Abstract
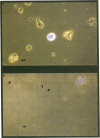
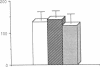
Postnatal rat retinal ganglion cells (RGCs) were identified with specific fluorescent labels and placed in culture. Under these conditions, the outgrowth of processes by RGCs was found to be promoted to a far greater degree by acidic fibroblast growth factor (aFGF) than by basic fibroblast growth factor (bFGF). The effect of aFGF and bFGF on process extension by solitary RGCs was quantified after 24 hr in culture, a time when neither a FGF nor bFGF enhanced RGC survival. The action of aFGF on process outgrowth was markedly potentiated by the addition of heparin (10 micrograms/ml) to the medium, but heparin alone had no effect. In the presence of heparin, half-maximal process outgrowth occurred at an aFGF concentration of less than 20 pg/ml (1 pM). Since all of the centrally projecting processes have already been formed in the living animal prior to use (at 7-12 days of age), at least a portion of the process outgrowth in culture appears to represent a regenerative phenomenon. Statistical analysis of the increase in process growth revealed that aFGF with heparin contributed to both neurite initiation and elongation. The mean number of glial cells, identified with polyclonal antiserum against glial fibrillary acidic protein, was slightly increased in cultures receiving aFGF plus heparin, but this effect was variable, and these glial cells were not in contact with the solitary RGCs that were scored for regeneration of processes. Thus, glial cells probably did not exert a direct physical influence on the degree of process outgrowth observed in the solitary RGCs, although a humoral effect cannot be totally excluded. These results suggest that aFGF has a potent influence on the outgrowth of processes by a neuron in the mammalian central nervous system. The potentiation of this effect by heparin leads us to speculate that the interaction of aFGF with a heparin-like molecule located in the extracellular matrix (such as heparan sulfate proteoglycan) may produce physiological effects in vivo. Furthermore, the lack of a substantial effect of bFGF in this system under these conditions shows that a specific population of mammalian central neurons may be differentially influenced by these two closely related peptide growth factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Dräger U. C. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984 Apr;11(4):847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Björklund H., Bignami A., Dahl D. Immunohistochemical demonstration of glial fibrillary acidic protein in normal rat Müller glia and retinal astrocytes. Neurosci Lett. 1985 Mar 15;54(2-3):363–368. [PubMed] [Google Scholar]
- Björklund H., Dahl D. Glial fibrillary acidic protein (GFAP)-like immunoreactivity in the rodent eye. Comparison between peripheral glia of the anterior uvea and central glia of the retina. J Neuroimmunol. 1985 Jun;8(4-6):331–345. doi: 10.1016/s0165-5728(85)80071-0. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Mehlman T., Friesel R., Johnson W. V., Maciag T. Multiple forms of endothelial cell growth factor. Rapid isolation and biological and chemical characterization. J Biol Chem. 1985 Sep 25;260(21):11389–11392. [PubMed] [Google Scholar]
- D'Amore P. A., Klagsbrun M. Endothelial cell mitogens derived from retina and hypothalamus: biochemical and biological similarities. J Cell Biol. 1984 Oct;99(4 Pt 1):1545–1549. doi: 10.1083/jcb.99.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenfeld A. J., Bunt-Milam A. H., Sarthy P. V. Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984 Nov;25(11):1321–1328. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Massoglia S., Cheng J., Lui G. M., Böhlen P. Isolation of pituitary fibroblast growth factor by fast protein liquid chromatography (FPLC): partial chemical and biological characterization. J Cell Physiol. 1985 Feb;122(2):323–332. doi: 10.1002/jcp.1041220223. [DOI] [PubMed] [Google Scholar]
- Gundersen R. W., Barrett J. N. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980 Dec;87(3 Pt 1):546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M. E., Heinrich S. P., Lee M. R., Yin H. S. Molecular cloning and expression of neuroleukin, a neurotrophic factor for spinal and sensory neurons. Science. 1986 Oct 31;234(4776):566–574. doi: 10.1126/science.3764429. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Barde Y. A., Schwab M., Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986 Oct;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large T. H., Bodary S. C., Clegg D. O., Weskamp G., Otten U., Reichardt L. F. Nerve growth factor gene expression in the developing rat brain. Science. 1986 Oct 17;234(4774):352–355. doi: 10.1126/science.3764415. [DOI] [PubMed] [Google Scholar]
- Leifer D., Lipton S. A., Barnstable C. J., Masland R. H. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984 Apr 20;224(4646):303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Lipton S. A. Blockade of electrical activity promotes the death of mammalian retinal ganglion cells in culture. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9774–9778. doi: 10.1073/pnas.83.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Tauck D. L. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol. 1987 Apr;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- Matthew W. D., Patterson P. H. The production of a monoclonal antibody that blocks the action of a neurite outgrowth-promoting factor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):625–631. doi: 10.1101/sqb.1983.048.01.066. [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Sharma A., de Vellis J., Bradshaw R. A. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Morris R. J., Raisman G. Is Thy-1 expressed only by ganglion cells and their axons in the retina and optic nerve? J Neurocytol. 1984 Oct;13(5):809–824. doi: 10.1007/BF01148495. [DOI] [PubMed] [Google Scholar]
- Pettmann B., Weibel M., Sensenbrenner M., Labourdette G. Purification of two astroglial growth factors from bovine brain. FEBS Lett. 1985 Sep 9;189(1):102–108. doi: 10.1016/0014-5793(85)80851-6. [DOI] [PubMed] [Google Scholar]
- Sarthy P. V. Establishment of Muller cell cultures from adult rat retina. Brain Res. 1985 Jun 24;337(1):138–141. doi: 10.1016/0006-8993(85)91618-x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Edgar D. Neurotrophic factors. Science. 1985 Jul 19;229(4710):238–242. doi: 10.1126/science.2409599. [DOI] [PubMed] [Google Scholar]
- Togari A., Dickens G., Kuzuya H., Guroff G. The effect of fibroblast growth factor on PC12 cells. J Neurosci. 1985 Feb;5(2):307–316. doi: 10.1523/JNEUROSCI.05-02-00307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., D'Amore P. A. Neurite outgrowth induced by an endothelial cell mitogen isolated from retina. J Cell Biol. 1986 Oct;103(4):1363–1367. doi: 10.1083/jcb.103.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke P., Cowan W. M., Ueno N., Baird A., Guillemin R. Fibroblast growth factor promotes survival of dissociated hippocampal neurons and enhances neurite extension. Proc Natl Acad Sci U S A. 1986 May;83(9):3012–3016. doi: 10.1073/pnas.83.9.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]