Abstract
Purpose
Postinfectiously irritable bowel syndrome (PI-IBS) develops in 3-30% of individuals with bacterial gastroenteritis. Recent studies demonstrated increases in inflammatory components in gut mucosa of PI-IBS patients even after complete resolution of infection. We aimed to investigate histological changes in colon and rectum of PI-IBS subjects after long term period of infection.
Materials and Methods
We recruited PI-IBS subjects who had been diagnosed IBS after complete resolution of enteritis caused by shigellosis outbreak 3 years earlier. We compared unmatched four groups, PI-IBS (n = 4), non PI-IBS (n = 7), D-IBS (n = 7, diarrhea predominant type) and healthy controls (n = 10). All of them underwent colonoscopic biopsy at three areas, including descending colon (DC), sigmoid colon (SC) and rectum, which were assessed for 5-hydroxytryptamine (5-HT)/peptide YY (PYY)-containing enterochromaffin (EC) cell, intraepithelial (IEL) and lamina propria T lymphocyte (CD3), CD8 lymphocytes, mast cells and CD68/calprotectin+ macrophages.
Results
All subjects had no structural or gross abnormalities at colonoscopy. In PI-IBS, 5-HT containing EC cells, PYY containing EC cells, IELs, CD3 lymphocytes, CD8 lymphocytes, mast cells, and CD68 + macrophages were increased compared to control (p < 0.05). In D-IBS, PYY containing EC cells, IELs, and CD3 lymphocytes were increased compared to control (p < 0.05). In PI-IBS, 5-HT containing EC cells tended to increase and PYY containing EC cells, CD8 lymphocytes, mast cells, and CD68+ macrophages were increased compared to non PI-IBS (p < 0.05). Calprotectin + marcrophages were decreased in PI-IBS, non PI-IBS and IBS compared to control.
Conclusion
The immunoendocrine cells were sporadically increased in PI-IBS, non PI-IBS and D-IBS compared with control. Our findings in a very small number of patients suggest that mucosal inflammation may play a role in long-term PI-IBS, and that other sub-groups of IBS and larger scale studies are needed to confirm this observation.
Keywords: Post infectious irritable bowel syndrome, enterochromaffin cell, T lymphocyte, mast cell, macrophage
INTRODUCTION
Irritable bowel syndrome (IBS) after previous episode of infectious enteritis is now well recognized and defined as one subgroup of postinfectiously IBS (PI-IBS). The incidence of PI-IBS has been reported to be between 4 and 31%.1-7 Intestinal mucosa has abundant immune system, called physiologically inflamatory status. For many years, low grade inflammation was considered to have a role in IBS. There are many studies investigating inflammation in mucosal biopsy from terminal ileum to rectum in IBS. Mast cells were increased8-12 and located close to mucosal nerve endings within 2-5 µm.11,12 Mast cell numbers in close proximity to nerves were correlated with IBS symptoms.11 Increased cellularity, including T lymphocytes, has also been shown in IBS.13 PI-IBS is subcategorized under the concept that morphologically normal intestine after recovery from acute infection has abnormal function, inducing IBS symptoms. However, only a few biopsy studies have been reported in PI-IBS patients. Spiller, et al. first defined the pathological basis of PI-IBS that increased enterochromaffin (EC) cells, and T lymphocytes during the acute period of enteritis can persist for more than a year. Up to date, there is no case-control, long term follow up study about the histologic assessment of PI-IBS. We previously reported the incidence of PI-IBS in a single hospital after 3 years of shigellosis outbreak.14 In this homogenous group, 13 cases (14.9%) had IBS after 3 years of infection.
In this study, we aim to investigate mucosal immunohistologic change in PI-IBS after long time period of infection. We assessed EC cells, lymphocytes, mast cells and macrophage numbers. Microscopic colitis (MC), including lymphocytic colitis (LC) and collagenous colitis (CC), is a common cause of chronic diarrhea.15,16 About 50% of IBS had microscopic inflammation compatible with MC,13 and about 41-56% of MC patients were diagnosed as IBS by IBS criteria.17 Therefore, we also counted intraepithelial T lymphocyte to exclude lymphocytic colitis. We examined mucosal histology in non PI-IBS who had normal bowel function after shigella infection, out-patient IBS, and control as well as PI-IBS.
MATERIALS AND METHODS
Subjects
In November 2001, there was a shigellosis outbreak at Yongdong Severance Hospital in Seoul. We obtained the list of infected subjects and reported the prevalence of PI-IBS after 3, 6, and 12 months of infection.18 Three years later, we then reported long term follow up data about the prevalence of PI-IBS.14 PI-IBS was defined who had normal bowel habit before infection and met Rome II criteria for IBS after shigellosis. All of them had at least 2 of the followings during acute illness; fever, vomiting, diarrhea or positive stool culture. Non PI-IBS was defined as a subject who had normal bowel habit before and after shigellosis. We also recruited patients who met the Rome II criteria for IBS, especially diarrhea-predominant type without history of acute enteritis. All subjects calssified as PI-IBS, non PI-IBS, diarrhea predominant IBS (D-IBS), and controls underwent screening colonoscopy. Under colonoscopy, we excluded patients with organic diseases such as inflammatory bowel disease, tuberculosis intestine, or colon cancer. However, we included colonic adenoma or polyps under 1 cm as well as anal diseases such as hemorrhoid. All participants in this study gave an informed consent. This study protocol was approved by the Ethics Committee, Yonsei University College of Medicine.
Colonic and rectal biopsy
All subjects underwent colonoscopy after bowel preparation with conventional sodium phosphate. Two biopsies each were obtained from descending colon, sigmoid colon and rectum using endoscopic biopsy forceps (FB-13K-1, Olympus, Tokyo, Japan).
Immunohistochemistry
The biopsy specimens were fixed in 4% formaldehyde. This paraffin block was cut at 4 µm thickness and mounted onto poly-L-lysine coated slides. For deparaffinization, the fixed paraffin-embedded section was dehydrated with xylene for 45 min at 60℃. Then, the tissue was successively rehydrated with 100%, 90%, and 70% alcohol, and heated in a microwave for 10 min to retrieve antigen, at which point cooling was achieved at room temperature. The slide was rinsed with 0.02 M phosphate buffered saline (PBS) for 10 min. Sections were immersed in 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity. The tissue was incubated with the following primary antibodies; 5-hydroxytryptamine (5-HT) (monoclonal antibody, DAKO, Cambridge, UK, 1 : 50), peptide YY (PYY) (monoclonal antibody, abcam, 1 : 400), CD3 (polyclonal antibody, DAKO, 1 : 25), CD8 (lyophilized monoclonals, DAKO, 1 : 5), mast cell tryptase (monoclonal antibody, DAKO, 1 : 250), CD 68 (monoclonal antibody DAKO, 1 : 100), and calprotectin (monoclonal antibody, DAKO, 1 : 200). Sections were washed with PBS and incubated with secondary antibodies within biotinylated goat antimouse and antirabbit antibody (DAKO, Cambridge, UK). The sections were washed with PBS and incubated for 30 minutes with streptavidin (DAKO, 1 : 200, room temperature, 30 minutes). After washing with PBS, the sections were processed with 3-amino-9-ethyl-carbazole (AEC) chromogen solution for 10 minutes and counterstained with Mayer's hematoxylin for 30 seconds.
Cell counting
The immunostained cells were examined at labeled 3 anatomic regions: descending colon, sigmoid colon, and rectum. The numbers of 5-HT positive EC cells and CD3 positive intraepithelial T lymphocytes (IEL) per 100 epithelial cells were counted in 5 areas, and then averaged. The numbers of PYY positive EC cells, T lymphocytes (CD3), CD8 lymphocyte, mast cells (mast cell tryptase), resident macrophages (CD68) and reactive macrophage (calprotectin) were counted within high power filed, 400 µm×400 µm in lamina propria. The counts under 5 fileds were averaged.
Statistic analysis
Data were presented as mean ± SE. Comparisons were made using Kruskall-Wallis test and Mann-Whitney U test for nonparametric data, using SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered significant.
RESULTS
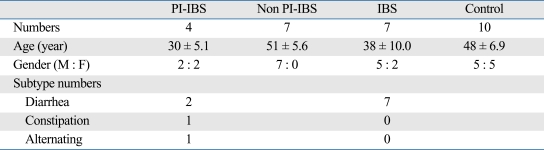
Patients (Table 1)
Table 1.
Characteristcs of Patients and Controls
PI-IBS, postinfectious irritable bowel syndrome; IBS, irritable bowel syndrome.
Total 28 subjects were included in this study. Thirteen patients were compatible with PI-IBS, but only four of them agreed to undergo colonoscopic biopsy. There were 2 diarrhea, one constipation, and one alternating subtype in this group. We collected 64 non PI-IBS subjects. About half of them left the hospital or moved out. Most of the remainder refused the exam because they had no IBS symptoms and fear of colonoscopy. Only 7 middle-aged women agreed to enroll as non PI-IBS. Diarrhea- predominant IBS patients were recruited from outpatients in clinic. Thirteen D-IBS subjects underwent colonoscopy, but finally 7 patients agreed to use specimen in our research. Finally, 10 volunteers were enrolled as controls.
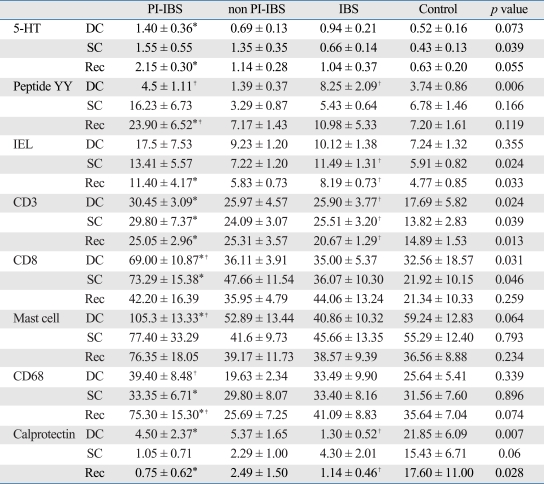
Histology (Table 2)
Table 2.
Histologic Findings
PI-IBS, postinfectious irritable bowel syndrome; IBS, irritable bowel syndrome; 5-HT, 5-hydroxytryptamine.
Cell counts/100 epithelial cells for 5-HT, IEL. Cell counts / 160,000 µm2 for peptide YY, CD3, mast cell, CD68, calprotectin.
*PI-IBS vs. control, p value < 0.05, Mann Whitney U test.
†IBS vs. control, p value < 0.05, Mann Whitney U test.
‡PI-IBS vs non PI-IBS, p value < 0.05, Mann Whitney U test.
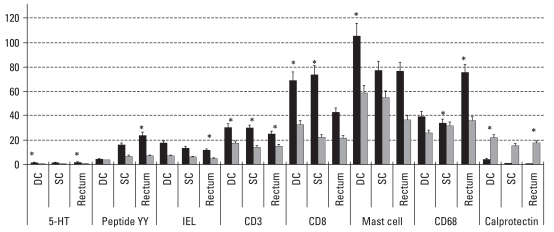
PI-IBS and control (Fig. 1)
Fig. 1.
Differences of counts of cellular markers between PI-IBS and controls. There were significant increase of all mucosal markers and decrease of calprotectin positive cells in patients with PI-IBS, compared to control. PI-IBS, postinfectious irritable bowel syndrome; IEL, intraepithelial. *p< 0.05, Cell counts/100 epithelial cells for 5-HT, IEL. Cell counts/160,000 µm2 for peptide YY, CD3, mast cell, CD68, calprotectin.
The main findings were remarkable increase of all mucosal markers and decrease of calprotectin positive cells in patients with PI-IBS, compared to control. EC cells were stained for 5-HT or PYY, both of which were increased in colon and rectum. IEL were more than twice the cotrol counts, but less 20/100 surface epithealial cells. Microscopic colitis can be diagnosed if IEL counts are over this degree.17 CD3 and CD8 positive lymphocyte counts in lamina propria were also elevated. Mast cells were increased in all areas. CD68 positive macrophages were increased, whereas calprotectin positve cells representing recently emigrated monocytes were decreased. 5-HT positive EC cell numbers showed negative correlation with CD3 lymphocyte numbers in lamina propria (r = -0.64, p = 0.025). There were no correlations between other mucosal markers.
D-IBS and control
EC cells stained for only PYY was increased in D-IBS patients, compared to control. CD3 positive lymphocyte counts in lamina propria and IELs were both elevated. IEL numbers were not over 20/100 surface epithealial cells. Calprotectin positive cells were decreased in D-IBS.
PI-IBS, non PI-IBS and control
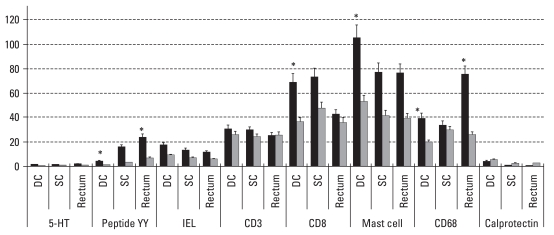
In non PI-IBS group, 5-HT positive EC cells, and lamina propria CD3 lymphocytes were increased compared to controls. Similar to PI-IBS and D-IBS patients, calprotectin positive cells were also decreased in non PI-IBS group. In PI-IBS patients, EC cells which were stained for 5-HT and PYY, CD8 positive lymphocytes, mast cells and CD68 positive macrophages were increased, compared to non PI-IBS group (Fig. 2).
Fig. 2.
Differences of counts of cellular markers between PI-IBS and non PI-IBS. EC cells stained for PYY, CD8 positive lymphocytes, mast cells and CD68 positive macrophages were increased in PI-IBS patients, compared to non PI-IBS group. 5-HT positive EC cells showed increased tendency in PI-IBS group compared to non PI-IBS group. (p = 0.085 in DC, p = 0.058 in rectum). PI-IBS, postinfectious irritable bowel syndrome; PPY, peptide YY; IEL, intraepithelial. *p< 0.05. Cell counts/100 epithelial cells for 5-HT, IEL. Cell counts/160000 µm2 for PYY, CD3, mast cell, CD68, calprotectin.
DISCUSSION
To our best knowledge, this is the first case control study to compare immunohistological features after long-term resolution of acute infectious enteritis. We confirmed that the acute inflammatory or endocrine change can persisit over three years in gut mucosa, independent on IBS symptoms. The most interesting finding in this study was that, although there was a difference in degree of changes among PI-IBS, non PI-IBS and D-IBS groups, low grade inflammation and increased enteroendocrine cells were found definitely in those groups.
What would play an important role in developing symptoms in post infectious IBS patients? We found cellular differences between PI-IBS and non PI-IBS groups. Both groups had common history of enteritis caused by same organisms. Enteric pathogen can induce tight junction disruption, thereby contributing to the development of secretory abnormality and activation of further immunologic responses.19 Increased permeability allows antigens to easily enter through gut mucosa, which results in inflammatory cascade characterized by increase of various immune cells.20 However, the role of increased gut permeability in IBS symptoms is not yet unclear. We suggest that broken gut barrier might closely be related to longstandinging inflammatory environment in gut mucosa. Many other functional gastrointestinal disorders develop after acute infection.21-23 Furthermore, inflammatory response can affect neural and motor functions, consequently inducing hypersensitivity or irritability.24 However, we also found clear changes of some marker cells in non PI-IBS, and thought that cellular difference between PI-IBS and non PI-IBS groups would be related to the development of PI-IBS. In this study, 5-HT containing EC cells, PYY containing EC cells, CD8-lymphocytes, mast cells, and resident macrophages were abundant more in PI-IBS group. Previously, persistently increased EC cell numbers were found in PI-IBS25,26: EC cells, CD3 lymphocytes and IEL counts were increased in PI-IBS patients after 1 year of campylobacter enteritis. However, there were no control or non PI-IBS groups to be compared.25 Dunlop, et al.2 showed that 5-HT containing EC cells, and T lymphocytes in lamina propria were significantly increased in PI-IBS compared to non PI-IBS in rectal biopsy 3 month after campylobacter enteritis. 5-HT has been investigated along with its receptors as target molecules to treat functional gastrointestinal disorders. Most of 5-HT is found in enterochromaffin cells which are best characterized subset of enteroendocrine cells.27 5-HT activates gastrointestinal motility through 5-HT3 or 5-HT4 receptors and induces gastric relaxation through 5-HT7 receptors.28 PYY is a gastrointestinal hormone, released from the endocrine cells which are distributed mainly in the distal ileum, colon and rectum.29 As shown in the present study, 5-HT containing EC cells were found to be increase in both groups but more in PI-IBS, whereas PYY containing EC cells were elevated only in PI-IBS group. PYY inhibits intestinal motility and increases postprandial colonic absorption of water and electrolyte.29 Postprandial increase in plasma PYY levels was consistent with the increase of PYY containing cell numbers after total colectomy.30 Mucosa can be destroyed by major surgery or severe enteritis. We suggest, therefore, that initial destruction of mucosal barrier induces diarrrhea and cellular adaptation, which can persist for a long time. Because both 5-HT and PYY are involved in gut motility and secretion, their plama levels and various receptor functions may be related to development of IBS symptoms.
Mast cells release potent inflammatory mediators which are related to visceral hypersensitivity.10,11 Especially, mast cell tryptase is considered to develop visceral hypersensitivity and increase gut permeability via protease-activated receptor (PAR).31 Longstanding increase of gut permeability and gut hypersensitivity are common in PI-IBS.19 However, the pathophysiologic role of mast cell tryptase in these phenomena remains unclear.
In view of immunologic activation, increased CD8 lymphocytes and resident macrophages and decreased calprotectin positive cells in laminal propria can not easily be explained. In acute phase of campylobacter enteritis, striking increase of CD8 lymphocytes and fall of resident macrophages were found and they were gradually recovered after 12 weeks.25 On the other hand, calprotectin positive cells showed inverse pattern.25 Memory T cells in lamina propria are rich source of cytokines and primary trigger of an immune response. However, intraepithelial CD8 lymphocytes are generally anergic in the gut, and selectively activated to express suppressor function without cytolytic activity.32 Macrophages expressed by CD68 in normal lamina propria are L1 protein (calprotectin) negative.33 CD68 + L1 + macrophages are recruited from blood only to moderately and severely inflammed areas against microbial invasion.33,34 Under steady state, resident monocytes function as sensors to monitor danger signals and contribute to the maintenance of macrophages.35 A differentiation of emigrating monocytes into intestinal macrophages with functional anergy may be decisive for intestinal mucosal integrity.36 We suggest that both CD8 lymphocytes and resident macrophages in resolution state of inflammation in PI-IBS are increased excessively for a long time to maintain steady state as well as prepare to be activated againt dangerous antigens. It is quite certain that other cells whose numbers were increased might also be involved in this process. The possibility of in situ activation of resident macrophage into L1 positive form should be investigated. In the present study, CD3 lymphocyte counts in lamina propria and 5-HT containing EC cells were elevated both in PI-IBS and non PI-IBS. Lamina propria T cells are mostly memory cells.32 Therefore, non PI-IBS groups might possibly switch over to PI-IBS groups, compared to normal controls, if immunoendocrine balance is broken by various reasons such as psychologic stress.
In the present study, 5-HT positive EC cell numbers were found to be negatively correlated with CD3 lymphocytes in lamina propria of PI-IBS. In other study, however, they were shown to be positively corelated at enteritis phase, but then not 12 weeks later.25 EC cell numbers are controlled by Th1 or Th2 predominance.37 Th2 predominant condition induces EC cell hyperplasia and 5-HT contents.37 On the other hand, Th1 response which is known to be related to macrophage activation diminishes the EC cell numbers.37 Although both cells were increased in PI-IBS, relatively small increase in EC cells could be related to this Th1/Th2 balance. The correlation among the activation of resident macrophages, memory T cell function and EC cell proliferation should be investigated further.
We also compared D-IBS groups with controls. There wasn't even increase of all cell types like PI-IBS. Although there are many reports to reveal the increase of 5-HT containing EC cells and mast cells in IBS patients, there are also data that could not prove the relation of those cells with IBS.26,38 In our study, PYY containing EC cells, IEL, and CD3 lymphocyte in lamina propria were increased, whereas calprotectin positive macrophages were decreased in D-IBS patients. Because D-IBS groups had no history of enterocolitis, they did not experience severe mucosal destruction. There could be a fluctuation in PYY cell numbers, depending on manifestation period of diarrhea or chronic adaptive hyperplasia of cells. Chronic immune activation by increased T cells may persist in epithelium and lamina propria of IBS.38,39 Calprotectin positive cells can be recruited in acute pathologic state. Consequently, we expect that this cell numbers are just above or below normal level, but definitely decreased compared to control. We should investigate further to find biological significance of decrease of calprotectin positive macrophages in long-standing low grade inflammatory area in the gut.
Finally, small numbers of enrolled patients and unmatched control group are the inevitable limitation of our study. This is a feasibility study. We used the statistic tools to compare groups, but not used the term significant. However, all subjects who experienced shigella infection were employees or students in a single hospital, therefore, they are homogenous groups: same infectious organism, follow up period, and ordinary environment. Furthermore, this is the first case-controlled study about long-term mucosal histopathologic changes after enteric infection.
In conclusion, we found that immunoendocrine activation by enteric infection persisted for a long time, at least three years. Once destroyed, immunologic steady state might not be completely normalized. Therefore, we should be more concerned about normal-looking patients who have history of infectious enterocolitis. Although both PI-IBS and D-IBS patients have IBS symptoms and colonoscopically normal mucosa, immunoendocrine network seems to be variously activated in PI-IBS.
ACKNOWLEDGEMENTS
This study was supported in part by a research fund from Department of Internal Medicine, Gangnam Severance Hospital.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 4.McKendrick MW, Read NW. Irritable bowel syndrome-post salmonella infection. J Infect. 1994;29:1–3. doi: 10.1016/s0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- 5.Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six-year follow-up study. Gut. 2002;51:410–413. doi: 10.1136/gut.51.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neak KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez LA, Ruigómez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999;318:565–566. doi: 10.1136/bmj.318.7183.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiatt RB, Katz J. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962;37:541–545. [PubMed] [Google Scholar]
- 9.Weston AP, Biddle WL, Bhatia PS, Miner PB., Jr Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–1595. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- 10.O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 11.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 12.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrheapredominant irritable bowel syndrome. J Korean Med Sci. 2003;18:204–210. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Kim MS, Ji SW, Park H. The development of irritable bowel syndrome after Shigella infection: 3 year follow-up study. Korean J Gastroenterol. 2006;47:300–305. [PubMed] [Google Scholar]
- 15.Pardi DS, Ramnath VR, Loftus EV, Jr, Tremaine WJ, Sandborn WJ. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol. 2002;97:2829–2833. doi: 10.1111/j.1572-0241.2002.07030.x. [DOI] [PubMed] [Google Scholar]
- 16.Veress B, Löberg R, Bergman L. Microscopic colitis syndrome. Gut. 1995;36:880–886. doi: 10.1136/gut.36.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limsui D, Pardi DS, Camilleri M, Loftus EV, Jr, Kammer PP, Tremaine WJ, et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007;13:175–181. doi: 10.1002/ibd.20059. [DOI] [PubMed] [Google Scholar]
- 18.Ji S, Park H, Lee D, Song YK, Choi JP, Lee SI. Post-infectious irritable bowel syndrome in patients with Shigella infection. J Gastroenterol Hepatol. 2005;20:381–386. doi: 10.1111/j.1440-1746.2005.03574.x. [DOI] [PubMed] [Google Scholar]
- 19.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucharzik T, Maaser C, Lüering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 21.Tack J, Demedts I, Dehondt G, Caenepeel P, Fischler B, Zandecki M, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology. 2002;122:1738–1747. doi: 10.1053/gast.2002.33663. [DOI] [PubMed] [Google Scholar]
- 22.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 23.Bardhan PK, Salam MA, Molla AM. Gastric emptying of liquid in children suffering from acute rotaviral gastroenteritis. Gut. 1992;33:26–29. doi: 10.1136/gut.33.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 25.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunlop SP, Jenkins DJ, Spiller RC. Distictive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 28.Tonini M. 5-Hydroxytryptamine effects in the gut: the 3,4, and 7 receptors. Neurogastroenterol Motil. 2005;17:637–642. doi: 10.1111/j.1365-2982.2005.00716.x. [DOI] [PubMed] [Google Scholar]
- 29.Imamura M. Effects of surgical manipulation of the intestine on peptide YY and its physiology. Peptides. 2002;23:403–407. doi: 10.1016/s0196-9781(01)00618-0. [DOI] [PubMed] [Google Scholar]
- 30.Imamura M, Nakajima H, Mikami Y, Yamauchi H. Morphological and immunohistochemical changes in intestinal mucosa and PYY release following total colectomy with ileal pouch-anal anastomosis in dogs. Dig Dis Sci. 1999;44:1000–1007. doi: 10.1023/a:1026620900259. [DOI] [PubMed] [Google Scholar]
- 31.Kong W, McConaloque K, Khitin LM, Hollenberg MD, Payan DG, Böhm SK, et al. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Yio XY, Mayer L. Human intestinal epithelial cell-induced CD8+ T cell activation is mediated through CD8 and the activation of CD8-associated p56lck. J Exp Med. 1995;182:1079–1088. doi: 10.1084/jem.182.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein(calprotectin) in human Peyer's patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993;34:1357–1363. doi: 10.1136/gut.34.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hausmann M, Bataille F, Spoettl T, Schreiter K, Falk W, Schoelmerich J, et al. Physiological role of macrophage inflammatory protein-3alpha induction during maturation of intestinal macrophages. J Immunol. 2005;175:1389–1398. doi: 10.4049/jimmunol.175.3.1389. [DOI] [PubMed] [Google Scholar]
- 37.Motomura Y, Ghia JE, Wang H, Akiho H, El-Sharkawy RT, Collins M, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–481. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- 38.Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 39.Chadwick VS, Chen W, Shu D, Paulus B, Bethwait P, Tie A, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]