), including ventromedial prefrontal cortex (vmPFC) (Gur et al., 2002; Woods et al., 2008), because these are key structures that support affect and emotion regulation (for a review, see Kringelbach and Rolls, 2004). Further, we sought to relate variation within the OFC not only to sex, but also to affective individual differences when controlling for sex. Finally, we sought to test the extent to which sex differences in affect could be mediated by variation in OFC subregions, i.e. testing whether sex–affect covariations can be wholly or partially explained by sex-related differences in OFC structure.
In the present study, our two major goals were first to corroborate prior findings concerning sex differences in brain structure, especially the OFC, doing so using tensor-based morphometry, and then to investigate potential sex differences in the OFC in relation to emotion. For the second goal, we wanted to sample affective individual differences broadly to include measures of emotion regulation, expression, and experience. For emotion regulation, we focused on Gross and John’s (2003) distinction between two emotion regulation strategies: reappraisal (changing the way one thinks about events in order to change the way one feels) and suppression (deliberately inhibiting or masking the outward display of emotion especially one’s facial expression). Reappraisal is generally a more effective strategy than suppression in reducing negative emotional experiences. Sex differences in reappraisal are typically non-existent or small, whereas women reliably use suppression less than men (Gross and John, 2003). For emotion expression, we focused on Gross and John’s (1995) distinction among types of expressivity, namely the expression of positive and negative emotions; women report being more expressive than men for both positive and negative emotions. Finally, for emotional experience, we assessed emotional impulse strength (women report stronger impulses, Gross and John, 1995) as well as anxiety (women report more anxiety, e.g. Hewitt and Norton, 1993). For anxiety, we focused on a well-validated distinction between two types of anxiety (e.g. Heller and Nitschke, 1998): cognitive anxiety/anxious apprehension, and somatic anxiety/anxious arousal.
Only a few studies have directly investigated putative gender differences in the neural underpinnings of emotion regulation processes such as reappraisal and suppression. Nonetheless, the research to date has supported the general claim that while regulating emotions with a comparable degree of success, men and women may differentially engage cognitive control regions of prefrontal cortex (Koch et al., 2007; McRae et al., 2008; Mak et al., 2009). Koch et al. (2007) investigated cognition–emotion interaction during a difficult working memory task in which participants were exposed to a noxious odor. Direct contrasts revealed that emotion regulation in this paradigm recruited the OFC, as well as the amygdala, to a greater degree in women than in men. Greater activity in the middle temporal lobe and the supramarginal gyrus were associated with emotion regulation processes in men. However, despite these underlying differences in neural activity, there were no obvious differences in the success of emotion regulation, at least as indicated by performance on the concurrent working memory task.
Similarly, McRae et al. (2008) found that men and women engaged different neural systems in a cognitive reappraisal task. Both male and female participants recruited superior, middle, and inferior frontal gyri, as well as the anterior cingulate and inferior parietal lobule during the cognitive reappraisal condition. Moreover, both genders demonstrated comparable emotional reactivity to the stimuli, and did not differ in their ability to reduce negative affect through reappraisal. However, compared to men, women demonstrated significantly greater activity during cognitive reappraisal within a subset of these regions, including the superior and inferior frontal gyri as well as the anterior cingulate cortex. Women also showed substantial increases in the activity of the ventral striatum during emotion regulation by cognitive reappraisal.
Most recently, Mak and colleagues (Mak et al., 2009) found that men and women recruit different brain regions in regulating positive and negative emotional responses to arousing images. Both men and women engaged left anterior cingulate cortex when regulating negative emotions and left dorsomedial prefrontal cortex when regulating positive emotions. However, distinct subregions of prefrontal cortex appeared to contribute to emotion regulation in a gender-specific fashion. Negative emotion regulation preferentially activated left dorsolateral and lateral OFC in men, but instead engaged left medial OFC in women. In comparison to women, men also demonstrated greater activity in lateral OFC when down-regulating positive emotions.
Previous research endeavors have thus painted a consistent picture of comparably successful emotion regulation in men and women accomplished by invoking distinct neural mechanisms. As two of the three studies discussed above revealed gender differences in OFC activity during emotion regulation, it constitutes a very reasonable candidate region. Its role in processing affective information and maintaining accurate representations of stimulus reward contingencies lend plausibility to the hypothesis that affective individual differences may be related, in part, to structural variation in this region.
Moreover, reports revealing gender differences in the structure of the OFC suggest the plausible hypothesis that differences between men and women in the use of reappraisal and suppression, and perhaps also in the functional activity of prefrontal brain regions during regulation, may be related (at least in part) to gender-related variation in structure. Most germane to our study are findings by Gur et al. (2002) indicating sex differences in the volume of both ventromedial and right lateral OFC. These results are important in light of extensive evidence linking OFC (including vmPFC) to emotion and emotion regulation (for a review, see Kringelbach and Rolls, 2004). More recently, Wood and colleagues (2008) replicated sex differences in the volume of the straight gyrus of the vmPFC, but found no differences in the lateral OFC. Intriguingly, the relative volume of the straight gyrus correlated with self-reported variation in masculinity-femininity and social perceptiveness—one of the few studies to relate gender differences in brain structure to any affect-related function.
In the present study, we sought to test the extent to which individual differences in emotion regulation, expression, and experience relate to sex and the volume of OFC subregions (lateral and ventromedial). To our knowledge, this is the first such investigation, and is theoretically motivated by the clear relevance of the OFC to emotion-related functions and the existence of sex differences in some emotion-related skills and experience. If sex differences in self-reported affect were entirely due to differences in the willingness to disclose or self-report, e.g. due to differences in emotion-related socialization, one would not expect to find sex differences in the structure of brain regions involved in affective functioning. We not only found such differences, but further found that variation in a key region, the vmPFC, was related to affective individual differences even when controlling for sex, and statistically mediated the relation between sex and some affective variables.
As a secondary objective, we sought to replicate previous findings of gender differences in a variety of other brain structures, including the basal ganglia (Filipek et al., 1994; Giedd et al., 1996; Raz et al., 1995; Xu et al., 2000), prefrontal regions other than the OFC (Goldstein et al., 2001; Im et al., 2006; Luders et al., 2004, 2005, and 2006; Schlaepfer et al., 1995), the planum temporale (PT) (Chance et al., 2006; Im et al., 2006; Kulynych et al., 1994; Schlaepfer et al., 1995) and the corpus callosum (DeLacoste-Utamsing and Holloway, 1982).
Sex differences in total brain volume have been very reliable in the literature (Allen et al., 2003; Filipek, 1994; Giedd et al., 1996; Good et al., 2001; Gur et al., 1999; Leonard et al., 2008, Nopoulous et al., 2000; Passe et al., 1997). Nonetheless, such global differences are probably uninformative about differences in psychological function. The mean male brain is 8–10% larger than the mean female brain, including GM, white matter (WM) and cerebrospinal fluid (CSF) (Filipek, 1994; Giedd et al., 1996; Good et al., 2001; Gur et al., 1999; Leonard et al., 2008; Passe et al., 1997). While volumes for each tissue type are typically larger for men in absolute terms, women have a higher percentage of GM relative to total brain volume as compared to men, and a correspondingly lower percentage of WM (Allen et al., 2003; Good et al., 2001; Gur et al. 1999).
Sex differences in GM–WM ratio probably reflect the fact that women have less WM than men as a proportion of brain volume. When Allen and colleagues (Allen et al., 2003) controlled for total GM volume, global and localized sex differences in tissue compositionality remained, whereas when they controlled for total WM volume, those differences disappeared (Allen et al., 2003). When Gur and colleagues plotted GM against total intracranial volume, the slope was identical for men and women (Gur et al., 1999). In contrast, when WM is plotted against total intracranial volume, women had a shallower slope than men (whose WM volume increases according to the same proportion as GM). Good et al. (2001) corroborate this finding, presenting a very similar result. These studies suggest that with increasing intracranial volume, men have greater increases in WM than women with increasing brain size, and this divergence underlies the greater GM–WM ratio in females than males. Such observations may be explained in part by Zhang and Sejnowski’s (2000) model, which holds on the basis of geometric considerations that larger brains require relatively more WM to support the same degree of connectivity between regions. They derived a power-law model of the relationship between GM and WM volume, and empirically validated the model across seven orders of magnitude of brain volume (using variation across species). Thus, it is possible that a non-linear (power law) relationship between total GM and WM exists for variation in the human brain (within-species), and if so, that it might account for at least some apparent human sex differences, given sex differences in overall volume.
Because sex differences in global brain structure probably do not have a profound impact on psychological function and in the case of GM–WM ratio may simply ensure equivalent structural connectivity, we focus our analyses on the relationship between affective individual differences and vmPFC structure, with a secondary interest in localized sex differences in other brain structures.
METHODS
Participants
Healthy, right-handed participants were recruited from Washington University in St. Louis and the surrounding community (n = 121 enrolled, 117 with complete data; 58 female; age range 18–40, mean = 22.8). All were pre-screened to exclude a history of neurological or psychiatric disorder and gave informed consent. These participants have contributed data to other empirical reports (DeYoung et al., in press; Fales et al., 2008; Shamosh et al., 2008), none of which reported brain structure or sex differences. The experimental protocol was approved by the Washington University Medical Center Human Subjects Committee.
Behavioral measures were obtained off-line, including a standard measure of fluid intelligence, Raven’s Advanced Progressive Matrices Set II (RAPM; Raven et al., 1998), and self-report individual-difference measures targeting emotion regulation, expression, and experience: the Emotion Regulation Questionnaire (ERQ; Gross and John, 2003), the Berkeley Expressivity Scale (BEQ; Gross and John, 1995), the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990) assessing cognitive anxiety, and the Taylor Manifest Anxiety Scale (TMAS; Taylor, 1953) assessing somatic anxiety. We also administered a Big Five personality assessment NEO-PI-R (Costa and McRae, 1992), and the Attentional Control Scale (Derryberry and Reed, 2002), but do not report these data here. Participants returned individually on a different day for the MRI session.
Image acquisition and analysis
We used 3 T Allegra System (Siemens, Erlangen, Germany) to acquire a T1-weighted MPRAGE for each subject (FOV = 256 mm; 256 × 256 matrix; 1 × 1 mm in-plane resolution, 1.25 mm-thick axial slices, 1 average). Functional scans were also obtained but are not reported here. The preprocessing steps included brain extraction, brain volume estimation, and registration to MNI space. Finally, subject-specific differences in brain volume relative to a female reference subject were derived from the registration.
To elaborate, extraction consisted of two stages, both using BET (Smith, 2002): first removing skull, eyes and other extra-cranial structures; and second, removing the remaining meninges as effectively as possible. Of note, because the subsequent registration steps rely on normalized mutual information (NMI), which is robust to small amounts of non-brain material, we tried to err on the side of retaining as much of the brain as possible by using lower than the default fractional intensity threshold, despite leaving some non-brain tissue as well (rather than removing almost all non-brain tissue but also removing brain tissue). Two independent raters judged the quality of extraction (i.e. the degree of under- or over-extraction) in left and right OFC, brainstem, and mid-line superior parietal cortex. These ratings were then used as covariates in control analyses to control statistically for extraction quality as a possible confound (men have larger brains, and extraction quality could covary with brain volume).
To estimate total brain volume, as well as GM, WM and CSF, we used the automated segmentation tool FAST (Zhang et al., 2001). For each subject, FAST segments the brain into regions of GM, WM and CSF, and computes the total volume of each type of region in units of cubic centimeters in native space (e.g. not relative to a reference brain).
Finally, we coregistered all the extracted brain images to standard space using BioImage Suite (Papademetris et al., http://www.bioimagesuite.org). We selected a reference subject from our sample on the basis of having average brain size, proportionality of GM, WM, and CSF, intelligence, and personality simultaneously (based on Mahalanobis distance), with a good image, and good extraction; this subject happened to be female. We have no reason to expect that the gender of the reference subject should exert any undue influence on the subsequent analyses and their results; and we replicated sex differences that have been previously reported (such as differences in GM–WM ratio). We first affine-transformed the reference subject to a brain-only version of the Colin 27 brain in approximate MNI space (Holmes et al., 1998). All of the other subjects were then non-linearly registered (Papademetris et al., 2004) to the standardized reference subject (i.e. to the subject from our sample, effectively in MNI space). As a result, each subject ended up in standard space, registered to a within-study reference image. The transformation needed to achieve coregistration contains information about the degree of local expansion or contraction of the template reference brain in order for it to match a given subject. The determinant of the jacobian of the transformation for each subject is effectively a scaling factor that indexes, at each point, the relative expansion or contraction of the brain of the subject, relative to the reference subject. The non-linear part of the transformation is of most interest, because the linear part captures differences in whole-brain volume and other linear effects of no interest, such as translations. The determinant of the jacobian images were then used as the dependent measure in a GLM, using sex as a fixed effect of main interest, and age and intelligence as covariates. The ratings of brain extraction quality were used as covariates in control analyses; these analyses did not change the conclusions, and so are not reported.
Unless noted, we used a whole-brain threshold of P < 0.0001, uncorrected, plus a minimum of eight contiguous voxels to define a cluster. To detect differences in specific structures of interest and having precedent in the literature (OFC, PT, corpus callosum), we used a whole-brain threshold of P < 0.001 with an eight voxel cluster size threshold.
RESULTS
Motivated by our main interest in OFC, sex and affective individual differences, we first sought evidence for sex differences in OFC structure, using a threshold of P < 0.001 uncorrected, given prior reports of sex differences (Gur et al., 2002; Wood et al., 2008). Controlling for age, intelligence and whole-brain volume, women had relatively greater volume in two regions, replicating Gur et al. (2002). The lateral locus was centered at MNI 39, 38, −9 in right OFC, with effect size r = −0.31 for sex, and total volume 0.66 cm3. The medial locus was centered at MNI 6, 37, −12 in the vmPFC (Brodmann’s area 11), effect size r = −0.33 for sex and total volume of 2.3 cm3.
As summarized in Table 1, sex differences in affective measures were of the expected direction and magnitude based on a comparison with prior work (e.g., women tended to report using suppression less frequently than did men, r = −0.18, P < 0.05, cf. Gross and John (2003); women reported higher anxiety scores).
Table 1.
Zero-order correlations among sex, affective individual differences, and the volume of orbitofrontal subregions
| Sex | R_OFC | vmPFC | RAPM | PSWQ | TMAS | Reappr | Suppr | BEQ_nex | BEQ_pex | |
|---|---|---|---|---|---|---|---|---|---|---|
| R_OFC | 0.30** | |||||||||
| vmPFC | 0.32** | 0.34** | ||||||||
| RAPM | −0.06 | 0.09 | −0.03 | |||||||
| PSWQ | 0.26** | 0.14 | 0.25** | −0.06 | ||||||
| TMAS | 0.31** | 0.10 | 0.14 | 0.15 | 0.57** | |||||
| Reappr | 0.17+ | 0.07 | 0.21* | −0.06 | −0.02 | −0.03 | ||||
| Suppr | −0.18* | −0.03 | −0.25** | 0.11 | 0.08 | 0.11 | −0.09 | |||
| BEQ_nex | 0.17+ | −0.07 | 0.11 | −0.13 | 0.14 | 0.13 | 0.01 | −0.48** | ||
| BEQ_pex | 0.37** | 0.07 | 0.35** | −0.16 | 0.09 | 0.06 | 0.15 | −0.51** | 0.51** | |
| BEQ_str | 0.43** | 0.07 | 0.22* | −0.13 | 0.49** | 0.37** | 0.15 | −0.28** | 0.38** | 0.48** |
Note: R_OFC, right orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex (straight gyrus); RAPM, Raven’s advanced progressive matrices; PSWQ, Penn State Worry Questionnaire; TMAS, Taylor Manifest Anxiety Scale; Reappr, Reappraisal; Suppr, Suppression; BEQ_nex, Berkeley Expressivity Questionnaire, negative emotion; BEQ_pex, Berkeley Expressivity Questionnaire, positive emotion; BEQ_str, Berkeley Expressivity Questionnaire, impulse strength.
*P < 0.05; **P < 0.01; +P < 0.10.
To relate OFC volumes to affective individual differences, we defined regions of interest (ROIs) in ventromedial and right lateral OFC, as defined by a significant sex difference (Figure 1A). From the two regions, we extracted the average volume for each subject, and then correlated local volume with self-reported affective individual differences. Strikingly, five of the seven self-reported affective measures were related to the volume estimates for vmPFC (Table 1). For example, more frequent use of reappraisal as an emotion regulation strategy was associated with greater vmPFC volume, whereas greater frequency of using suppression was associated with smaller vmPFC volume. No relations held for rlOFC, all P > 0.10. Of note, for vmPFC the relations remained (or held marginally) when controlling for sex (Table 2), with the exception of BEQ Impulse strength (r = 0.10, P = n.s.). This analysis confirms many prior reports that these regions are affective in function. Again, none of the measures were related to rlOFC.
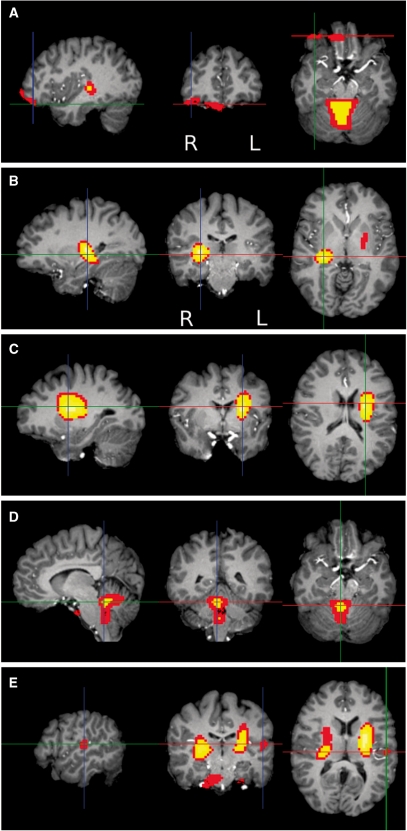
Fig. 1.
Women had relatively larger local volumes in all regions shown except for planum temporale. In axial and coronal images, the left side of the brain is on the right side of the image. (A) Sex differences in the orbitofrontal cortex, coronal view, thresholded at P < 0.001, showing both the right lateral OFC (at crosshair, MNI 34, 40, –5) and ventromedial PFC (center, MNI 6, 36, –12). (B) Sex differences in right WM, near basal ganglia, P < 0.0001, MNI 28, −21, 8. (C) Sex differences in the left basal ganglia and WM, P < 0.0001, MNI −26, −7, 21. (D) Sex differences in the cerebellum, P < 0.0001, MNI 5, −44, −8. (E) Sex differences in the left planum temporale (at crosshair), P < 0.001, MNI −52, −17, 14.
Table 2.
Partial correlations of affective individual differences with the volume of orbitofrontal subregions
| Partial correlations, controlling for sex | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| R_OFC | vmPFC | RAPM | PSWQ | TMAS | Reappr | Suppr | BEQ_nex | BEQ_pex | |
| vmPFC | 0.27** | ||||||||
| RAPM | 0.11 | −0.01 | |||||||
| PSWQ | 0.07 | 0.19* | −0.04 | ||||||
| TMAS | 0.01 | 0.04 | 0.18 | 0.54** | |||||
| Reappr | 0.02 | 0.16+ | −0.06 | −0.07 | −0.10 | ||||
| Suppr | 0.02 | −0.20* | 0.10 | 0.14 | 0.17+ | −0.06 | |||
| BEQ_nex | −0.13 | 0.06 | −0.12 | 0.10 | 0.08 | −0.02 | −0.47** | ||
| BEQ_pex | −0.04 | 0.27** | −0.15 | −0.02 | −0.06 | 0.10 | −0.49** | 0.49** | |
| BEQ_str | −0.07 | 0.10 | −0.11 | 0.43** | 0.28** | 0.09 | −0.22* | 0.35** | 0.39** |
Note: Abbreviations are the same as for Table 1.
*P < 0.05; **P < 0.01; +P < 0.10.
Finally, using a mediation analysis, we tested the extent to which sex differences in affect could plausibly be explained by variation in vmPFC volume, Table 3. We used the bootstrap method to test the significance of indirect effects [using the bias-corrected confidence interval method, bootstrap N = 2000; Shrout and Bolger (2002) as implemented in Amos 7]. A significant mediation effect indicates that brain volume in that region is plausibly responsible for (some of) the observed relation between sex and a given affective variable. vmPFC volume fully mediated the relation between sex and the two emotion regulation strategies, reappraisal and suppression, albeit only marginally for reappraisal. vmPFC volume partially mediated the relation of sex to cognitive anxiety (PSWQ) and to expressivity of positive emotions (BEQ) positive, meaning that the indirect effect was significant, yet the direct effect also remained significant. Although sex differences in other variables were present, none of these relations were statistically mediated by vmPFC volume.
Table 3.
Ventromedial PFC volume as a mediator of sex differences in affect
| Affect measure | Zero-order (sex – affect) | Beta, direct path (sex – affect) | P-value, indirect path (sex – affect via vmPFC) | Mediation by vmPFC |
|---|---|---|---|---|
| PSWQ | 0.26** | 0.20* | 0.03 | Partial |
| TMAS | 0.31** | 0.30** | 0.61 | No |
| Reappr | 0.17+ | 0.11 | 0.058 | Full, marginal |
| Suppr | −0.18* | −0.12 | 0.015 | Full |
| BEQ_nex | 0.17+ | 0.15 | 0.50 | No |
| BEQ_pex | 0.37** | 0.28** | 0.001 | Partial |
| BEQ_str | 0.43** | 0.40** | 0.20 | No |
Note: Abbreviations are the same as for Table 1.
*P < 0.05; **P < 0.01; +P < 0.10.
Global sex-related differences in brain structure
As expected, men had greater total brain volume overall (effect size r = 0.64), as well as greater overall volume of GM (r = 0.57), WM (r = 0.67) and CSF (r = 0.45), all P-values < 0.05, Table 4. As predicted, women had greater GM as a proportion of total brain volume, r = −0.31. Mean percentages of total brain volume and intra-cranial volume by tissue type are given in Table 5. Although a power law describes gray/white ratio across species (Zhang and Sejnowski, 2000), the gray/white ratio was effectively linear over the range of brain volumes in our sample. In particular, in a hierarchical regression with WM volume and WM volume squared as predictors of GM volume, WM volume predicted GM volume, R2 = 0.70, P < 0.001. Adding the non-linear term (WM volume squared) lead to a marginal improvement, change in R2 = 0.009, F(1,118) = 3.44, P = 0.066. This analysis suggests that, over the range of variation in human brain volume, the gray–white relation is effectively linear (effect size r = 0.84), and to the extent that non-linearities exist, they are considerably weaker than the linear trend (by a factor of about 78:1, based on a comparison of R2 values). For this reason, using a General Linear Model (GLM) for analyses of differences in local brain structure is unlikely to be compromised seriously by non-linearities associated with overall brain volume interacting with GM/WM ratio.
Table 4.
Absolute global gender differences in volume by tissue type
| Intracranial volume (cm3) | Total brain volume (cm3) | Total GM volume (cm3) | Total WM volume (cm3) | |
|---|---|---|---|---|
| Female | 1462 | 1244 | 789 | 455 |
| Male | 1675 | 1416 | 884 | 532 |
| Effect size (r) | 0.66** | 0.64** | 0.57** | 0.67** |
**P < 0.001.
Table 5.
Proportional global gender differences by tissue type
| GM % of ICV | WM % of ICV | TBV % of ICV | GM % of TBV | WM % of TBV | |
|---|---|---|---|---|---|
| Female | 53.96 | 31.15 | 85.12 | 63.40 | 36.60 |
| Male | 52.84 | 31.79 | 84.63 | 62.43 | 37.57 |
| Effect size r | −0.28 | 0.22 | −0.12 | −0.31 | 0.31 |
Note: ICV, intracortical volume (GM + WM + cerebrospinal fluid volume); TBV, total brain volume (GM + WM volume).
Other brain regions demonstrating localized sex-related variation in structure
In order to assess sex differences in brain areas outside the primary a priori regions of interest (vmPFC and lateral OFC), we employed a whole-brain GLM (ANCOVA) analysis, with the dependent variable being the non-linear-only component of the determinant of the jacobian (which has whole-brain volume and other linear effects removed). Sex, age and intelligence (RAPM scores) were entered as the independent variables. For sex differences, we report effect size r-values controlling for age and intelligence.
We found localized sex differences in right hemisphere WM tracts and in the left hemisphere putamen, globus pallidus and surrounding WM (especially posteriorly). In both hemispheres, women exhibited relatively greater volume than men. In the right hemisphere, sex differences were found in the WM tracts of the medial superior temporal lobe (MNI 28, 22, 7), effect size r = −0.51 (Figure 1B). Strong sex differences in relative volume extended to the parahippocampal gyrus (MNI 25, −29, −2), into the putamen (MNI 32, −17, 10), and bordered medially on the ventral lateral nucleus and ventral medial nucleus of the thalamus (MNI 17, −20, 10), occupying a total volume of 7.4 cm3. Sex differences in the left hemisphere (r = −0.49) were observed in the WM tracts of the internal capsule (MNI −25, −6, 20) and extended ventrolaterally into the putamen at MNI −29, −6, 11 while barely reaching the body of the caudate nucleus along its superior medial border (Figure 1C).
In more posterior sections, correlations with sex extended more ventrally into the lateral globus pallidus (MNI −22, −10, 6), bounded medially by the ventral lateral nucleus of the thalamus (MNI −17, −14, 16). The correlation in the left hemisphere (MNI −26, −6, 20) extended over a slightly larger area, occupying a total volume of 27.9 cm3. The right hemisphere correlation was centered at MNI 28, −21, 8, but extended weakly much further to anterior and posterior WM tracts at threshold P < 0.001. The negative sign of the effect size reflects that the areas were larger in women than in men as a proportion of total brain volume.
Sex differences were also discovered in the left hemisphere PT (r = 0.34, men relatively larger; MNI −52, −17, 14) when thresholded at P < 0.001, in Brodmann’s area 41 within the transverse temporal gyrus. The identified region was confined to a roughly spherical volume of 0.84 cm3 (Figure 1E). The PT was the only region to have a larger proportional volume in men than women.
A correlation with sex in the cerebellum extended over a greater area (10.6 cm3) reaching the dorsal surface of the midbrain at MNI 5, −36, −9, as well as into the posterior lobe of the cerebellum, declive folium at MNI 5, −67, −6 (Figure 1D). Slight correlations extend caudally into the dorsal medulla. We did not find sex differences in the corpus callosum, even at a threshold of P < 0.001 uncorrected.
DISCUSSION
In the present study we confirm the existence of sex differences in OFC volume, and for the first time demonstrate that these sex-related structural differences are meaningfully related to affective individual differences, including emotion-regulation strategies, expression and experience. Notably, these associations held for vmPFC but not lateral OFC, and mostly remained when controlling for sex. In addition, vmPFC volume plausibly mediated intriguing sex differences in affect, especially emotion regulation. In our whole-brain GLM we also replicate previous findings regarding sex differences in a number of other regions, including especially the basal ganglia and surroundingWM, as well as the PT. Compared to previous studies, the present dataset has relatively high spatial resolution and a large sample size. We controlled statistically control for age and intelligence, which may plausibly exert a confounding impact on research into brain structure.
While the role of the OFC in emotion regulation is likely to be complex, sex differences in these structures are intriguing in light of what is known about OFC functioning. Our results confirm and considerably extend previous reports of sex differences in the structure of the OFC, specifically vmPFC and rlOFC (Goldstein et al., 2001; Gur et al., 2002; Wood et al., 2008). Wood and colleagues (2008) reported sex differences in the extent of the straight gyrus in right vmPFC. Moreover, these structural differences were correlated with social perceptiveness and indices of masculinity and femininity. It is possible (though not obviously the case) that social perceptiveness is related to emotion regulation and expressivity, e.g. as empirical work on Emotional Intelligence suggests (Brackett et al., 2006). Gur et al. (2002) found sex differences in vmPFC as well as rlOFC, and speculated about a possible relation to sex differences in affect. It is gratifying that vmPFC volume correlates with a number of affective variables, including emotion regulation, expression and experience.
Of note, we found no relation between right OFC volume and any of the affective measures (despite finding equally robust sex differences in volume), which is surprising because the right ventral PFC/OFC appears to play an important role in emotion regulation. For example, Eisenberger et al. (2003) found that activity in the right ventral PFC was inversely correlated with subjective distress during perceived social exclusion, and likewise inversely correlated with concurrent activity in the anterior cingulate cortex (ACC). Taylor et al. (2008) found that individuals high in psychosocial resources demonstrated greater activity in the right ventral PFC during an affect-labeling task in response to threatening stimuli. Moreover, activity in a separate section of the RVPFC in these subjects was inversely related to amygdala activation and positively related to cortisol reactivity. Ochsner et al. (2004) also implicated the rlPFC in down-regulation of emotion during cognitive control via reappraisal.
More recently, Koch et al. (2007) found evidence for sex-related differences in functional activation associated with cognition-emotion interactions during working memory. While engaged in a difficult n-back task, participants were exposed to an aversive odor, and successful performance was reliably impaired. Women and men demonstrated an equal capacity to regulate emotion during this task, but women recruited the OFC to a greater extent than men in doing so. This important finding provides direct evidence for functional differences in the OFC during emotion regulation, on the basis of gender.
In light of functional activation patterns, it is surprising that we did not find reliable relations between affective individual differences and the volume of rlOFC, which is near (albeit not identical with) regions active during emotion regulation. For whatever reason, based on our data vmPFC volume reflects emotion regulation expertise more strongly than does lateral OFC volume. At least one recent functional imaging study suggests that there may be reliable gender differences in the contributions of ventromedial prefrontal structure and function to affective processing. Mak et al. (in press) report greater medial OFC activity in women than men during regulation of negative emotion, though these differences emerge in the left hemisphere. Considered alongside the correlations between social perceptiveness, masculinity/femininity and sex-related structural variation in the straight gyrus, it is possible that ventromedial subregions of prefrontal cortex play a special role in affective processes in women.
Global sex-related differences in brain structure
As expected, males had greater volumes of GM, WM and CSF. The values reported here for both total brain and compartmental volumes for men and women are consistent with those in the literature, suggesting that our sample is not unusual. Our results also replicate reports of a greater proportion of GM in women relative to whole brain volume, and a correspondingly greater proportion of WM in men relative to whole brain volume. Women also had a greater volume in GM (and men a greater volume in WM) as a proportion of total intracranial volume. While we found consistent sex-related variance in the proportion of total GM to WM, the effect size was small, which may explain why some previous studies have not observed differences between male and female brains. It is also not entirely clear what the functional consequences might be, if any, of global differences in the ratio of GM to WM.
Halpern and colleagues (2007) offer the intriguing hypothesis that male brains support greater intrahemispheric structural connectivity, a specialization that may subserve spatial reasoning through elements of the frontoparietal network. In contrast, they suggest that female brains may demonstrate greater connectivity between hemispheres (specifically, in possessing more bulbous callosal splenia), and thereby demonstrate greater proficiency in linguistic tasks that demand interhemispheric communication. We did not find sex differences in the corpus callosum, even at liberal thresholds, and so cannot assess this fascinating hypothesis.
There is no evidence for sex differences in general cognitive ability, either in our sample, r = −0.06, P = n.s., see Table 1, or in the literature. Regional or microstructural differences between men and women will likely contribute substantially more to the different processing strategies males and females may employ, and their consequences in terms of specific skills, such as mental rotation (e.g. Richardson, 1994), rather than general abilities. Thus, we caution against speculative interpretations of the macrostructural differences (Table 6).
Table 6.
Summary of regional sex differences
| Regions | Center (MNI)* | Area (cm3) | Effect size (r) |
|---|---|---|---|
| Right hemisphere subcortical (WM and basal ganglia)a,° | 28, −21, 8 | 7.425 | −0.51 |
| Left hemisphere subcortical (WM and basal ganglia)a,• | −26, −7, 21 | 27.864 | −0.49 |
| Right lateral prefrontal cortex• | 34, 40, −5 | 0.665 | −0.31 |
| Right medial prefrontal cortex• | 6, 36, −12 | 2.335 | −0.33 |
| Right anterior prefrontal cortex• | 34, 54, 0 | 0.311 | −0.30 |
| Cerebellumb,° | 5, −47, −8 | 10.573 | −0.45 |
| Left Planum Temporaleb,• | −52, −17, 14 | 0.837 | 0.34 |
Note: Summary of regions exhibiting substantial gender differences in a GLM (ANCOVA). The MNI coordinates of the center of each region is specified, as are the total area over which significant correlations were observed at the indicated P-value, and the associated correlation coefficient, where negative indicates that women had a relatively larger region than men.
aGender differences persist at P < 0.00001.
bGender differences persist at P < 0.0001.
*All centers reported at P < 0.001; •areas at P < 0.001; °areas at P < 0.0001.
Differences in localized structures are likely to be more relevant than overall brain volume to explaining behavioral differences between women and men. Nonetheless we caution that the influence of the relative volume of a particular structure on information processing within a larger, integrative system is not simple. It will require additional work to establish whether it is the absolute volume of a structure that determines its contribution to neural function, its relative volume in comparison to other components of the neural circuits in which it is active, or a combination of these factors; almost certainly other factors are involved as well, not just mere size. Because the OFC is unambiguously implicated in the rapid, context-relevant assessment of the emotional and social relevance of arousing stimuli, a larger volume of this area could potentially have profound effects on emotional and social behavior. Greater volume can also reflect greater skill or experience with engaging in such activity, rather than an innate difference in ability (e.g. Draganski et al., 2004).
Planum temporale
Our analysis identified only one region with locally greater volumes in male subjects as a proportion of overall brain volume: Brodmann’s area 41 in the vicinity of the PT. The PT has been the focus of intense interest due to pronounced hemispheric asymmetry in this structure and gender differences at both the macro-and microstructural level (Chance et al., 2006; Im et al., 2006; Kulynych et al., 1994; Steinmetz, 1996). While Kovalev et al. (2003) presented evidence that the male brain is generally more asymmetric than the female brain, the superior temporal cortex was among the regions exhibiting the greatest gender differences in brain asymmetry in their study. In finding that the PT is larger in the left hemisphere in males relative to total brain volume, our findings corroborate much of the previous literature on gender differences in this structure. However, as we did not find gender differences in the corpus callosum, our data cannot further inform the relationship between PT asymmetry and the density of interhemispheric connections through the corpus callosum. Further studies will be required to elucidate precisely what the relationship, if any, there may be between gender-based differences in the volume of the PT and sex-based differences in performance on linguistic tasks.
Basal ganglia
Sex differences in the relative volume of the basal ganglia and surrounding WM were especially robust, exhibiting relatively high correlation coefficients and persisting at very conservative significance thresholds. Previous studies are not in full agreement about gender differences in this area, and have used a variety of means to delineate the basal ganglia for analysis. Several of the earlier studies depended upon estimation from traced structures in a limited number of slices for their estimates of the volume. With a substantial sample size and robust results, our data represent the first clear demonstration of systematic sex differences in these areas, especially in the left hemisphere. Note that these differences are relative to whole brain volume; i.e. the regions are larger in women as a proportion of brain volume, but need not differ in absolute terms. The use of automated procedures without a narrow predefined region of interest renders the results more general and less spatially biased. It is interesting that sex differences in this region demonstrated significant asymmetry, with the left hemisphere exhibiting correlations more anterior than the right hemisphere, including more of the basal ganglia. Correlations in the right hemisphere comprised mostly WM tracts of the superior temporal lobe, extending ventrally towards the hippocampus and parahippocampal gyrus. This may explain the finding of sex differences in the hippocampus as well as the basal ganglia (Filipek et al., 1994). It is important to note that these gender differences in relative volume do not necessarily amount to differences in absolute volume; these areas did not appear to vary significantly between men and women in absolute terms (unpublished raw data). We also note that most of the caudate did not exhibit relative gender differences in volume in our analysis, which likely results in larger caudate volumes overall in men than women (as in Raz et al., 1995). Also, given the asymmetric nature of the sex-related differences uncovered, assessments that combine volumes of relevant structures from both hemispheres or rely on automated structural segmentation may obscure hemisphere-specific differences.
Cerebellum
Our results show strong sex differences in parts of the cerebellum, although we note that there is disagreement in previous work concerning the cerebellum [i.e. Xu et al. (2000) and Giedd et al. (1996) found differences, while Nopoulos et al. (2000) and Filipek et al. (1994) did not]. Our findings are not entirely commensurate with those of Leonard and colleagues (2008), in which they found that the large gender differences (male greater) in cerebellar volume can be entirely accounted for by differences in brain size. Our results, which controlled for brain size, suggest that if men may have greater cerebellar volume in absolute size, not only does this advantage fail to persist when total brain volume is controlled, but the local volume of the cerebellum is actually greater in women than men relative to total brain volume. Whether this effect is a unique difference between the sexes, or only the result of systematic differences in average brain size, remains to be determined.
CONCLUSIONS
The present study assessed sex differences in brain structure at both global and local levels of analysis, using a large sample of subjects in which important factors such as age, handedness, intelligence, and personality were controlled or standardized. Most importantly our findings corroborate evidence for sex differences in the OFC, and to our knowledge, is the first report to relate the volume of vmPFC to emotion regulation, expression, and experience. It will be important for future research to assess how structural differences in the lateral and vmOFC between men and women influence network activation during emotion regulation, in order to explain the proximate means by which differences in structure impact regulation strategies.
In addition, we replicated other principle findings in the extant literature on sex differences in brain structure using tensor-based morphometry, showing that they persist independently of differences in age, intelligence, and whole-brain volume, allowing more accurate estimates of sex-specific effect sizes. We confirmed sex differences in absolute brain volume and tissue types, and found that women possessed a greater proportion of GM relative to total brain size.
Acknowledgments
The authors thank James J. Gross for helpful discussion. National Institutes of Health (grant numbers R01 MH66088 to J.R.G.; R01 EB006494 to X.P.); and the National Science Foundation (grant number DRL 0644131 to J.R.G.).
REFERENCES
- Allen JS, Damasio H, Grabowski TJ, et al. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–94. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proceedings of the National Academy of Sciences of the United States of America. 2005;120(45):16496–501. doi: 10.1073/pnas.0408456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Brill II LB, et al. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. American Journal of Psychiatry. 1997;154(5):661–7. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Bloise SM, Johnson MK. Memory for emotional and neutral information: gender and individual differences in emotional sensitivity. Memory. 2007;15:192–204. doi: 10.1080/09658210701204456. [DOI] [PubMed] [Google Scholar]
- Brackett MA, Rivers SE, Shiffman S, et al. Relating emotional abilities to social functioning: a comparison of self-report and performance measures of emotional intelligence. Journal of Personality and Social Psychology. 2006;91(4):780–95. doi: 10.1037/0022-3514.91.4.780. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Soufer R, McCarthy G, et al. Gender differences in cognitive and neural correlates of remembrance of emotional words. Psychopharmalogical Bulletin. 2001;35(3):55–78. [PubMed] [Google Scholar]
- Butler T, Imperato-McGinley J, Pan H, et al. Sex differences in mental rotation: top-down versus bottom-up processing. Neuroimage. 2006;32:445–56. doi: 10.1016/j.neuroimage.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Annals of the New York Academy of Sciences. 2003;985:163–73. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, et al. Sex-related hemispheric lateralization of Amygdala function in emotionally influenced memory: an fMRI investigation. Learning and Memory. 2004;11:261–6. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, et al. Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10789–94. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, et al. Minicolumnar structure in Heschl's; gyrus and planum temporale: asymmetries in relation to sex and callosal fiber number. Neuroscience. 2006;143:1041–1050. doi: 10.1016/j.neuroscience.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- De Lacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. 1982;216(4553):1431–2. doi: 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–36. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Shamosh NA, Green AE, et al. Intellect as distinct from openness: differences revealed by fMRI of working memory. Journal of Personality and Social Psychology. in press doi: 10.1037/a0016615. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, et al. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, et al. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage. 2004;23:S34–45. doi: 10.1016/j.neuroimage.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does Rejection Hurt? An fMRI Study of Social Exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Burgess GC, et al. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cognitive Affective & Behavioral Neuroscience. 2008;8(3):239–53. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, et al. The Young Adult Human Brain: An MRI-based Morphometric Analysis. Cerebral Cortex. 1994;4(4):334–60. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Herlitz A, et al. Sex-differential brain activation during exposure to female and male faces. Neuroreport. 2004;15(2):235–8. doi: 10.1097/00001756-200402090-00004. [DOI] [PubMed] [Google Scholar]
- Fivush R, Berlin LJ, McDermott Sales J, et al. Functions of parent-child reminiscing about emotionally negative events. Memory. 2003;11:179–92. doi: 10.1080/741938209. [DOI] [PubMed] [Google Scholar]
- Frederikse ME, Lu A, Aylward E, et al. Sex differences in the inferior parietal lobule. Cerebral Cortex. 1999;9(8):896–901. doi: 10.1093/cercor/9.8.896. [DOI] [PubMed] [Google Scholar]
- Frederikse ME, Lu A, Aylward E, et al. Sex differences in inferior parietal lobule volume in schizophrenia. American Journal of Psychiatry. 2000;157(3):422–27. doi: 10.1176/appi.ajp.157.3.422. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Junque C, GimŽnez M, et al. Sex differences in the human olfactory system. Brain Research. 2006;1116:103–111. doi: 10.1016/j.brainres.2006.07.115. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6(4):551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11(6):490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Facets of emotional expressivity: three self-report factors and their correlates. Personality and Individual Differences. 1995;19:555–68. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, et al. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–31. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience. 1999;19(10):4060–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, et al. Sex differences in temporolimbic and frontal brain volumes of healthy adults. Cerebral Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, et al. The science of sex differences in science and mathematics. Psychological Science in the Public Interest. 2007;8(1):1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: the importance of subtypes and comorbidity. Cognition and Emotion. 1998;12:421–44. [Google Scholar]
- Hewitt PL, Norton GN. The beck anxiety inventory: a psychometric analysis. Psychological Assessment. 1993;5:408–12. [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage. 2006a;32:854–62. doi: 10.1016/j.neuroimage.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, et al. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychological Medicine. 2006b;37(1):109–19. doi: 10.1017/S0033291706008919. [DOI] [PubMed] [Google Scholar]
- Holmes C, Hoge R, Collins L, et al. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324–33. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Linn MC. Gender differences in verbal ability: a meta-analysis. Psychological Bulletin. 1988;104:53–69. [Google Scholar]
- Im K, Lee J-M, Lee J, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31:31–8. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, et al. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–61. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, et al. Gender differences in the cognitive control of emotion: an fMRI study. Neuropsychologia. 2007;45:2744–54. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kovalev VA, Kruggel F, Von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage. 2003;19:895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. Journal of Clinical Psychology. 1988;44:907–15. [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl's; gyrus and the planum temporale. Cerebral Cortex. 1994;4:107–18. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- Leach LS, Christensen H, Mackinnon AJ, et al. Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Social Psychiatry and Psychiatric Epidemiology. 2008;43(12):983–98. doi: 10.1007/s00127-008-0388-z. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Towler S, Welcome S, et al. Size matters: cerebral volume influences sex differences in neuroanatomy. Cerebral Cortex. 2008;18(12):2920–31. doi: 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, et al. Gender differences in cortical complexity. Nature Neuroscience. 2004;7(8):799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26:493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, et al. Gender effects on cortical thickness and the influence of scaling. Human Brain Mapping. 2006;27:314–24. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, et al. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14200–5. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AKY, Hu ZG, Zhang JX, et al. Sex-related differences in neural activity during emotion regulation. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2009.06.017. (in press). doi:10.1016/j.neuropsychologia.2009.06.017. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, et al. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11(2):143–62. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, et al. Development and validation of the Penn state worry questionnaire. Behavior Research and Therapy. 1990;28:487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychological Science. 2001;10:173–6. [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, et al. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski M, Rajeevan N, et al. BioImage Suite: An Integrated Medical Image Analysis Suite Section of Bioimaging Sciences. Department of Diagnostic Radiology, Yale School of Medicine. http://www.bioimagesuite.org. [Google Scholar]
- Papademetris X, Jackowski AP, et al. Integrated intensity and point-feature nonrigid registration. MICCAI. 2004;1:763–70. doi: 10.1901/jaba.2001.3216-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passe TJ, Rajagopalan P, Tupler LA, et al. Age and sex effects on brain morphology. Progress in Neuro-psychopharmcology and Biological Psychiatry. 1997;21:1231–7. doi: 10.1016/s0278-5846(97)00160-7. [DOI] [PubMed] [Google Scholar]
- Petrides KV, Furnham A. Gender differences in measured and self-estimated trait emotional intelligence. Sex Roles: A Journal of Research. 2000;42(5):449–61. [Google Scholar]
- Raz N, Torres IJ, Acker JD. Age, gender, and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiology of Learning and Memory. 1995;63:133–42. doi: 10.1006/nlme.1995.1013. [DOI] [PubMed] [Google Scholar]
- Richardson JT. Gender differences in mental rotation. Perceptual Motor Skills. 1994;78(2):435–8. doi: 10.2466/pms.1994.78.2.435. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, et al. Gender differences in the processing of disgust-and fear-inducing pictures: an fMRI study. Neuroreport. 2004;16(3):277–80. doi: 10.1097/00001756-200502280-00015. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris HG, Tien AY, et al. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Research. 1995;61(3):129–35. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CD, Green AE, et al. Individual differences in delay discounting: Relation to intelligence, working memory, and frontopolar cortex. Psychological Science. 2008;19:904–11. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods. 2002;7:422–45. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H. Structure, function and cerebral asymmetry: in vivo morphometry of the Planum Temporale. Neuroscience and Biobehavioral Reviews. 1996;20(4):587–91. doi: 10.1016/0149-7634(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Taylor JA. A personality scale of manifest anxiety. Journal of Abnormal and Social Psychology. 1953;48:285–90. doi: 10.1037/h0056264. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, et al. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, et al. Gender differences in cerebellar metabolism: test-retest reproducibility. The American Journal of Psychiatry. 1997;154(1):119–21. doi: 10.1176/ajp.154.1.119. [DOI] [PubMed] [Google Scholar]
- Watson NV, Kimura D. Nontrivial sex differences in throwing and intercepting: relation to psychometrically-defined spatial functions. Personality and Individual Differences. 1991;12:375–85. [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage. 2005;28:618–26. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. The Journal of Neuroscience. 1995;15(5):3418–28. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JL, Heitmiller D, Andreasen NC, et al. Morphology of the ventral frontal cortex: relationship to femininity and social cognition. Cerebral Cortex. 2008;18(3):534–40. doi: 10.1093/cercor/bhm079. [DOI] [PubMed] [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, et al. Gender effects on age-related changes in brain structure. American Journal of Neuroradiology. 2000;21:112–8. [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(10):5621–6. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]