Abstract
STDP (spike-timing-dependent synaptic plasticity) is thought to be a synaptic learning rule that embeds spike-timing information into a specific pattern of synaptic strengths in neuronal circuits, resulting in a memory. STDP consists of bidirectional long-term changes in synaptic strengths. This process includes long-term potentiation and long-term depression, which are dependent on the timing of presynaptic and postsynaptic spikings. In this review, we focus on computational aspects of signaling mechanisms that induce and maintain STDP as a key step toward the definition of a general synaptic learning rule. In addition, we discuss the temporal and spatial aspects of STDP, and the requirement of a homeostatic mechanism of STDP in vivo.
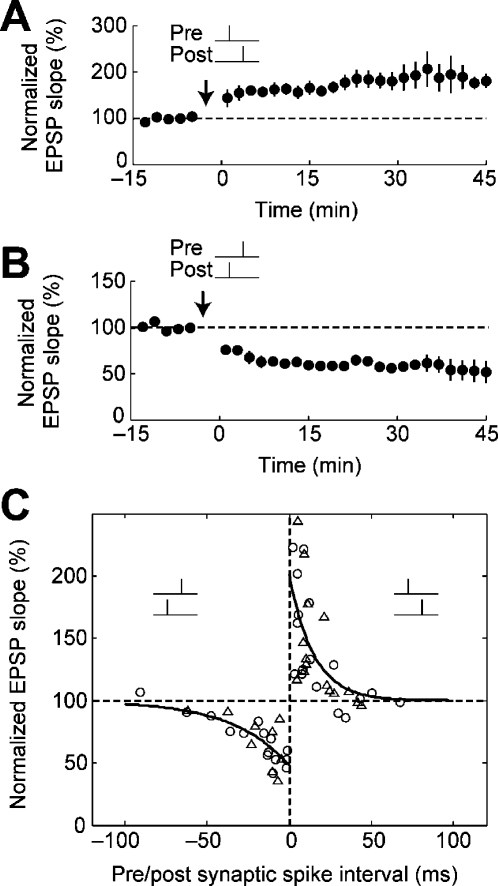
Activity-dependent persistent changes in the strength of synaptic transmission (synaptic strength) are believed to refine neural connections and mediate learning and memory (Hebb, 1949). STDP (spike-timing-dependent synaptic plasticity) has emerged experimentally as a form of such changes in synaptic strength (Magee and Johnston, 1997; Markram et al., 1997; Bi and Poo, 1998; Zhang et al., 1998a). STDP consists of bidirectional long-term changes in synaptic strength, depending on the timing of presynaptic and postsynaptic spikings (prespiking and postspiking). STDP incorporates both persistent increases in synaptic strength [LTP (long-term potentiation)] [Fig. 1A] and persistent decreases in synaptic strength [LTD (long-term depression)] [Fig. 1B]. STDP may serve as a synaptic learning rule that embeds spike-timing information into a specific pattern of synaptic strengths in a neuronal circuit. As such, it has attracted the attention of experimental researchers as well as computational modelers.
Figure 1. A canonical form of STDP.
Reprinted by permission, from Macmillan Publishers Ltd: Nature (Froemke and Dan, 2002), copyright (2002). (A) Repetitive pairings of prespiking→postspiking within a 20 ms interval (20 ms>Tpost−Tpre>0 ms) at 0.2 Hz lead to persistent increase In synaptic strength (LTP). (B) Repetitive pairings of postspiking→prespiking within a 40 ms interval (−40 ms<Tpost−Tpre<0 ms) at 0.2 Hz lead to persistent decrease in synaptic strength (LTD). (C) The critical timing window of STDP. Circles and triangles indicate experimental data (triangles: high Ca2+ and Mg2+ with bicuculline), and solid lines are exponential fits to the data. The data are taken from layer II∕III neurons in the visual cortex (Froemke and Dan, 2002).
Although there are several forms of STDP that vary depending on the types of synapses and neurons involved (for reviews, see Abbott and Nelson, 2000; Caporale and Dan, 2008), here we focus on the canonical form of STDP because it has been the most extensively studied. The canonical form of STDP is induced by 60–100 repetitive pairings of prespiking and postspiking events at a rate of 0.2–5 Hz (for a review, see Caporale and Dan, 2008). tLTP (spike-timing-dependent LTP) is induced when a presynaptic spike is followed by a postsynaptic spike [Fig. 1A]. Conversely, tLTD (spike-timing-dependent LTD) is induced when a postsynaptic spike is followed by a presynaptic spike [Fig. 1B]. The amplitude of both tLTP and tLTD exhibits bidirectional exponential decay as a function of the interval between the prespiking and postspiking events. The range of the spike interval that leads to tLTP and tLTD is referred to as the “critical timing window” of STDP [Fig. 1C]. Hereafter, prespiking preceding postspiking is denoted as prespiking→postspiking, and postspiking preceding presynaptic spiking as postspiking→prespiking.
The canonical form of STDP has been widely observed in the excitatory glutamatergic synapses of the following types of cells: neurons in the visual cortex (Sjostrom et al., 2001; Froemke and Dan, 2002), neurons in the somatosensory cortex (Feldman, 2000; Bender et al., 2006), neurons in the corticostriatum (Pawlak and Kerr, 2008), cultured hippocampal neurons (Bi and Poo, 1998), hippocampal CA3 neurons (Debanne et al., 1998), neurons in the dorsal cochlear nucleus (Tzounopoulos et al., 2004), neurons in the retinotectal projection of the Xenopus laevis tadpole (Zhang et al., 1998a), and neurons in the olfactory system of the locust Schistocerca americana (Cassenaer and Laurent, 2007). Moreover, plasticity involving a similar critical timing window has been observed in hippocampal CA1 neurons, but with distinct features (Pike et al., 1999; Wittenberg and Wang, 2006; Carlisle et al., 2008). In these cells, it has been found that a distinct form of tLTD can be induced by prespiking→postspiking pairings with a 20 ms interval, and postsynaptic bursting is required to induce tLTP. In addition, STDP has been shown to contribute to acquired brain functions such as direction selectivity in the visual system of the Xenopus laevis tadpole (Engert et al., 2002; Mu and Poo, 2006) and the synchronous flow of olfactory information in the locust Schistocerca americana (Cassenaer and Laurent, 2007).
STDP is regulated by signaling mechanisms that consist of electrophysiological and biochemical activities. The signaling mechanisms of STDP constitute a synaptic learning rule. The synaptic learning rule is a mathematical description of synaptic plasticity, describing when and what neural activities are able to induce changes in synaptic strength and explaining how altered synaptic strengths are stabilized. Because the synaptic learning rule governs the changes in synaptic strengths in a neural circuit that represent learning and memory, a complete description of the synaptic learning rule is essential for understanding the representation of learning and memory in the brain. Biophysical modeling and phenomenological modeling are two approaches to describe the synaptic learning rule. Biophysical modeling is a mathematical description of the signaling mechanisms of STDP, which consist of electrophysiological and biochemical processes. Phenomenological modeling of STDP is a mathematical description of its characteristics as an observed phenomenon, without reference to signaling mechanisms.
STDP can be temporally divided into two processes: induction and maintenance. Induction is a process in which prespiking and postspiking initiate signaling events, resulting in changes in synaptic strengths. Maintenance occurs after induction, and is the process by which changes in synaptic strengths are stabilized. In this review we describe signaling mechanisms underlying the induction and maintenance of STDP. In addition, we discuss temporal and spatial aspects of STDP. In vitro experiments examining STDP typically involve stimulation with spiking events at regular intervals. However, neurons in vivo are known to fire spikes irregularly (e.g., Softky and Koch, 1993), and it is unclear how STDP is induced in vivo under natural conditions. Furthermore, the form of STDP differs depending on the location of dendrites (Froemke et al., 2005; Letzkus et al., 2006), and STDP depends on an interaction between multiple presynaptic inputs located at different parts of dendrites (Golding et al., 2002; Letzkus et al., 2006; Sjostrom and Hausser, 2006). Such spatiotemporal aspects must be considered in any realistic discussion of synaptic plasticity in vivo.
A number of reviews have been published on the experimental aspects of STDP (Linden, 1999; Zucker, 1999; Bi and Wang, 2002; Sjostrom and Nelson, 2002; Johnston et al., 2003; Lisman and Spruston, 2005; Dan and Poo, 2006; Duguid and Sjostrom, 2006; Kampa et al., 2007; Letzkus et al., 2007; Caporale and Dan, 2008; Larkum and Nevian, 2008; Sjostrom et al., 2008) as well as the computational aspects involved (Abbott and Nelson, 2000; Worgotter and Porr, 2005; Morrison et al., 2008; see also reviews in Biol. Cybern. 87 (5–6), 2002). At present, however, few reviews have considered both experimental and computational features of STDP together (see Abbott and Nelson, 2000 for one exception). As such, in this review we focus on the intersection between experimental investigations and computational modeling of the signaling mechanisms of STDP.
SIGNALING MECHANISMS OF INDUCTION
Signaling mechanisms of induction of STDP include a spike-timing detector, which is a signaling function that directly detects the timing between prespiking and postspiking events. Many signaling activities have been identified as essential for STDP, but not all would be expected to be involved in spike-timing detection. Although several candidate spike-timing detectors have been proposed (for a review, see Caporale and Dan, 2008), the mechanism underlying this spike-timing detection remains to be elucidated. Because the properties of a spike-timing detector would determine the form of STDP, its characterization is required for a complete description of STDP as a synaptic learning rule.
Signaling mechanisms of induction of tLTP
As described above, tLTP is induced by repetitive pairings of prespiking→postspiking events within a 20 ms interval. Prespiking triggers neurotransmitter release from presynaptic terminals, whereas postspiking triggers a bpAP (back-propagating action potential) that travels back into the dendrites (Spruston et al., 1995). Glutamate, which is a neurotransmitter released in response to prespiking, activates N-methyl-D-aspartate receptors (NMDARs), and mediates a Ca2+ influx to postsynaptic spines via the channel activity of NMDARs. Because of the timing of prespiking→postspiking, the activation of the NMDARs coincides with a bpAP. The simultaneous bpAP removes the voltage-dependent Mg2+ block of the NMDAR channels, and NMDAR activation leads to a larger Ca2+ influx via NMDAR channels than would be induced by prespiking alone (Koester and Sakmann, 1998; Schiller et al., 1998). Therefore, the NMDAR possesses properties, which enable it to function as a detector for the timing of prespiking→postspiking. In the spines, Ca2+ binds to calmodulin, producing Ca2+∕calmodulin. Ca2+∕calmodulin activates multiple kinases including CaMKII (Ca2+∕calmodulin-dependent protein kinase II, see below; Wang et al., 2005; Tzounopoulos et al., 2007) and PKA (protein kinase A) via Ca2+-dependent adenylyl cyclase (Seol et al., 2007). These kinases regulate α-amino-3-hydoxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) (Seol et al., 2007) by increasing AMPAR conductance (Benke et al., 1998), and∕or the delivery of AMPARs to postsynaptic sites (Hayashi et al., 2000). Computational models of tLTP induction have been developed based on the signaling mechanisms described above (Karmarkar and Buonomano, 2002; Shouval et al., 2002).
The timing detection of prespiking→postspiking by NMDARs involves several mechanisms. The voltage-dependent removal of the Mg2+ block of NMDAR channels is facilitated by a boost in the amplitudes of bpAPs induced by stimulation with prespiking→postspiking (Hoffman et al., 1997; Magee and Johnston, 1997; Watanabe et al., 2002; Sjostrom and Hausser, 2006) occurring within a 20 ms interval (Migliore et al., 1999; Stuart and Hausser, 2001). This, in turn, leads to a larger Ca2+ influx, leading to a timing window in the response range of tLTP (20 ms). The boost of bpAP is mediated by fast Na+ channels in the neocortex (Stuart and Hausser, 2001), and by A-type K+ channels in hippocampal CA1 neurons (Hoffman et al., 1997; Watanabe et al., 2002). Mg2+, in addition to providing a voltage-dependent block, also reduces the affinity of the NMDAR for glutamate and reduces the open probability of the NMDAR channels. This also contributes to producing the timing window of tLTP within a 20 ms interval (Kampa et al., 2004). Together, these mechanisms could lead to a consistently larger Ca2+ influx via NMDARs by causing prespiking→postspiking within a 20 ms interval (see also Fuenzalida et al., 2007). The function of these mechanisms, however, may vary depending on the dendritic branching pattern and dendritic location involved. These have been demonstrated by computer simulations (Schaefer et al., 2003; Urakubo et al., 2004). Experimental work has shown that pairings of prespiking→single postspiking events at some synapses do not induce tLTP and that a burst of postspiking is required (Pike et al., 1999; Sjostrom et al., 2001; Watanabe et al., 2002; Kampa et al., 2006; Nevian and Sakmann, 2006; Wittenberg and Wang, 2006). This may be because a burst of postspiking is sufficient to remove the Mg2+ block of NMDAR channels for tLTP, whereas single postspiking is not (Nevian and Sakmann, 2006).
Signaling mechanisms of induction of tLTD
The signaling mechanisms of the induction of tLTD are relatively unclear in comparison with those of tLTP, but evidence has emerged suggesting that at least two alternative signaling mechanisms are involved in its induction. This results in two forms of tLTD: a form of postsynaptic NMDAR-dependent tLTD (Froemke and Dan, 2002; Corlew et al., 2007; Urakubo et al., 2008) and a form of postsynaptic NMDAR-independent tLTD (Sjostrom et al., 2003; Bender et al., 2006; Nevian and Sakmann, 2006; Corlew et al., 2007; Rodriguez-Moreno and Paulsen, 2008). These two distinct signaling mechanisms of tLTD induction depend on the developmental stage involved (Corlew et al., 2007) and are likely to depend on the types of synapses (Tzounopoulos et al., 2007; Brasier and Feldman, 2008) and neurons involved (for a review, see Corlew et al., 2008). Biophysical models have been proposed to explain the mechanisms of both the postsynaptic NMDAR-dependent form (Karmarkar and Buonomano, 2002; Shouval et al., 2002; Rubin et al., 2005; Kubota and Kitajima, 2008; Urakubo et al., 2008) and the postsynaptic NMDAR-independent form of tLTD (Karmarkar and Buonomano, 2002).
Postsynaptic NMDAR-dependent tLTD. tLTD occurs at synapses in layer II∕III in the visual cortex and is dependent on postsynaptic NMDAR activity (Froemke et al., 2005; Corlew et al., 2007; Urakubo et al., 2008). Within a 40 ms postspiking→prespiking interval, the preceding bpAP caused by postspiking suppresses subsequent NMDAR activation caused by prespiking (Froemke et al., 2005). The suppression of NMDAR activation leads to a reduced Ca2+ influx via NMDAR channels, compared with when prespiking occurs alone (Koester and Sakmann, 1998; Nevian and Sakmann, 2004). This reduced Ca2+ influx activates phosphatases such as calcineurin (Wang et al., 2005) and PP1 (protein phosphatase 1) (for a review, see Lisman (2001)) without activating kinases. Evidence has emerged that the activation of phosphatases can lead to the induction of postsynaptically-expressed tLTD (Seol et al., 2007; Pawlak and Kerr, 2008) via the following processes: a decrease in AMPAR conductance via dephosphorylation (Lee et al., 1998; Banke et al., 2000), removal of AMPARs from the postsynaptic sites (Ashby et al., 2004), and internalization of AMPARs by endocytosis (Beattie et al., 2000; Lee et al., 2002; for a review, see Cousin and Robinson, 2001). Postsynaptic NMDAR-dependent tLTD has been modeled on the basis of these signaling mechanisms (Urakubo et al., 2008).
The amplitude of postsynaptic NMDAR-dependent tLTD is correlated with the suppression of NMDAR activation (Froemke et al., 2005). This suggests that the detection of postspiking→prespiking timing for postsynaptic NMDAR-dependent tLTD is achieved by NMDAR suppression. This suppression requires the activity of postsynaptic Ca2+, voltae-gated calcium channels (VGCCs), and calcineurin (Froemke et al., 2005). Ca2+ binds to calmodulin and activates calcineurin. The Ca2+∕calmodulin and active calcineurin then interact with the intracellular domains of NMDARs, which negatively regulate NMDAR activation (Tong et al., 1995; Ehlers et al., 1996; Zhang et al., 1998b; Krupp et al., 1999; Umemiya et al., 2001; Rycroft and Gibb, 2002, 2004). Importantly, the decay time-course of the Ca2+ influx via VGCCs is consistent with the timing window of tLTD (for a review, see Helmchen, 2002). Therefore, the detection of postspiking→prespiking timing for tLTD can be achieved by the following signaling cascade: VGCC activation leads to an increase in intracellular Ca2+ and a subsequently increased level of active calmodulin∕calcineurin, which suppresses NMDAR activation.
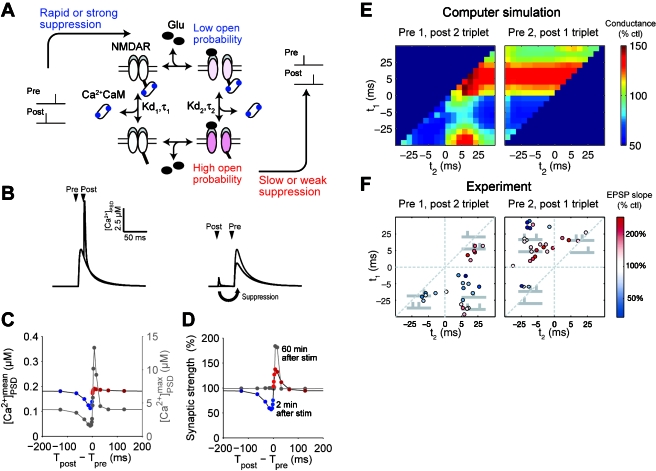
We modeled the mechanism by which Ca2+∕calmodulin, produced by Ca2+ influx via VGCCs, suppresses NMDARs (Urakubo et al., 2008) and found that both prespiking and postspiking induce a Ca2+ influx via both NMDARs and VGCCs, respectively. Ca2+ influx via both NMDARs and VGCCs similarly suppresses NMDARs, and changes in synaptic strengths are the same regardless of spike timing (Urakubo et al., 2008). This indicates that an additional mechanism is required for timing detection, by which a Ca2+ influx from VGCCs (but not from NMDARs) suppresses NMDARs. We have proposed that an allosteric kinetics of NMDARs, in which glutamate binding and calmodulin binding differ depending on the order of their binding, provides such a mechanism [see Urakubo et al., 2008 and Fig. 2A]. We supposed that a Ca2+ influx suppresses glutamate-unbound NMDARs, but not glutamate-bound NMDARs. This would mean that a Ca2+ influx via VGCCs, but not via NMDARs, could suppress NMDARs with the timing of postspiking→prespiking. This would lead to a reduced Ca2+ influx via NMDAR channels, and thus to tLTD. In the model incorporating the allosteric kinetics, NMDARs were successfully suppressed only by postspiking→prespiking within a 40 ms interval, but not by other timing intervals [Figs. 2B, 2C]. This leads to a timing window in the response range of tLTD, and our model successfully reproduced the canonical form of STDP observed experimentally (Froemke and Dan, 2002) [Fig. 2D]. Surprisingly, without parameter tuning, our model was also able to precisely reproduce synaptic plasticity induced by complex spike patterns, such as spike triplets, spike quadruplets, spike bursts, and spike pairs with various frequencies [Figs. 2E, 2F] (Urakubo et al., 2008). Moreover, the changes in the kinetics of NMDARs induced by postspiking→prespiking stimulation observed in experimental studies is consistent with the allosteric kinetics of NMDARs in our model (Urakubo et al., 2008). Overall, the results of our model indicate that the allosteric kinetics of NMDARs (or similar mechanisms) can function as a spike-timing detector in STDP.
Figure 2. Allosteric kinetics of NMDARs and synaptic plasticity induced by spike triplets.
[(A), (C), (D), and (E)] Adapted with permission of the Society for Neuroscience (Urakubo et al., 2008), copyright (2008). (A) Hypothesis of the allosteric kinetics of NMDARs as a spike-timing detector (Urakubo et al., 2008). Ca2+/calmodulin binds to a glutamate-unbound NMDAR more rapidly or strongly than to a glutamate-bound NMDAR. (B) Ca2+ time courses by prespiking→postspiking (left, black) and postspiking→prespiking (right, black) with the allosteric kinetics. Ca2+ increase is given primarily by Ca2+ influx via NMDAR channels and secondarily by Ca2+ influx via VGCCs. Gray traces indicate Ca2+ time courses by prespiking alone. (C) Spike-timing dependency of mean and maximum Ca2+ concentration with the allosteric kinetics. Ca2+ increase is primarily given by Ca2+ influx via NMDAR channels. (D) The critical timing window of STDP with the allosteric kinetics. Synaptic plasticity induced by spike triplets (E) in the model with the allosteric kinetics (Urakubo et al., 2008) and (F) in experiments (Froemke and Dan, 2002). Reprinted by permission, from Macmillan Publishers Ltd: Nature (Froemke and Dan, 2002), copyright (2002). In the left panels (pre-1 and post-2 triplets), t1 indicates the spike interval of the first postspiking event and the prespiking event, and t2 indicates the spike interval of the second postspiking event and the prespiking event. In the right panels (pre-2 and post-1 triplets), t1 indicates the spike interval of the first prespiking event and the postspiking event, and t2 indicates the spike interval of the postspiking event and the second prespiking event.
The proposal that the mechanism of tLTD involves the allosteric kinetics of NMDARs still requires additional experimental support, and there are several caveats to the hypothesis. First, the allosteric kinetics of NMDARs have not been tested experimentally. Second, the hypothesis involves the assumption that timing detection depends on postsynaptic Ca2+. Although there is evidence that postsynaptic Ca2+ is required for the suppression of NMDARs, we cannot exclude the possibility that postsynaptic Ca2+ is merely a prerequisite condition for NMDAR suppression by postspiking (Froemke et al., 2005). Third, although the partial blockage of NMDAR activation by pharmacological agents induces LTD, the suppression of NMDARs via postspiking→prespiking may not be related to tLTD (Froemke et al., 2005; Urakubo et al., 2008). Further study is necessary before we can conclude that the mechanism of postspiking→prespiking timing detection in tLTD involves the allosteric kinetics of NMDARs. However, the finding that our model was able to make accurate predictions about synaptic plasticity induced by complex spike patterns implicates this mechanism, or one that is functionally similar, as a timing detection mechanism in tLTD.
A similar hypothesis for postsynaptic NMDAR-dependent tLTD has been proposed in biophysical models of STDP for hippocampal CA1 neurons (Karmarkar and Buonomano, 2002; Shouval et al., 2002). These models capture the characteristics of tLTD in hippocampal CA1 neurons, including the additional tLTD induced by the pairings of prespiking→postspiking with a 20 ms interval (Nishiyama et al., 2000; Wittenberg and Wang, 2006). However, one study has shown that tLTD in hippocampal CA1 neurons is insensitive to NMDARs but is sensitive to mGluRs (metabotropic glutamate receptors) (Normann et al., 2000). Further experiments are required to determine whether tLTD in hippocampal CA1 neurons is dependent on postsynaptic NMDAR activity.
Postsynaptic NMDAR-independent tLTD. At some other glutamate synapses, including those in the somatosensory cortex (Bender et al., 2006; Nevian and Sakmann, 2006; Rodriguez-Moreno and Paulsen, 2008) and the visual cortex (Sjostrom et al., 2003; Corlew et al., 2007), it has been found that tLTD is insensitive to postsynaptic NMDARs. Such postsynaptic NMDAR-independent tLTD is sensitive to mGluRs (Bender et al., 2006; Nevian and Sakmann, 2006), VGCCs (Bender et al., 2006), cannabinoid receptors (Sjostrom et al., 2003; Bender et al., 2006; Nevian and Sakmann, 2006), and presynaptic NMDARs (Rodriguez-Moreno and Paulsen, 2008). At these synapses, prespiking leads to mGluR activation, and mGluRs activate G-protein coupled signaling mechanisms, resulting in PLC (phospholipase C) and IP3R (inositol trisphosphate receptors) activations (Bender et al., 2006; Nevian and Sakmann, 2006). Postspiking, in turn, leads to a Ca2+ influx via VGCCs, and Ca2+ positively regulates PLC and IP3R activations (Hashimotodani et al., 2005; Bender et al., 2006; Nevian and Sakmann, 2006). The activation of these molecules leads to the release of retrograde messengers (e.g., endocannabinoids) from postsynaptic to presynaptic neurons (Sjostrom et al., 2003; Bender et al., 2006; Nevian and Sakmann, 2006). This retrograde signal decreases the probability of presynaptic neurotransmitter release in concert with presynaptic NMDA autoreceptors (Rodriguez-Moreno and Paulsen, 2008), resulting in tLTD (Sjostrom et al., 2003; Bender et al., 2006; Corlew et al., 2007). In the somatosensory cortex, tLTD is induced by the pairings of postspiking→prespiking, even when the Ca2+ influx exceeds the threshold for tLTP (Nevian and Sakmann, 2006). This may be because tLTD counteracts tLTP under these conditions. Endocannabinoids (Sjostrom et al., 2003) and PLCβ (Hashimotodani et al., 2007) have both been proposed as possible spike-timing detectors for postsynaptic NMDAR-insensitive tLTD. Further study is necessary to address how these molecules discriminate the timing between prespiking and postspiking.
SIGNALING MECHANISMS OF MAINTENANCE
Although stimulation for STDP is transient, changes in synaptic strengths such as postsynaptic AMPAR accumulation and increased neurotransmitter release probability are maintained over hours. This indicates that mechanisms of maintenance must exist by which the changed synaptic strengths are stabilized. The maintenance of plasticity can be generally categorized into early and late phases (for reviews, see Malenka and Bear, 2004 and Blitzer, 2005). The late-phase of maintenance requires gene transcription and protein synthesis. The early phase of maintenance, where the amplitude of changed synaptic strengths initially becomes stable, is likely to be regulated by signaling molecules. Because signaling mechanisms of STDP maintenance have not been extensively investigated, here we focus on the signaling mechanisms of the maintenance of conventional LTP and LTD, which may be similar to those of STDP.
An additional important question regarding the signaling mechanisms of STDP maintenance is whether the amplitude of tLTP and tLTD are graded or binary. The characteristics of graded and binary responses differ substantially; graded responses are continuous and fragile against noise, whereas binary responses are discrete and robust against noise. The canonical form of STDP observed experimentally suggests that tLTP and tLTD must be graded. Some experimental results, however, suggest that LTP is binary (Petersen et al., 1998; O’Connor et al., 2005). This inconsistency may be reconciled if the amplitudes of graded tLTP and tLTD consist of an ensemble of binary tLTP or tLTD. This issue is important because the different characteristics between graded and binary responses would be expected to lead to substantial differences in the way that memory is stored in the brain. Here, we summarize a possible mechanism of the early phase of maintenance and discuss whether tLTP and tLTD are graded or binary.
Signaling mechanisms and binary or graded features of maintenance
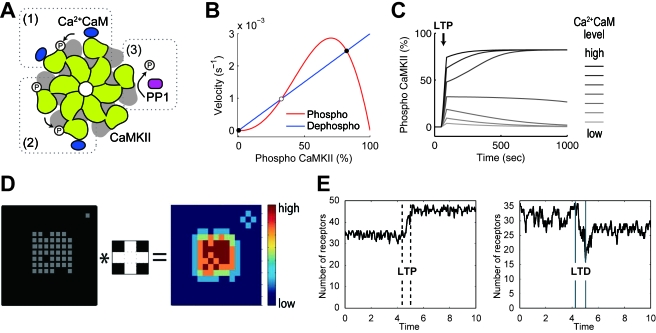
The persistent phosphorylation of CaMKII, which leads to persistent changes in synaptic strength, has been proposed as one of the mechanisms of the early phase of maintenance of LTP (Okamoto and Ichikawa, 2000; Zhabotinsky, 2000; Lisman and Zhabotinsky, 2001). In this theory, the persistent phosphorylation of CaMKII results from the bistablity of CaMKII phosphorylation. CaMKII is a holoenzyme composed of 12 subunits, and Ca2+∕calmodulin-bound subunits cooperatively phosphorylate adjacent subunits [Fig. 3A]. This autophosphorylation of the CaMKII holoenzyme generates two stable steady states: a predominantly unphosphorylated state and an almost fully phosphorylated state [Fig. 3B]. If LTP induction stimulation triggers a Ca2+ increase and Ca2+ exceeds a certain threshold, CaMKII is changed from an unphosphorylated state to an almost fully phosphorylated state and remains persistently phosphorylated after the stimulation is removed [Fig. 3C]. Moreover, evidence has emerged suggesting that the bistable phosphorylation of CaMKII could be an underlying mechanism of depotentiation, which is the reversible transition of a synapse from a potentiated state to a naïve state by LTD stimulation (Hesse and Teyler, 1976; Lee et al., 2000; Huang et al., 2001; Zhou et al., 2003; Graupner and Brunel, 2007; Pi and Lisman, 2008). The modest Ca2+ increase induced by LTD stimulation leads to PP1 activation. PP1, in turn, transiently erases the upper stable steady state of CaMKII phosphorylation. This causes the transition of CaMKII phosphorylation from the almost fully phosphorylated state to the unphosphorylated state, resulting in depotentiation. The results of the experiments regarding CaMKII bistability are equivocal. Some experiments support the bistability of CaMKII (Fukunaga et al., 1993; Barria et al., 1997; Sanhueza et al., 2007), whereas others do not (Malinow et al., 1989; Otmakhov et al., 1997; Lee et al., 2009). Further study is necessary to draw firm conclusions about the existence and role of CaMKII bistability in the early phase of the maintenance of LTP.
Figure 3. The possible roles of CaMKII and AMPAR clustering in maintenance of LTP and LTD.
[(A)–(C)] CaMKII bistability (Lisman and Zhabotinsky, 2001). (A) CaMKII is composed of 12 subunits, and the subunits that bind to Ca2+/calmodulin can phosphorylate neighboring Ca2+/calmodulin-bound subunits (1). Once a subunit is phosphorylated, the subunit can phosphorylate neighboring Ca2+/calmodulin-bound subunits regardless of whether the subunit binds to Ca2+/calmodulin (2). The phosphorylated subunits are dephosphorylated by PP1 (3). (B) The cooperative phosphorylation mechanism makes three balanced states in rates of phosphorylation (red line) and dephosphorylation (blue line). One is unstable (open circle) and two are stable (filled circles). (C) Transient increase (40 s) of Ca2+/calmodulin as LTP stimulation (arrow) leads to transition of CaMKII from the unphosphorylated stable steady state to the fully phosphorylated stable steady state, depending on the amplitudes of the stimulation. The simulation is based on the simple CaMKII model by Dupont et al. (2003). [(d) and (e)] AMPAR clustering (Shouval, 2005). Reproduced with permission, Proceedings of National Academy of Sciences, U.S.A., copyright (2005). (D) Insertion rate of new AMPARs to grids in a lattice space (right panel) is computed from present occupation state of AMPARs (gray, left panel) and the probability of inserting a new AMPAR around an AMPAR-occupied grid (center panel). In the center panel, white grids denote the higher probability of inserting a new AMPAR. Convolution operator (∗) denotes that the insertion probability of an AMPAR (center panel) is applied to all AMPAR-occupied grids (right panel). (E) The AMPAR dynamics keeps the AMPAR number stable after an increase and decrease by transient stimulation, which corresponds to LTP and LTD (left and right, respectively). One unit time is the mean dwell time of an AMPAR in the lattice space.
Although a single holoenzyme of CaMKII can show bistable phosphorylation, an entire population of CaMKII holoenzymes in a postsynaptic spine can show either bistable or graded phosphorylation. The results of a recent experiment suggest the existence of interholoenzyme autophosphorylation of CaMKII (Rose et al., 2009). This autophosphorylation between holoenzymes results in the bistable phosphorylation of an entire population of CaMKII molecules. On the other hand, other experiments have not found interholoenzyme autophosphorylation of CaMKII to occur (Hanson et al., 1994). If interholoenzyme autophosphorylation does not take place, the phosphorylation state of each holoenzyme could be different, resulting in the graded phosphorylation of a population of CaMKII molecules in a spine. Note that CaMKII phosphorylation is known to be ultrasensitive to Ca2+ stimulation (Bradshaw et al., 2003). Therefore, even without interholoenzyme autophosphorylation, a population of CaMKII molecules can show binary (all-or-none) phosphorylation. Therefore, if LTP does indeed rely on CaMKII phosphorylation (for a review, see Lisman et al., 2002), LTP is likely to be binary.
As another possible mechanism of the early phase of the maintenance of LTP and LTD, a computational model has demonstrated that an interaction between AMPARs associated with PSD (postsynaptic density) and free AMPARs can produce graded and stable AMPAR accumulations at the PSD (Shouval, 2005). In this model, the PSD is assumed to be a simple lattice space, which can anchor AMPARs. AMPARs are inserted into vacant grids of the lattice at a rate that follows a function in which the insertion rate of an AMPAR into a grid in the vicinity of other AMPARs is larger than that for a grid isolated from other AMPARs [Fig. 3D]. The inserted AMPARs are removed from the grids at a constant rate. With these assumptions, the AMPAR number is kept stable even after an increase or decrease in the AMPAR number caused by transient stimulation [Fig. 3E]. The AMPAR number at the PSD is constant regardless of dynamic AMPAR trafficking, and AMPARs are transiently trapped by the PSD. Both of these features of the model are consistent with experimental observation (Ehlers et al., 2007). According to this model, LTP and LTD can be graded.
Several additional hypotheses for the maintenance of LTP or LTD have been proposed. A modeling study has suggested that a two-step kinase and phosphatase cycle of AMPARs can result in bistability in AMPAR number at the PSD. This would lead to binary LTD (Hayer and Bhalla, 2005). Another modeling study has revealed that a kinase and phosphatase cycle can enable the maintenance of graded LTP and LTD, if the kinase and phosphatase are activated by stimulation with high Hill coefficients (≥4), and if the reactions are negligible in the basal state (Delord et al., 2007). In addition, one modeling study has revealed that autophosphorylation of PP2A (protein phosphatase 2 A) can function as a bistable switch that could underlie LTD (Pi and Lisman, 2008). The existence of this switch would allow binary LTD to take place.
Recent experiments have revealed that synaptic plasticity is associated with structural plasticity of postsynaptic spines. Changes in spine structure are bidirectional, with stimuli that induce LTP causing spine enlargement (Matsuzaki et al., 2004; Nagerl et al., 2004; Okamoto et al., 2004; Yang et al., 2008) and with stimuli that induce LTD causing spine shrinkage (Nagerl et al., 2004; Okamoto et al., 2004; Zhou et al., 2004; Wang et al., 2007). Spine enlargement occurs in a graded manner (Okamoto et al., 2004) and seems to be necessary for LTP induction (Matsuzaki et al., 2004; Okamoto et al., 2007; Yang et al., 2008). Spine shrinkage shares mechanisms with LTD induction (Zhou et al., 2004) but seems to have less of a direct causal link to LTD induction (Yang et al., 2008).
No biophysical models have been proposed for the maintenance of presynaptically expressed LTP and LTD, because the underlying molecular mechanisms are poorly understood. The mechanisms of maintenance of presynaptically expressed LTP and LTD may involve actin polymerization and depolymerization (for a review, see Cingolani and Goda, 2008). There are currently no experiments that provide an answer to the question of whether presynaptically expressed synaptic plasticity is binary or graded. Further study is required to explore the mechanisms of maintenance of presynaptically expressed LTP and LTD and to examine whether such LTP or LTD is binary or graded.
Additional features of maintenance
Additional features of the maintenance of STDP must be taken into account for an STDP model to provide a synaptic learning rule. Our model operates on the assumption that a synapse has three strength states: LTP, LTD, and naïve states. If synaptic strength operated in a binary fashion, however, then synapses would possess only two strength states, and LTP and LTD would be determined simply by the ratio of the numbers of unpotentiated and potentiated synapses. However, in calcineurin Aα knockout mice, LTD can be induced even though depotentiation is not observed (Zhuo et al., 1999). This finding is in accord with the notion that synaptic connections have three stable strength states, rather than just two binary states.
Spontaneous spiking after induction of tLTP or tLTD leads to depotentiation or de-depression, respectively, and causes potentiated or depressed synapses to return to their naïve states (Zhou et al., 2003). Moreover, after the induction of LTP and LTD by repetitive prespiking, hippocampal CA1 neurons return to their naïve states more easily relative to other states (Mulkey et al., 1993; Heynen et al., 2000; Krucker et al., 2002). Conversely, in the retinotectal projection of the Xenopus tadpole, LTP is stabilized by repetitive stimulation with 5 min intervals that causes the induction of tLTP (Zhou et al., 2003) and is also stabilized over 15 min after induction in hippocampal CA1 synapses (Staubli and Chun, 1996). In addition, the depressed synapses may be eliminated (Montgomery and Madison, 2002; Luthi et al., 2004; Bastrikova et al., 2008).
Some theoretical studies have suggested that the application of random synaptic inputs to a neuron with STDP as a synaptic learning rule eventually leads to a bimodal distribution of synaptic strengths (Song et al., 2000; Cateau and Fukai, 2003). The predicted bimodal distribution, however, is inconsistent with the unimodal distribution of synaptic strengths observed in in vivo experiments (van Rossum et al., 2000). This bimodal distribution of synaptic strengths can become unimodal if the same spike pairings induce weaker tLTP at the stronger synapses (van Rossum et al., 2000). Furthermore, an alternative explanation can be provided by additional features of maintenance. Even if the distribution of synaptic strengths, which are stable during maintenance, becomes bimodal, the synapses that are associated with the weaker peak of the bimodal distribution may be subjected to elimination, resulting in a unimodal distribution of the remaining synapses that are associated with the stronger peak.
TEMPORAL AND SPATIAL ASPECTS OF STDP
In the methodology of many in vitro STDP experiments, the intervals between prespiking and postspiking pairings are constant and are applied to the same dendritic site. However, as mentioned earlier, neurons in vivo are known to fire spikes at irregular intervals (Softky and Koch, 1993). Moreover, multiple synaptic inputs occur at different sites along dendrites (for a review, see Spruston, 2008). Here, we review STDP taking into account temporal features involving complex spike patterns and spatial features involving the interaction of multiple synaptic inputs at different sites along the dendrites.
Temporal aspects of STDP
In the canonical form of STDP, the repetitive pairings of prespiking and postspiking can be separated into interactions of potentiation effects of prespiking→postspiking and depression effects of postspiking→prespiking. The linear summation of these potentiation and depression effects reproduces the canonical form of STDP with spike pairs but is not able to reproduce the results observed when synaptic plasticity is induced by spike triplets (a set of three spiking events including either two prespiking and one postspiking events, or one prespiking and two postspiking events) in layer II∕III neurons of the visual cortex (Froemke and Dan, 2002) and cultured hippocampal neurons (Wang et al., 2005). This indicates that there is a nonlinear interaction between spikes in STDP.
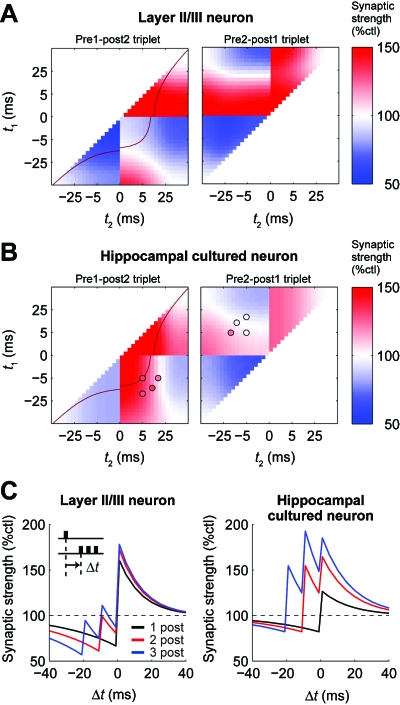
Although the canonical form of STDP elicited by pairings of a single prespiking event with a single postspiking event are similar between layer II∕III neurons (Froemke and Dan, 2002) and cultured hippocampal neurons (Bi and Poo, 1998), synaptic plasticity induced by spike triplets differs substantially between these two types of neurons [see Froemke and Dan, 2002 and Wang et al., 2005, and also Figs. 2F, 4A, 4B]. The amplitude of synaptic plasticity induced by spike triplets is a function of two variables (t1,t2), where t1 represents the relative timing of the first prespiking event to the postspiking event (two prespiking and one postspiking) or the timing of the prespiking event to the first postspiking event (one prespiking and two postspiking), and t2 represents the relative timing of the second prespiking event to the postspiking event (two prespiking and one postspiking) or the timing of the prespiking event to the second postspiking event (one prespiking and two postspiking) [Figs. 2F, 4A, 4B]. In layer II∕III neurons of the visual cortex, synaptic plasticity induced by spike triplets can be phenomenologically explained by nonlinear interactions of two independent spike pairs [Figs. 2F, 4A] (Froemke and Dan, 2002). The nonlinear interactions of two independent spike pairs can be formalized as a summation of independent spike pairs with a multiplication of spike efficacy, which is transiently “decreased” by the preceding spike in a presynaptic and a postsynaptic neuron [Fig. 4A] (Froemke and Dan, 2002; Froemke et al., 2006). Similarly, in cultured hippocampal neurons, synaptic plasticity induced by spike triplets has been found to show nonlinear interactions between two independent spike pairs. However, synaptic plasticity induced by spike triplets is different from that in layer II∕III neurons of the visual cortex [Fig. 4B] (Wang et al., 2005). For example, by postspiking→prespiking→postspiking pairings (−5 ms, 5 ms), tLTP is induced in cultured hippocampal neurons, whereas tLTD is induced in layer II∕III of the visual cortex [Fig. 4B]. Also, by prespiking→postspiking→prespiking pairings (5 ms, −5 ms), no plasticity is induced in cultured hippocampal neurons, whereas tLTP is induced in layer II∕III of the visual cortex [Fig. 4B, right, circles]. In cultured hippocampal neurons, synaptic plasticity induced by spike triplets can also be phenomenologically formalized as a summation of the effects of two independent spike pairs with a multiplication of spike efficacy. However, unlike in layer II∕III of the visual cortex, spike efficacy in cultured hippocampal cells is transiently “increased” by the preceding spike in presynaptic and postsynaptic neurons [Fig. 4B] (Pfister and Gerstner, 2006). Both phenomenological models in layer II∕III of the visual cortex and cultured hippocampal neurons can explain synaptic plasticity induced by complex spike patterns (Froemke and Dan, 2002; Froemke et al., 2006; Pfister and Gerstner, 2006). This implies that there are distinct signaling mechanisms for STDP in each of these two types of neurons.
Figure 4. Difference of synaptic plasticity induced by spike triplets in layer II/III neurons and cultured hippocampal neurons.
(A) Synaptic plasticity induced by spike triplets in a phenomenological model for layer II/III visual cortex (Froemke et al., 2006). Definition of t1 and t2 and the corresponding experimental data are shown in Fig. 2F. Red line in the left panel corresponds to a red line in (C), which shows the timing window of STDP with pairings of a prespiking event and two postspiking events, the latter of which have a 10 ms interval. (B) Synaptic plasticity induced by spike triplets in a phenomenological model for cultured hippocampal neurons (Pfister and Gerstner, 2006). Colored circles indicate experimental data (Wang et al., 2005). (C) The timing windows of STDP with single prespiking and postburst spiking in the phenomenological models (Wang et al., 2005; Froemke et al., 2006). Δt indicates the interval between the prespiking event and the first postspiking event. Black lines show the conventional STDP induced by pairings of a single prespiking event and a single postspiking event; red lines show synaptic plasticity induced by pairings of a single prespiking event and two postspiking events, the latter of which have a 10 ms interval; and blue lines show synaptic plasticity induced by pairings of a single prespiking event and three postspiking events, the latter of which have 10 ms intervals.
These phenomenological models explain the distinct characteristics of STDP in layer II∕III neurons in the visual cortex and cultured hippocampal neurons. In particular, they describe the effects of a prespiking event on postburst spiking, which is a series of multiple postspiking events. For example, consider synaptic plasticity induced by spike triplets with a single prespiking event and two postspiking events, where the interval of the latter is 10 ms [Figs. 4A, 4B, red lines]. In layer II∕III of the visual cortex, because the synaptic efficacy is transiently decreased by the preceding spiking, the effect of a second postspiking event is decreased by the first postspiking event. Therefore, the first postspiking event always becomes dominant compared with the following postspiking event in postburst spiking, so that the timing between the first postspiking event and the prespiking event determines the change in synaptic strength [Fig. 4C, left]. By contrast, in cultured hippocampal neurons, because synaptic efficacy is transiently increased by the preceding spiking, the effect of the second postspiking event is increased by the first postspiking event. Therefore, the last postspiking event always becomes dominant compared with the preceding postspiking event in postburst spiking. This means that the timing between the last postspiking event and the prespiking event determines the resultant change in synaptic strength [Fig. 4B, right]. Because burst-spiking is often observed in vivo (for a review, see Steriade, 2004), the first and last postspiking events on synaptic efficacy are likely to be physiologically dominant in layer II∕III and hippocampal neurons, respectively. This suggests that distinct signaling mechanisms underlie each of these types of synaptic plasticity. Thus, the description of synaptic plasticity induced by spike triplets in phenomenological models highlights specific features of synaptic plasticity in layer II∕III and hippocampal neurons. This modeling is an important first step toward understanding how synaptic plasticity is induced by natural firing patterns in vivo and toward describing a general synaptic learning rule.
Biophysical models of signaling mechanisms of synaptic plasticity have been proposed for both layer II∕III of the visual cortex (Urakubo et al., 2008) and for cultured hippocampal neurons (Rubin et al., 2005). Models for both of these cell types have been found to reproduce synaptic plasticity induced by spike triplets. In the biophysical model for layer II∕III neurons, synaptic plasticity by spike triplets has been explained by a suppression of NMDAR activation by preceding prespikes or postspikes (Urakubo et al., 2008). This NMDAR suppression by postspiking is similar to the transient decrease in synaptic efficacy by the preceding spiking in the phenomenological model for layer II∕III neurons (Froemke and Dan, 2002; Froemke et al., 2006). In the biophysical model for cultured hippocampal neurons, synaptic plasticity induced by spike triplets is explained by the detection of the postsynaptic Ca2+ time-course via downstream signaling molecules (Rubin et al., 2005). However, the signaling mechanisms responsible for causing transient increases in synaptic efficacy in response to the preceding spiking are not simple or obvious in this biophysical model.
Spatial aspects of STDP
Because bpAPs do not typically propagate into distal apical dendrites, prespiking→postspiking pairings fail to induce tLTP at distal apical synapses (Golding et al., 2002; Sjostrom and Hausser, 2006). However, tLTP is induced by prespiking→postspiking pairings at distal apical synapses when excitatory inputs are added to proximal apical dendrites in the neocortex (Letzkus et al., 2006; Sjostrom and Hausser, 2006). This appears to be due to the boosting of bpAPs by simultaneous excitatory inputs at proximal apical dendrites, which propagate into distal apical synapses (Hoffman et al., 1997; Magee and Johnston, 1997; Stuart and Hausser, 2001). Thus, excitatory inputs from proximal apical synapses can act as a “gate signal” for tLTP at distal apical synapses by prespiking and postspiking. Such bpAP boosting has been observed in vivo as a supralinear Ca2+ influx in dendrites (Waters and Helmchen, 2004).
In addition, LTP at distal apical synapses can be induced by cooperative synaptic inputs, which lead to local dendritic spiking (Golding et al., 2002). Similarly, LTP at distal basal synapses is also induced by cooperative prespiking rather than simple prespiking→postspiking pairings. Cooperative prespiking has been found to lead to local NMDAR spikes, in the presence of BDNF (brain-derived neurotrophic factor) (Gordon et al., 2006; but see Holthoff, 2004). Thus, LTP at distal synapses depends not only on the timing of prespiking and postspiking, but also on the spike timing between multiple prespiking events.
Moreover, there is evidence that the features of STDP differ depending on the location of dendrites. In layer II∕III of the visual cortex, the tLTP amplitude at middle apical synapses is lower than that at proximal apical synapses, and the tLTD timing window at middle apical synapses is broader than that at proximal apical synapses (Froemke et al., 2005). In the somatosensory cortex, STDP exhibits the canonical form at proximal apical synapses, whereas STDP exhibits the reversed form at distal apical synapses where tLTP and tLTD are induced by postspiking→prespiking and prespiking→postspiking, respectively (Letzkus et al., 2006).
Incorporating these spatial features of STDP into biophysical models will allow us to explore the complex interaction between the spatial and temporal information involved in spiking.
REQUIREMENT OF A HOMEOSTATIC MECHANISM OF STDP IN VIVO
STDP shows a similar canonical form both in vitro and in vivo (Zhang et al., 1998a; Yao and Dan, 2001; Wolters et al., 2003; Celikel et al., 2004; Yao et al., 2004; Meliza and Dan, 2006; Cassenaer and Laurent, 2007). This makes sense if the membrane potential of neurons in vivo is maintained around the resting membrane potential, in conditions similar to those of neurons in vitro (Zhang et al., 1998a). However, membrane potentials of neurons in vivo are likely to fluctuate because of excitatory and inhibitory inputs from other neurons (Yao and Dan, 2001; Wolters et al., 2003; Celikel et al., 2004; Yao et al., 2004; Meliza and Dan, 2006; Cassenaer and Laurent, 2007). Membrane potential fluctuations affect NMDAR and VGCC activities (which are dependent on membrane potential), and thereby change the Ca2+ influx, which is a major determinant of tLTP and tLTD (see above). Therefore, under conditions of membrane potential fluctuation, STDP in vivo is likely to differ from STDP in vitro, even with the same spike pairings. In particular, membrane potentials of neurons in vivo sometimes hover near a spiking threshold because of ongoing excitatory inputs (for a review, see Destexhe et al., 2003), and under these conditions, lessening the voltage-dependent Mg2+ block of the NMDAR would lead to a larger Ca2+ influx via NMDAR channels. According to the present biophysical models of STDP, tLTP is induced regardless of spike timing in these synapses, because the large Ca2+ influx exceeds the threshold for tLTP induction (Karmarkar and Buonomano, 2002; Shouval et al., 2002; Rubin et al., 2005; Urakubo et al., 2008). In contrast, uncorrelated pairings of prespiking and postspiking induce no plasticity in STDP in vivo (Celikel et al., 2004; Meliza and Dan, 2006; Cassenaer and Laurent, 2007). Despite the theoretical effects of membrane potential fluctuations, experimentally STDP has been found to show a similar canonical form both in vitro and in vivo. This suggests the existence of homeostatic mechanism in vivo, which compensates for fluctuations of membrane potential. Taken together, these findings demonstrate the utility of biophysical models for extending theoretical knowledge of the mechanisms underlying synaptic plasticity.
CONCLUDING REMARKS
STDP has been robustly observed in vivo in many species, including Xenopus laevis tadpoles (Zhang et al., 1998a), Schistocerca americana locusts (Cassenaer and Laurent, 2007), rats (Celikel et al., 2004; Meliza and Dan, 2006; Jacob et al., 2007), ferrets (Dahmen et al., 2008), cats (Schuett et al., 2001; Yao and Dan, 2001; Fu et al., 2002; Yao et al., 2004), and humans (Yao and Dan, 2001; Fu et al., 2002; Wolters et al., 2003). In particular, STDP in vivo has been well studied in the cat visual cortex (Schuett et al., 2001; Yao and Dan, 2001; Yao et al., 2004), rat somatosensory cortex (Celikel et al., 2004; Jacob et al., 2007), and the retinotectal projection of the Xenopus laevis tadpole (Zhang et al., 1998a; Engert et al., 2002; Zhou et al., 2003; Mu and Poo, 2006; Vislay-Meltzer et al., 2006). Nevertheless, some authors are critical of STDP in vivo (for a review, see Lisman and Spruston, 2005). They have pointed out that tLTP is not unconditionally induced by prespiking→postspiking pairings, but only occurs in the presence of sufficient depolarization such as that caused by large excitatory postsynaptic potentials (EPSPs) (Sjostrom et al., 2001). Furthermore, some authors have also pointed out that LTP and LTD in vivo depend not only on precise timing between prespiking and postspiking, but also on the timing between prespiking and other postsynaptic depolarization events, such as dendritic spikes induced by prespiking (Golding et al., 2002; Sjostrom et al., 2004; Hardie and Spruston, 2009). Despite this, biophysical models can potentially provide a framework for unifying various types of synaptic plasticity, describing plasticity that depends not only on precise timing between prespiking and postspiking, but also plasticity dependent on the timing between prespiking and other postsynaptic depolarization. We propose that biophysical models can be applied to both of these types of synaptic plasticity.
The signaling mechanisms of STDP are mediated by a number of signaling molecules, and STDP depends not only on the temporal interaction of prespiking and postspiking events, but also on spatial interaction at the dendrites. Thus, STDP involves multiple layers of complexity. One possible approach to handling the complex nature of STDP is detailed biophysical modeling that can explicitly incorporate experimental findings by basing features of the model on biophysical mechanisms. The accumulation of experimental evidence makes it possible to construct realistic electrophysiological and morphological models of neurons as well as kinetic models of intracellular biochemical reactions (e.g., Bhalla and Iyengar, 1999; Kuroda et al., 2001; Poirazi et al., 2003; Schaefer et al., 2003). While these models may have limitations in their mechanics and parameters, they have nevertheless been proved useful for prediction. Biophysical modeling is a powerful technique, particularly in cases where rich experimental data are available. A biophysical model that is sufficiently constrained by experimental data can provide quantitative verification of experimentally proposed mechanisms and can facilitate experimental studies by proposing potential mechanisms to fill gaps in the experimental evidence. For example, the involvement of allosteric kinetics of NMDARs was logically predicted by a biophysical model and was subsequently found to be consistent with experimental data (Urakubo et al., 2008).
We note that phenomenological models of STDP, which are based on experiments in vitro, may not reliably predict STDP in vivo, because of membrane potential fluctuations (Sjostrom et al., 2001; Froemke and Dan, 2002; Froemke et al., 2006; Pfister and Gerstner, 2006). Phenomenological models are based on the principle of “Occam’s razor,” which holds that simpler models are better than complex ones and that fewer parameters are better than more. This modeling approach, generally used for statistical models, is effective in making inferences about synaptic plasticity under conditions where experimental data are available but is not suitable for making predictions in novel environments. To predict synaptic plasticity in a novel environment, phenomenological models first require experimental data from tests in that environment. In contrast, biophysical models are based on not only experimental data but also on prior knowledge of signaling mechanisms and are therefore more powerful for the prediction of synaptic plasticity in novel environments. Once a detailed biophysical model is described, it can be simplified by pruning redundant mechanisms in a way that preserves its fundamental framework. By extracting the essential parts of the model, we are able to interpret the functional roles of synaptic plasticity in neuronal circuits in vivo more realistically.
The brain can be considered to be a memory-driven system. Unlike a computer, the brain stores memory as a pattern of synaptic strengths associated with neurons as simple computational units. Input signals through synapses allow neurons to process information and to further modify synaptic strengths. Differently modified synapses guide input signals to the neuron in different ways to enable more efficient information processing. Although the activity-dependent modification of synaptic strengths cannot be easily described by a simple synaptic learning rule due to the complex signaling machinery involved, the biophysical modeling approach has the potential to bridge the gap between the phenomenological and mechanistic aspects of synaptic changes, and may potentially lead to a complete understanding of synaptic plasticity in neuronal circuits.
ACKNOWLEDGMENTS
This work was supported by a grant in-aid for scientific research in the “Brain and Neural Systems Group, Computational Science Research Program” and priority areas of “Systems Genomics” (Contract No. 17017005) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- Abbott, LF, and Nelson, SB (2000). “Synaptic plasticity: taming the beast.” Nat. Neurosci. 3, 1178–1183. 10.1038/81453 [DOI] [PubMed] [Google Scholar]
- Ashby, MC, De La Rue, SA, Ralph, GS, Uney, J, Collingridge, GL, and Henley, JM (2004). “Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs.” J. Neurosci. 24, 5172–5176. 10.1523/JNEUROSCI.1042-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke, TG, Bowie, D, Lee, H, Huganir, RL, Schousboe, A, and Traynelis, SF (2000). “Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase.” J. Neurosci. 20, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria, A, Muller, D, Derkach, V, Griffith, LC, and Soderling, TR (1997). “Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation.” Science 276, 2042–2045. 10.1126/science.276.5321.2042 [DOI] [PubMed] [Google Scholar]
- Bastrikova, N, Gardner, GA, Reece, JM, Jeromin, A, and Dudek, SM (2008). “Synapse elimination accompanies functional plasticity in hippocampal neurons.” Proc. Natl. Acad. Sci. U.S.A. 105, 3123–3127. 10.1073/pnas.0800027105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie, EC, Carroll, RC, Yu, X, Morishita, W, Yasuda, H, von Zastrow, M, and Malenka, RC (2000). “Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD.” Nat. Neurosci. 3, 1291–1300. 10.1038/81823 [DOI] [PubMed] [Google Scholar]
- Bender, VA, Bender, KJ, Brasier, DJ, and Feldman, DE (2006). “Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex.” J. Neurosci. 26, 4166–4177. 10.1523/JNEUROSCI.0176-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke, TA, Lüthi, A, Isaac, J TR, and Collingridge, GL (1998). “Modulation of AMPA receptor unitary conductance by synaptic activity.” Nature (London) 393, 793–797. 10.1038/31709 [DOI] [PubMed] [Google Scholar]
- Bhalla, US, and Iyengar, R (1999). “Emergent properties of networks of biological signaling pathways.” Science 283, 381–387. 10.1126/science.283.5400.381 [DOI] [PubMed] [Google Scholar]
- Bi, GQ, and Poo, MM (1998). “Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type.” J. Neurosci. 18, 10464–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi, GQ, and Wang, HX (2002). “Temporal asymmetry in spike timing-dependent synaptic plasticity.” Physiol. Behav. 77, 551–555. 10.1016/S0031-9384(02)00933-2 [DOI] [PubMed] [Google Scholar]
- Blitzer, RD (2005). “Long-term potentiation: mechanisms of induction and maintenance.” Sci. STKE 2005, tr26. 10.1126/stke.3092005tr26 [DOI] [PubMed] [Google Scholar]
- Bradshaw, JM, Kubota, Y, Meyer, T, and Schulman, H (2003). “An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling.” Proc. Natl. Acad. Sci. U.S.A. 100, 10512–10517. 10.1073/pnas.1932759100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier, DJ, and Feldman, DE (2008). “Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex.” J. Neurosci. 28, 2199–2211. 10.1523/JNEUROSCI.3915-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale, N, and Dan, Y (2008). “Spike timing-dependent plasticity: a Hebbian learning rule.” Annu. Rev. Neurosci. 31, 25–46. 10.1146/annurev.neuro.31.060407.125639 [DOI] [PubMed] [Google Scholar]
- Carlisle, HJ, Fink, AE, Grant, SG, and O’Dell, TJ (2008). “Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity.” J. Physiol. 586, 5885–5900. 10.1113/jphysiol.2008.163469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassenaer, S, and Laurent, G (2007). “Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts.” Nature (London) 448, 709–713. 10.1038/nature05973 [DOI] [PubMed] [Google Scholar]
- Cateau, H, and Fukai, T (2003). “A stochastic method to predict the consequence of arbitrary forms of spike-timing-dependent plasticity.” Neural Comput. 15, 597–620. 10.1162/089976603321192095 [DOI] [PubMed] [Google Scholar]
- Celikel, T, Szostak, VA, and Feldman, DE (2004). “Modulation of spike timing by sensory deprivation during induction of cortical map plasticity.” Nat. Neurosci. 7, 534–541. 10.1038/nn1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani, LA, and Goda, Y (2008). “Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy.” Nat. Rev. Neurosci. 9, 344–356. 10.1038/nrn2373 [DOI] [PubMed] [Google Scholar]
- Corlew, R, Brasier, DJ, Feldman, DE, and Philpot, BD (2008). “Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity.” Neuroscientist 14, 609–625. 10.1177/1073858408322675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew, R, Wang, Y, Ghermazien, H, Erisir, A, and Philpot, BD (2007). “Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression.” J. Neurosci. 27, 9835–9845. 10.1523/JNEUROSCI.5494-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin, MA, and Robinson, PJ (2001). “The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis.” Trends Neurosci. 24, 659–665. 10.1016/S0166-2236(00)01930-5 [DOI] [PubMed] [Google Scholar]
- Dahmen, JC, Hartley, DE, and King, AJ (2008). “Stimulus-timing-dependent plasticity of cortical frequency representation.” J. Neurosci. 28, 13629–13639. 10.1523/JNEUROSCI.4429-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan, Y, and Poo, MM (2006). “Spike timing-dependent plasticity: from synapse to perception.” Physiol. Rev. 86, 1033–1048. 10.1152/physrev.00030.2005 [DOI] [PubMed] [Google Scholar]
- Debanne, D, Gahwiler, BH, and Thompson, SM (1998). “Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures.” J. Physiol. 507(Pt 1), 237–247. 10.1111/j.1469-7793.1998.237bu.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delord, B, Berry, H, Guigon, E, and Genet, S (2007). “A new principle for information storage in an enzymatic pathway model.” PLOS Comput. Biol. 3, 1123–1135. 10.1371/journal.pcbi.0030124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe, A, Rudolph, M, and Pare, D (2003). “The high-conductance state of neocortical neurons in vivo.” Nat. Rev. Neurosci. 4, 739–751. 10.1038/nrn1198 [DOI] [PubMed] [Google Scholar]
- Duguid, I, and Sjostrom, PJ (2006). “Novel presynaptic mechanisms for coincidence detection in synaptic plasticity.” Curr. Opin. Neurobiol. 16, 312–322. 10.1016/j.conb.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Dupont, G, Houart, G, and De Koninck, P (2003). “Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model.” Cell Calcium 34, 485–497. 10.1016/S0143-4160(03)00152-0 [DOI] [PubMed] [Google Scholar]
- Ehlers, MD, Heine, M, Groc, L, Lee, MC, and Choquet, D (2007). “Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity.” Neuron 54, 447–460. 10.1016/j.neuron.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers, MD, Zhang, S, Bernhadt, JP, and Huganir, RL (1996). “Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit.” Cell 84, 745–755. 10.1016/S0092-8674(00)81052-1 [DOI] [PubMed] [Google Scholar]
- Engert, F, Tao, HW, Zhang, LI, and Poo, MM (2002). “Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons.” Nature (London) 419, 470–475. 10.1038/nature00988 [DOI] [PubMed] [Google Scholar]
- Feldman, DE (2000). “Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex.” Neuron 27, 45–56. 10.1016/S0896-6273(00)00008-8 [DOI] [PubMed] [Google Scholar]
- Froemke, RC, and Dan, Y (2002). “Spike-timing-dependent synaptic modification induced by natural spike trains.” Nature (London) 416, 433–438. 10.1038/416433a [DOI] [PubMed] [Google Scholar]
- Froemke, RC, Poo, MM, and Dan, Y (2005). “Spike-timing-dependent synaptic plasticity depends on dendritic location.” Nature (London) 434, 221–225. 10.1038/nature03366 [DOI] [PubMed] [Google Scholar]
- Froemke, RC, Tsay, IA, Raad, M, Long, JD, and Dan, Y (2006). “Contribution of individual spikes in burst-induced long-term synaptic modification.” J. Neurophysiol. 95, 1620–1629. 10.1152/jn.00910.2005 [DOI] [PubMed] [Google Scholar]
- Fu, YX, Djupsund, K, Gao, H, Hayden, B, Shen, K, and Dan, Y (2002). “Temporal specificity in the cortical plasticity of visual space representation.” Science 296, 1999–2003. 10.1126/science.1070521 [DOI] [PubMed] [Google Scholar]
- Fuenzalida, M, Fernandez de Sevilla, D, and Buno, W (2007). “Changes of the EPSP waveform regulate the temporal window for spike-timing-dependent plasticity.” J. Neurosci. 27, 11940–11948. 10.1523/JNEUROSCI.0900-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga, K, Stoppini, L, Miyamoto, E, and Muller, D (1993). “Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II.” J. Biol. Chem. 268, 7863–7867. [PubMed] [Google Scholar]
- Golding, NL, Staff, NP, and Spruston, N (2002). “Dendritic spikes as a mechanism for cooperative long-term potentiation.” Nature (London) 418, 326–331. 10.1038/nature00854 [DOI] [PubMed] [Google Scholar]
- Gordon, U, Polsky, A, and Schiller, J (2006). “Plasticity compartments in basal dendrites of neocortical pyramidal neurons.” J. Neurosci. 26, 12717–12726. 10.1523/JNEUROSCI.3502-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner, M, and Brunel, N (2007). “STDP in a bistable synapse model based on CaMKII and associated signaling pathways.” PLOS Comput. Biol. 3, 2299–2323. 10.1371/journal.pcbi.0030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, PI, Meyer, T, Stryer, L, and Schulman, H (1994). “Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals.” Neuron 12, 943–956. 10.1016/0896-6273(94)90306-9 [DOI] [PubMed] [Google Scholar]
- Hardie, J, and Spruston, N (2009). “Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons.” J. Neurosci. 29, 3233–3241. 10.1523/JNEUROSCI.6000-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani, Y, Ohno-Shosaku, T, and Kano, M (2007). “Ca2+-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities.” Curr. Opin. Neurobiol. 17, 360–365. 10.1016/j.conb.2007.03.012 [DOI] [PubMed] [Google Scholar]
- Hashimotodani, Y, Ohno-Shosaku, T, Tsubokawa, H, Ogata, H, Emoto, K, Maejima, T, Araishi, K, Shin, HS, and Kano, M (2005). “Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal.” Neuron 45, 257–268. 10.1016/j.neuron.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Hayashi, Y, Shi, SH, Esteban, JA, Piccini, A, Poncer, JC, and Malinow, R (2000). “Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction.” Science 287, 2262–2267. 10.1126/science.287.5461.2262 [DOI] [PubMed] [Google Scholar]
- Hayer, A, and Bhalla, US (2005). “Molecular switches at the synapse emerge from receptor and kinase traffic.” PLOS Comput. Biol. 1, 137–154. 10.1371/journal.pcbi.0010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb, D (1949). The Organization of Behavior, Wiley, New York. [Google Scholar]
- Helmchen, F (2002). “Raising the speed limit—fast Ca2+ handling in dendritic spines.” Trends Neurosci. 25, 438–441, discussion 441. 10.1016/S0166-2236(02)02232-4 [DOI] [PubMed] [Google Scholar]
- Hesse, GW, and Teyler, TJ (1976). “Reversible loss of hippocampal long term potentiation following electroconvulsive seizures.” Nature (London) 264, 562–564. 10.1038/264562a0 [DOI] [PubMed] [Google Scholar]
- Heynen, AJ, Quinlan, EM, Bae, DC, and Bear, MF (2000). “Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo.” Neuron 28, 527–536. 10.1016/S0896-6273(00)00130-6 [DOI] [PubMed] [Google Scholar]
- Hoffman, DA, Magee, JC, Colbert, CM, and Johnston, D (1997). “K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons.” Nature (London) 387, 869–875. 10.1038/42571 [DOI] [PubMed] [Google Scholar]
- Huang, CC, Liang, YC, and Hsu, KS (2001). “Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses.” J. Biol. Chem. 276, 48108–48117. [DOI] [PubMed] [Google Scholar]
- Jacob, V, Brasier, DJ, Erchova, I, Feldman, D, and Shulz, DE (2007). “Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat.” J. Neurosci. 27, 1271–1284. 10.1523/JNEUROSCI.4264-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, D, Christie, BR, Frick, A, Gray, R, Hoffman, DA, Schexnayder, LK, Watanabe, S, and Yuan, LL (2003). “Active dendrites, potassium channels and synaptic plasticity.” Philos. Trans. R. Soc. London, Ser. B 358, 667–674. 10.1098/rstb.2002.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa, BM, Clements, J, Jonas, P, and Stuart, GJ (2004). “Kinetics of Mg2+unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity.” J. Physiol. 556, 337–345. 10.1113/jphysiol.2003.058842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa, BM, Letzkus, JJ, and Stuart, GJ (2006). “Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity.” J. Physiol. 574, 283–290. 10.1113/jphysiol.2006.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa, BM, Letzkus, JJ, and Stuart, GJ (2007). “Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity.” Trends Neurosci. 30, 456–463. 10.1016/j.tins.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Karmarkar, UR, and Buonomano, DV (2002). “A model of spike-timing dependent plasticity: one or two coincidence detectors?” J. Neurophysiol. 88, 507–513. [DOI] [PubMed] [Google Scholar]
- Koester, HJ, and Sakmann, B (1998). “Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials.” Proc. Natl. Acad. Sci. U.S.A. 95, 9596–9601. 10.1073/pnas.95.16.9596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker, T, Siggins, GR, McNamara, RK, Lindsley, KA, Dao, A, Allison, DW, De Lecea, L, Lovenberg, TW, Sutcliffe, JG, and Gerendasy, DD (2002). “Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus.” J. Neurosci. 22, 5525–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp, JJ, Vissel, B, Thomas, CG, Heinemann, SF, and Westbrook, GL (1999). “Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors.” J. Neurosci. 19, 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, S, and Kitajima, T (2008). “A model for synaptic development regulated by NMDA receptor subunit expression.” J. Comput. Neurosci. 24, 1–20. 10.1007/s10827-007-0036-8 [DOI] [PubMed] [Google Scholar]
- Kuroda, S, Schweighofer, N, and Kawato, M (2001). “Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation.” J. Neurosci. 21, 5693–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum, ME, and Nevian, T (2008). “Synaptic clustering by dendritic signaling mechanisms.” Curr. Opin. Neurobiol. 18, 321–331. 10.1016/j.conb.2008.08.013 [DOI] [PubMed] [Google Scholar]
- Lee, HK, Kameyama, K, Huganir, RL, and Bear, MF (1998). “NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus.” Neuron 21, 1151–1162. 10.1016/S0896-6273(00)80632-7 [DOI] [PubMed] [Google Scholar]
- Lee, HK, Barbarosie, M, Kameyama, K, Bear, MF, and Huganir, RL (2000). “Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity.” Nature (London) 405, 955–959. 10.1038/35016089 [DOI] [PubMed] [Google Scholar]
- Lee, SH, Liu, L, Wang, YT, and Sheng, M (2002). “Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD.” Neuron 36, 661–674. 10.1016/S0896-6273(02)01024-3 [DOI] [PubMed] [Google Scholar]
- Lee, SR, Escobedo-Lozoya, Y, Szatmari, EM, and Yasuda, R (2009). “Activation of CaMKII in single dendritic spines during long-term potentiation.” Nature (London) 458, 299–304. 10.1038/nature07842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus, JJ, Kampa, BM, and Stuart, GJ (2006). “Learning rules for spike timing-dependent plasticity depend on dendritic synapse location.” J. Neurosci. 26, 10420–10429. 10.1523/JNEUROSCI.2650-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus, JJ, Kampa, BM, and Stuart, GJ (2007). “Does spike timing-dependent synaptic plasticity underlie memory formation?” Clin. Exp. Pharmacol. Physiol. 34, 1070–1076. 10.1111/j.1440-1681.2007.04724.x [DOI] [PubMed] [Google Scholar]
- Linden, DJ (1999). “The return of the spike: postsynaptic action potentials and the induction of LTP and LTD.” Neuron 22, 661–666. 10.1016/S0896-6273(00)80726-6 [DOI] [PubMed] [Google Scholar]
- Lisman, J, Schulman, H, and Cline, H (2002). “The molecular basis of CaMKII function in synaptic and behavioural memory.” Nat. Rev. Neurosci. 3, 175–190. 10.1038/nrn753 [DOI] [PubMed] [Google Scholar]
- Lisman, J, and Spruston, N (2005). “Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity.” Nat. Neurosci. 8, 839–841. [DOI] [PubMed] [Google Scholar]
- Lisman, JE (2001). “Three Ca2+levels affect plasticity differently: the LTP zone, the LTD zone and no man’s land.” J. Physiol. 532, 285. 10.1111/j.1469-7793.2001.0285f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman, JE, and Zhabotinsky, AM (2001). “A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly.” Neuron 31, 191–201. 10.1016/S0896-6273(01)00364-6 [DOI] [PubMed] [Google Scholar]
- Luthi, A, Wikstrom, MA, Palmer, MJ, Matthews, P, Benke, TA, Isaac, JT, and Collingridge, GL (2004). “Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity.” BMC Neurosci. 5, 44. 10.1186/1471-2202-5-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee, JC, and Johnston, D (1997). “A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons.” Science 275, 209–213. 10.1126/science.275.5297.209 [DOI] [PubMed] [Google Scholar]
- Malenka, RC, and Bear, MF (2004). “LTP and LTD: an embarrassment of riches.” Neuron 44, 5–21. 10.1016/j.neuron.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Malinow, R, Schulman, H, and Tsien, RW (1989). “Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP.” Science 245, 862–866. 10.1126/science.2549638 [DOI] [PubMed] [Google Scholar]
- Markram, H, Lubke, J, Frotscher, M, and Sakmann, B (1997). “Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs.” Science 275, 213–215. 10.1126/science.275.5297.213 [DOI] [PubMed] [Google Scholar]
- Matsuzaki, M, Honkura, N, Ellis-Davies, GC, and Kasai, H (2004). “Structural basis of long-term potentiation in single dendritic spines.” Nature (London) 429, 761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliza, CD, and Dan, Y (2006). “Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking.” Neuron 49, 183–189. 10.1016/j.neuron.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Migliore, M, Hoffman, DA, Magee, JC, and Johnston, D (1999). “Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons.” J. Comput. Neurosci. 7, 5–15. 10.1023/A:1008906225285 [DOI] [PubMed] [Google Scholar]
- Montgomery, JM, and Madison, DV (2002). “State-dependent heterogeneity in synaptic depression between pyramidal cell pairs.” Neuron 33, 765–777. 10.1016/S0896-6273(02)00606-2 [DOI] [PubMed] [Google Scholar]
- Morrison, A, Diesmann, M, and Gerstner, W (2008). “Phenomenological models of synaptic plasticity based on spike timing.” Biol. Cybern. 98, 459–478. 10.1007/s00422-008-0233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, Y, and Poo, MM (2006). “Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system.” Neuron 50, 115–125. 10.1016/j.neuron.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Mulkey, RM, Herron, CE, and Malenka, RC (1993). “An essential role for protein phosphatases in hippocampal long-term depression.” Science 261, 1051–1055. 10.1126/science.8394601 [DOI] [PubMed] [Google Scholar]
- Nagerl, UV, Eberhorn, N, Cambridge, SB, and Bonhoeffer, T (2004). “Bidirectional activity-dependent morphological plasticity in hippocampal neurons.” Neuron 44, 759–767. 10.1016/j.neuron.2004.11.016 [DOI] [PubMed] [Google Scholar]
- Nevian, T, and Sakmann, B (2004). “Single spine Ca2+ signals evoked by coincident EPSPs and backpropagating action potentials in spiny stellate cells of layer 4 in the juvenile rat somatosensory barrel cortex.” J. Neurosci. 24, 1689–1699. 10.1523/JNEUROSCI.3332-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian, T, and Sakmann, B (2006). “Spine Ca2+ signaling in spike-timing-dependent plasticity.” J. Neurosci. 26, 11001–11013. 10.1523/JNEUROSCI.1749-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama, M, Hong, K, Mikoshiba, K, Poo, MM, and Kato, K (2000). “Calcium stores regulate the polarity and input specificity of synaptic modification.” Nature (London) 408, 584–588. 10.1038/35046067 [DOI] [PubMed] [Google Scholar]
- Normann, C, Peckys, D, Schulze, CH, Walden, J, Jonas, P, and Bischofberger, J (2000). “Associative long-term depression in the hippocampus is dependent on postsynaptic N-type Ca2+ channels.” J. Neurosci. 20, 8290–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor, DH, Wittenberg, GM, and Wang, SS (2005). “Graded bidirectional synaptic plasticity is composed of switch-like unitary events.” Proc. Natl. Acad. Sci. U.S.A. 102, 9679–9684. 10.1073/pnas.0502332102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, H, and Ichikawa, K (2000). “Switching characteristics of a model for biochemical-reaction networks describing autophosphorylation versus dephosphorylation of Ca2+/calmodulin-dependent protein kinase II.” Biol. Cybern. 82, 35–47. 10.1007/PL00007960 [DOI] [PubMed] [Google Scholar]
- Okamoto, K, Nagai, T, Miyawaki, A, and Hayashi, Y (2004). “Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity.” Nat. Neurosci. 7, 1104–1112. 10.1038/nn1311 [DOI] [PubMed] [Google Scholar]
- Okamoto, K, Narayanan, R, Lee, SH, Murata, K, and Hayashi, Y (2007). “The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure.” Proc. Natl. Acad. Sci. U.S.A. 104, 6418–6423. 10.1073/pnas.0701656104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov, N, Griffith, LC, and Lisman, JE (1997). “Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation.” J. Neurosci. 17, 5357–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak, V, and Kerr, JN (2008). “Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity.” J. Neurosci. 28, 2435–2446. 10.1523/JNEUROSCI.4402-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, CC, Malenka, RC, Nicoll, RA, and Hopfield, JJ (1998). “All-or-none potentiation at CA3–CA1 synapses.” Proc. Natl. Acad. Sci. U.S.A. 95, 4732–4737. 10.1073/pnas.95.8.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, JP, and Gerstner, W (2006). “Triplets of spikes in a model of spike timing-dependent plasticity.” J. Neurosci. 26, 9673–9682. 10.1523/JNEUROSCI.1425-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi, HJ, and Lisman, JE (2008). “Coupled phosphatase and kinase switches produce the tristability required for long-term potentiation and long-term depression.” J. Neurosci. 28, 13132–13138. 10.1523/JNEUROSCI.2348-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, FG, Meredith, RM, Olding, AW, and Paulsen, O (1999). “Rapid report: postsynaptic bursting is essential for ‘Hebbian’ induction of associative long-term potentiation at excitatory synapses in rat hippocampus.” J. Physiol. 518(Pt 2), 571–576. 10.1111/j.1469-7793.1999.0571p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirazi, P, Brannon, T, and Mel, BW (2003). “Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell.” Neuron 37, 977–987. 10.1016/S0896-6273(03)00148-X [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno, A, and Paulsen, O (2008). “Spike timing-dependent long-term depression requires presynaptic NMDA receptors.” Nat. Neurosci. 11, 744–745. 10.1038/nn.2125 [DOI] [PubMed] [Google Scholar]
- Rose, J, Jin, SX, and Craig, AM (2009). “Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II.” Neuron 61, 351–358. 10.1016/j.neuron.2008.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, JE, Gerkin, RC, Bi, GQ, and Chow, CC (2005). “Calcium time course as a signal for spike-timing-dependent plasticity.” J. Neurophysiol. 93, 2600–2613. 10.1152/jn.00803.2004 [DOI] [PubMed] [Google Scholar]
- Rycroft, BK, and Gibb, AJ (2002). “Direct effects of calmodulin on NMDA receptor single-channel gating in rat hippocampal granule cells.” J. Neurosci. 22, 8860–8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft, BK, and Gibb, AJ (2004). “Inhibitory interactions of calcineurin (phosphatase 2B) and calmodulin on rat hippocampal NMDA receptors.” Neuropharmacology 47, 505–514. 10.1016/j.neuropharm.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Sanhueza, M, McIntyre, CC, and Lisman, JE (2007). “Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor.” J. Neurosci. 27, 5190–5199. 10.1523/JNEUROSCI.5049-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, AT, Larkum, ME, Sakmann, B, and Roth, A (2003). “Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern.” J. Neurophysiol. 89, 3143–3154. 10.1152/jn.00046.2003 [DOI] [PubMed] [Google Scholar]
- Schiller, J, Schiller, Y, and Clapham, DE (1998). “NMDA receptors amplify calcium influx into dendritic spines during associative pre- and postsynaptic activation.” Nat. Neurosci. 1, 114–118. 10.1038/693 [DOI] [PubMed] [Google Scholar]
- Schuett, S, Bonhoeffer, T, and Hubener, M (2001). “Pairing-induced changes of orientation maps in cat visual cortex.” Neuron 32, 325–337. 10.1016/S0896-6273(01)00472-X [DOI] [PubMed] [Google Scholar]
- Seol, GH, Ziburkus, J, Huang, S, Song, L, Kim, IT, Takamiya, K, Huganir, RL, Lee, HK, and Kirkwood, A (2007). “Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity.” Neuron 55, 919–929. 10.1016/j.neuron.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval, HZ (2005). “Clusters of interacting receptors can stabilize synaptic efficacies.” Proc. Natl. Acad. Sci. U.S.A. 102, 14440–14445. 10.1073/pnas.0506934102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval, HZ, Bear, MF, and Cooper, LN (2002). “A unified model of NMDA receptor-dependent bidirectional synaptic plasticity.” Proc. Natl. Acad. Sci. U.S.A. 99, 10831–10836. 10.1073/pnas.152343099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom, PJ, and Hausser, M (2006). “A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons.” Neuron 51, 227–238. 10.1016/j.neuron.2006.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom, PJ, and Nelson, SB (2002). “Spike timing, calcium signals and synaptic plasticity.” Curr. Opin. Neurobiol. 12, 305–314. 10.1016/S0959-4388(02)00325-2 [DOI] [PubMed] [Google Scholar]
- Sjostrom, PJ, Rancz, EA, Roth, A, and Hausser, M (2008). “Dendritic excitability and synaptic plasticity.” Physiol. Rev. 88, 769–840. 10.1152/physrev.00016.2007 [DOI] [PubMed] [Google Scholar]
- Sjostrom, PJ, Turrigiano, GG, and Nelson, SB (2001). “Rate, timing, and cooperativity jointly determine cortical synaptic plasticity.” Neuron 32, 1149–1164. 10.1016/S0896-6273(01)00542-6 [DOI] [PubMed] [Google Scholar]
- Sjostrom, PJ, Turrigiano, GG, and Nelson, SB (2003). “Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors.” Neuron 39, 641–654. 10.1016/S0896-6273(03)00476-8 [DOI] [PubMed] [Google Scholar]
- Sjostrom, PJ, Turrigiano, GG, and Nelson, SB (2004). “Endocannabinoid-dependent neocortical layer-5 LTD in the absence of postsynaptic spiking.” J. Neurophysiol. 92, 3338–3343. 10.1152/jn.00376.2004 [DOI] [PubMed] [Google Scholar]
- Softky, WR, and Koch, C (1993). “The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs.” J. Neurosci. 13, 334–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S, Miller, KD, and Abbott, LF (2000). “Competitive Hebbian learning through spike-timing-dependent synaptic plasticity.” Nat. Neurosci. 3, 919–926. 10.1038/78829 [DOI] [PubMed] [Google Scholar]
- Spruston, N (2008). “Pyramidal neurons: dendritic structure and synaptic integration.” Nat. Rev. Neurosci. 9, 206–221. 10.1038/nrn2286 [DOI] [PubMed] [Google Scholar]
- Spruston, N, Schiller, Y, Stuart, G, and Sakmann, B (1995). “Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites.” Science 268, 297–300. 10.1126/science.7716524 [DOI] [PubMed] [Google Scholar]
- Staubli, U, and Chun, D (1996). “Factors regulating the reversibility of long-term potentiation.” J. Neurosci. 16, 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade, M (2004). “Neocortical cell classes are flexible entities.” Nat. Rev. Neurosci. 5, 121–134. 10.1038/nrn1325 [DOI] [PubMed] [Google Scholar]
- Stuart, GJ, and Hausser, M (2001). “Dendritic coincidence detection of EPSPs and action potentials.” Nat. Neurosci. 4, 63–71. 10.1038/82910 [DOI] [PubMed] [Google Scholar]
- Tong, G, Shepherd, D, and Jahr, CE (1995). “Synaptic desensitization of NMDA receptors by calcineurin.” Science 267, 1510–1512. 10.1126/science.7878472 [DOI] [PubMed] [Google Scholar]
- Tzounopoulos, T, Kim, Y, Oertel, D, and Trussell, LO (2004). “Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus.” Nat. Neurosci. 7, 719–725. 10.1038/nn1272 [DOI] [PubMed] [Google Scholar]
- Tzounopoulos, T, Rubio, ME, Keen, JE, and Trussell, LO (2007). “Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity.” Neuron 54, 291–301. 10.1016/j.neuron.2007.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya, M, Chen, N, Raymond, LA, and Murphy, TH (2001). “A calcium-dependent feedback mechanism participates in shaping single NMDA miniature EPSCs.” J. Neurosci. 21, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo, H, Aihara, T, Kuroda, S, Watanabe, M, and Kondo, S (2004). “Spatial localization of synapses required for supralinear summation of action potentials and EPSPs.” J. Comput. Neurosci. 16, 251–256. 10.1023/B:JCNS.0000025688.64836.df [DOI] [PubMed] [Google Scholar]
- Urakubo, H, Honda, M, Froemke, RC, and Kuroda, S (2008). “Requirement of an allosteric kinetics of NMDA receptors for spike timing-dependent plasticity.” J. Neurosci. 28, 3310–3323. 10.1523/JNEUROSCI.0303-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum, MC, Bi, GQ, and Turrigiano, GG (2000). “Stable Hebbian learning from spike timing-dependent plasticity.” J. Neurosci. 20, 8812–8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vislay-Meltzer, RL, Kampff, AR, and Engert, F (2006). “Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system.” Neuron 50, 101–114. 10.1016/j.neuron.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Wang, HX, Gerkin, RC, Nauen, DW, and Bi, GQ (2005). “Coactivation and timing-dependent integration of synaptic potentiation and depression.” Nat. Neurosci. 8, 187–193. 10.1038/nn1387 [DOI] [PubMed] [Google Scholar]
- Wang, XB, Yang, Y, and Zhou, Q (2007). “Independent expression of synaptic and morphological plasticity associated with long-term depression.” J. Neurosci. 27, 12419–12429. 10.1523/JNEUROSCI.2015-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, S, Hoffman, DA, Migliore, M, and Johnston, D (2002). “Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons.” Proc. Natl. Acad. Sci. U.S.A. 99, 8366–8371. 10.1073/pnas.122210599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, J, and Helmchen, F (2004). “Boosting of action potential backpropagation by neocortical network activity in vivo.” J. Neurosci. 24, 11127–11136. 10.1523/JNEUROSCI.2933-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg, GM, and Wang, SS (2006). “Malleability of spike-timing-dependent plasticity at the CA3–CA1 synapse.” J. Neurosci. 26, 6610–6617. 10.1523/JNEUROSCI.5388-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters, A, Sandbrink, F, Schlottmann, A, Kunesch, E, Stefan, K, Cohen, LG, Benecke, R, and Classen, J (2003). “A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex.” J. Neurophysiol. 89, 2339–2345. 10.1152/jn.00900.2002 [DOI] [PubMed] [Google Scholar]
- Worgotter, F, and Porr, B (2005). “Temporal sequence learning, prediction, and control: a review of different models and their relation to biological mechanisms.” Neural Comput. 17, 245–319. 10.1162/0899766053011555 [DOI] [PubMed] [Google Scholar]
- Yang, Y, Wang, XB, Frerking, M, and Zhou, Q (2008). “Spine expansion and stabilization associated with long-term potentiation.” J. Neurosci. 28, 5740–5751. 10.1523/JNEUROSCI.3998-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H, and Dan, Y (2001). “Stimulus timing-dependent plasticity in cortical processing of orientation.” Neuron 32, 315–323. 10.1016/S0896-6273(01)00460-3 [DOI] [PubMed] [Google Scholar]
- Yao, H, Shen, Y, and Dan, Y (2004). “Intracortical mechanism of stimulus-timing-dependent plasticity in visual cortical orientation tuning.” Proc. Natl. Acad. Sci. U.S.A. 101, 5081–5086. 10.1073/pnas.0302510101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhabotinsky, AM (2000). “Bistability in the Ca2+/calmodulin-dependent protein kinase-phosphatase system.” Biophys. J. 79, 2211–2221. 10.1016/S0006-3495(00)76469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, LI, Tao, HW, Holt, CE, Harris, WA, and Poo, M (1998a). “A critical window for cooperation and competition among developing retinotectal synapses.” Nature (London) 395, 37–44. 10.1038/25665 [DOI] [PubMed] [Google Scholar]
- Zhang, S, Ehlers, MD, Bernhardt, JP, Su, CT, and Huganir, RL (1998b). “Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors.” Neuron 21, 443–453. 10.1016/S0896-6273(00)80553-X [DOI] [PubMed] [Google Scholar]
- Zhou, Q, Homma, KJ, and Poo, MM (2004). “Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses.” Neuron 44, 749–757. 10.1016/j.neuron.2004.11.011 [DOI] [PubMed] [Google Scholar]
- Zhou, Q, Tao, HW, and Poo, MM (2003). “Reversal and stabilization of synaptic modifications in a developing visual system.” Science 300, 1953–1957. 10.1126/science.1082212 [DOI] [PubMed] [Google Scholar]
- Zhuo, M, Zhang, W, Son, H, Mansuy, I, Sobel, RA, Seidman, J, and Kandel, ER (1999). “A selective role of calcineurin Aα in synaptic depotentiation in hippocampus.” Proc. Natl. Acad. Sci. U.S.A. 96, 4650–4655. 10.1073/pnas.96.8.4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker, RS (1999). “Calcium- and activity-dependent synaptic plasticity.” Curr. Opin. Neurobiol. 9, 305–313. 10.1016/S0959-4388(99)80045-2 [DOI] [PubMed] [Google Scholar]