Abstract
PURPOSE
Cross-sectional studies have documented the co-occurrence of obstructive sleep apnea (hereafter sleep apnea) with glucose intolerance, insulin resistance, and type II diabetes mellitus (hereafter diabetes). It has not been determined, however, whether sleep apnea is independently associated with the subsequent development of diabetes, accounting for established risk factors.
METHODS
This observational cohort study examined 1233 consecutive patients in the Veteran Affairs Connecticut Health Care System referred for evaluation of sleep-disordered breathing; 544 study participants were free of preexisting diabetes and completed a full, attended, diagnostic polysomnogram. The study population was divided into quartiles based on severity of sleep apnea as measured by the apnea-hypopnea index. The main outcome was incident diabetes defined as fasting glucose level > 126 mg/dL and a corresponding physician diagnosis. Compliance with positive airway pressure therapy, and its impact on the main outcome, was also examined.
RESULTS
In unadjusted analysis, increasing severity of sleep apnea was associated with an increased risk of diabetes (P for linear trend < 0.001). After adjusting for age, sex, race, baseline fasting blood glucose, body mass index (BMI), and weight change, an independent association was found between sleep apnea and incident diabetes (hazard ratio per quartile 1.43; CI 1.10 – 1.86). Among patients with more severe sleep apnea (upper two quartiles of severity), 60% had evidence of regular positive airway pressure use, and this treatment was associated with an attenuation of the risk of diabetes (log-rank test P=0.04).
CONCLUSION
Sleep apnea increases the risk of developing diabetes, independent of other risk factors. Among patients with more severe sleep apnea, regular positive airway pressure use may attenuate this risk.
Keywords: Sleep Apnea Syndrome, Type 2 Diabetes Mellitus
INTRODUCTION
The prevalence of type 2 diabetes continues to increase in the U.S. and currently affects an estimated 18 million people.1 Although lifestyle changes, such as weight loss and physical activity, are the cornerstone of diabetes prevention, efforts are needed to better understand other disease determinants and to develop additional strategies for prevention. Understanding the link between obstructive sleep apnea and diabetes may represent one such effort.
Obstructive sleep apnea (hereafter sleep apnea) is a common and treatable form of sleep-disordered breathing, involving upper airway collapse during sleep. This collapse results in a cycle of physiologic events that occur repeatedly throughout the night, including intermittent hypoxemia and arousal from sleep. Sleep apnea is present in 9 – 24% of the middle aged adult population2 and has been associated with increased rates of hypertension, atherosclerosis, cardiovascular morbidity and mortality (including stroke), and all-cause mortality.3-7
Recent short-term studies have demonstrated striking alterations in metabolic function, including glucose intolerance and insulin resistance, associated with short-term sleep restriction8, 9 and sleep apnea.8, 10-15 These findings raise the question of whether sleep apnea may have a role in development of overt clinical diabetes. The current study evaluated longitudinally whether sleep apnea is an independent risk factor for diabetes. In addition, we explored whether positive airway pressure, as the main medical therapy for sleep apnea, had an impact on this risk.
METHODS
Study Design and Patient Population
This observational cohort study examined non-diabetic patients referred to the Veterans Affairs (VA) Connecticut Sleep Center from January 2000 to December 2005. Eligible participants include patients referred for initial evaluation of sleep-disordered breathing who had at least 2 hours of sleep monitoring and a fasting glucose level less than 126 mg/dL. Participants were excluded if the entire polysomnographic study was performed with airway pressurization for therapeutic purposes. The study was approved by the institutional review board at the VA Connecticut Health Care System.
Baseline Assessment
Data on demographics, sleep and medical history, and medication use/habits were extracted from each patient’s medical record. Hypertension occurred if the average blood pressure was ≥ 140 mm Hg (systolic) or ≥ 90 mm Hg (diastolic) from three office visits prior to polysomnography or if patients had a history of hypertension. Hyperlipidemia was based on history or an appropriate drug prescription. Baseline fasting blood glucose values (with all patients routinely tested) were obtained within three months of initial polysomnogram. Each patient’s body mass index (BMI) was calculated at the time of polysomnogram and at follow up. The Epworth Sleepiness Scale, a measure of sleepiness,16 was also recorded.
Polysomnography
At baseline, participants underwent a full, attended, overnight polysomnography, with the use of Grass data-acquisition systems (Astro-Med®). Recordings were manually scored according to standard criteria.17-19 Calculated variables included the arousal index (number of arousals/hour of sleep), minimum arterial oxygen saturation, mean arterial oxygen saturation, T90% (percentage of total sleep time with oxygen saturation less than 90%), and the apnea hypopnea index (the sum of apneas and hypopneas/hour of sleep). The study population was divided into quartiles based on severity of sleep apnea as measured by the apnea hypopnea index.
Outcomes
The primary outcome was the development of diabetes, measured from the interval of the initial polysomnogram to the date of first office visit in which the diagnosis of diabetes occurred. Diabetes was defined by a physician diagnosis during routine office visit and fasting blood glucose > 126 mg/dL20 (using Hexokinase/G-6-PDH methodology through the Architect/Aeroset system, Abbott Laboratories, IL, USA). A physician investigator, unaware of patients’ sleep apnea status, reviewed electronic medical records to ascertain incident diabetes. Censoring occurred at end of follow up (last office visit prior to July 31, 2006), or the time of death (if death occurred).
Positive Airway Pressure Treatment Compliance
This VA study population received treatment according to consensus guidelines,21 and appliance and supplies were ordered by the institution and issued through a single respiratory home care company. Importantly, the orders for these services are documented in the electronic medical record. In addition, patients are followed closely for assessment of efficacy and treatment compliance through established sleep medicine and pulmonary clinics where positive airway pressure use is documented. Two physician-investigators, blinded to outcome status, categorized each patient’s airway pressurization treatment use in categories of: 1) not ordered, 2) no use, 3) intermittent use, and 4) continuous or “regular” use of positive airway pressure. For the purposes of these analyses, airway pressurization compliance was dichotomized into “regular use” or not.
Statistical Analysis
Student’s t-test was used to compare mean values at baseline between subjects in the first quartile (comparison group) with the upper three quartiles (sleep apnea group). Categorical data was compared using the chi-square test. A nonparametric comparison of medians was done with the Wilcoxon-Mann-Whitney test.
Kaplan-Meier analysis was used to compare event-free survival among patients with and without sleep apnea. An analysis using the chi-square test for linear trend was done to analyze whether increased severity of sleep apnea was associated with an increased risk of developing diabetes.
Subsequently, using proportional-hazards analysis, hazard ratios and 95% confidence intervals were generated for the unadjusted association between sleep apnea and incident diabetes. Adjusted hazard ratios were then calculated to account for the confounding effects of other baseline characteristics, including, age, gender, race, BMI, change in BMI (over the follow up period), and baseline fasting glucose.
To examine the impact of positive airway pressure appliance use on diabetes, compliance (defined as percentage of patients with regular appliance use) was first examined by quartile of sleep apnea severity. Anticipating that regular use would be significantly higher among those with more severe sleep apnea, a pre-specified subgroup analysis sought to investigate the impact of regular positive airway pressure use on incident diabetes among the upper two quartiles of severity (quartiles 3 and 4). This association was examined first using Kaplan-Meier analysis, and subsequently in multivariable analysis using Cox proportional hazards analysis.
Based on an available sample size of 544 participants, 90% power existed to detect a hazard ratio (HR) of 1.38 for two-sided alpha at the 0.05 level22 for the primary outcome of diabetes. All statistical tests were performed with the use of SAS software, with the exception of the Kaplan–Meier curves, for which S-Plus software was used.
RESULTS
Between January 2000 and July 2005, 1233 consecutive patients were referred to the VA Connecticut Sleep Center; 407 (33%) patients were ineligible because there were referred for conditions other than sleep-disordered breathing, or they had no initial diagnostic study. An additional 233 (19%) patients were ineligible because they had previously diagnosed diabetes, and 49 (4%) patients were excluded because they had incomplete polysomnographic data. Data for the remaining 544 patients were included in the study.
Table 1 shows the baseline characteristics of participants according to quartile of severity of the apnea-hypopnea index. The mean apnea hypopnea index in the sleep apnea group was 41.5, compared to 2.9 in the comparison group. The baseline fasting blood glucose values were higher in the sleep apnea group compared to the comparison group (99.4 mg/dL vs. 95.3 mg/dL, respectively; P=0.05). As expected, the prevalence of obesity (defined as BMI > 30) was higher in the sleep apnea group, as was the prevalence of hypertension. The sleep apnea group was also older, with a mean age of 62.9 years vs. 57.6 years, respectively. Obstructive apneas were the predominant apneic event; central apneas were rare.
Table 1.
Baseline characteristics of patients in quartiles* of apnea-hypopnea index
| Quartile #1 | Quartile #2 | Quartile #3 | Quartile #4 | Quartiles #2–4 | ||
|---|---|---|---|---|---|---|
| Characteristics | (n = 142) | (n = 136) | (n = 132) | (n = 134) | (n = 402) | P Value** |
| Mean Age (years) | 57.6 | 62.3 | 65.0 | 61.2 | 62.9 | <0.001 |
| Male sex (%) | 85.2 | 94.9 | 96.2 | 97.8 | 96.3 | <0.001 |
| White race (%) | 76.8 | 83.8 | 78.8 | 76.9 | 79.9 | 0.45 |
| Hypertension (%) | 53.5 | 66.9 | 66.7 | 73.1 | 68.9 | 0.001 |
| Beta-blocker use (%) | 20.4 | 33.1 | 37.1 | 33.6 | 34.6 | <0.001 |
| Hyperlipidemia (%) | 42.3 | 42.7 | 45.5 | 35.8 | 41.3 | 0.43 |
| Current Alcohol Consumption (%) | 18.3 | 16.9 | 21.2 | 29.1 | 22.4 | 0.31 |
| Baseline Fasting Glucose | 95.3 | 97.8 | 99.7 | 100.6 | 99.4 | 0.05 |
| Obesity (%) | 48.6 | 58.8 | 67.4 | 84.3 | 70.2 | <0.001 |
| Body Mass Index (BMI) | 31.1 | 32.2 | 33.9 | 36.0 | 34.0 | <0.001 |
| Change in BMI | − 0.21 | − 0.21 | − 0.40 | − 0.80 | − 0.47 | 0.07 |
| Epworth Sleepiness Scale | 7.9 | 7.7 | 8.5 | 8.1 | 8.1 | 0.75 |
| Apnea-Hypopnea Index (AHI) | 2.9 | 13.7 | 32.6 | 78.2 | 41.5 | <0.001 |
| Arousal Index | 22.4 | 29.9 | 45.4 | 82.4 | 50.5 | <0.001 |
| Median T90% | 0.4 | 2.0 | 5.0 | 12.0 | 5.0 | <0.001 |
Quartile #1, AHI < 8 (reference group); quartile #2, 8 ≤ AHI ≥ 20; quartile #3, 21 ≤ AHI ≥ 45; quartile #4, AHI > 46.
P value for comparison of quartile #1 vs. quartiles #2–4.
Follow-up after the index sleep study took place between January 1, 2005 and July 31, 2006. Patients in groups with or without sleep apnea had a mean duration of follow up of 2.7 years from baseline. Incident diabetes occurred in 61 patients, with 55 events occurring in the sleep apnea group compared with 6 events in the comparison group (5.5 vs. 1.8 events per 100 person years, respectively). Figure 1 shows the Kaplan-Meier (unadjusted) estimates of time to the endpoint of diabetes; the probability of developing diabetes was significantly higher for the sleep apnea group than for the comparison group (log rank test, P = 0.002).
Figure 1.
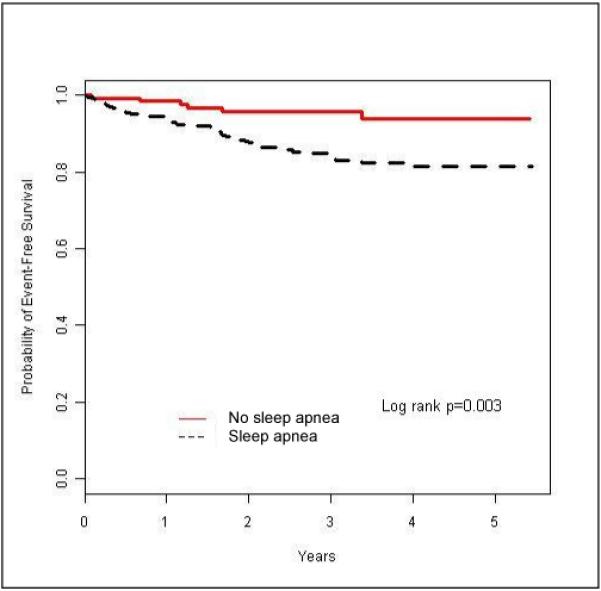
Kaplan – Meier Curves for the association of sleep apnea and diabetes. Sleep apnea defined as apnea-hypopnea index ≥8.
An initial analysis of linear trend revealed an increased risk of incident diabetes as a function of an increased severity of sleep apnea (P < 0.001; data not shown). In unadjusted analysis (Table 2), a significant association was found between sleep apnea (categorized by quartiles with increasing severity) and incident diabetes (HR per quartile 1.53; 95% CI 1.21–1.94; P=0.0005). In particular, the unadjusted risk of incident diabetes for subjects in the fourth quartile of severity was more than four and a half times that in the comparison group (HR 4.67; 95% CI 1.92 – 11.34; p=0.0007).
Table 2.
Unadjusted and adjusted hazard ratios for the risk of diabetes (N = 544)
| Unadjusted Hazard Ratio; | Adjusted Hazard Ratio; | |
|---|---|---|
| Factor | (95% CI; P value) | (95% CI; P value) |
| Age | 0.99 (0.98–1.02; P = 0.92) | 1.00 (0.97–1.02; P = 0.71) |
| Male Gender | 1.41 (0.44–4.51; P =0.56) | 0.43 (0.13–1.48; P = 0.18) |
| Non-Caucasian | 1.35 (0.64–2.85; P = 0.43) | 1.13 (0.56–2.30; P = 0.73) |
| Fasting Glucose | 1.04 (1.04–1.05; P = < 0.001) | 1.04 (1.03–1.05; P = < 0.001) |
| BMI | 1.06 (1.03–1.09; P = < 0.001) | 0.99 (0.96–1.03; P = 0.72) |
| Change in BMI | 0.73 (0.69–0.78; P = < 0.001) | 0.76 (0.70–0.83; P = <0.001) |
| Sleep apnea* | 1.53 (1.21–1.94 P = < 0.001) | 1.43 (1.10–1.86; P = 0.008) |
See text for coding of variables.
BMI=body mass index CI=confidence interval
Per Quartile of Sleep Apnea Severity
After adjusting for age, gender, race, baseline fasting blood glucose, BMI, and change in BMI, sleep apnea retained a statistically significant association with diabetes (adjusted HR per quartile 1.43; 95% CI 1.10–1.86; P=0.008); see Table 2. The association of both fasting blood glucose and change in BMI with incident diabetes persisted after adjustment. In a secondary, explanatory analysis, both T90 (as measures of hypoxia) and arousal index also had statistically significant hazard ratios for their association with diabetes; data not shown.
Regular use of positive airway pressure was observed in 28% of patients in the second quartile, and no significant impact of this use was observed. Regular use increased to 58% and 62% in the third and fourth quartiles, respectively, and Figure 2 shows the Kaplan-Meier estimates of the time to incident diabetes in this subgroup. The probability of incident diabetes was attenuated for those with regular positive airway pressure use compared to those without (log-rank test, P=0.04). In adjusted analyses among these same participants, regular use of positive airway pressure was independently associated with a significantly attenuated risk of incident diabetes (HR 0.53; 95% CI 0.28–0.99; P=0.04), even after adjusting for baseline fasting blood glucose, BMI, and change in BMI at follow-up.
Figure 2.

Kaplan – Meier Curves examining the impact of regular continuous positive airway pressure on the risk of incident diabetes among patients in the upper two quartiles of apnea hypopnea index severity (quartiles 3 and 4).
COMMENTS
We conducted an observational cohort study examining the impact of sleep apnea on the development of type II diabetes mellitus. Our results show that a) sleep apnea is a risk factor for the development of diabetes; b) increasing severity of sleep apnea is associated with an increasing risk for the development of diabetes; and, c) among patients with moderate to severe sleep apnea (upper 2 quartiles of severity), regular use of positive airway pressure is associated with an attenuated risk for the development of incident diabetes.
Our findings are consistent with previous cross-sectional data showing an association between sleep apnea and insulin resistance, glucose intolerance, and diabetes.8, 11, 13, 14, 23-25 The causal direction of these associations, however, has been questioned.26-28 The few studies assessing longitudinal associations between sleep apnea and diabetes have had limitations. For example, sleep apnea was associated with development of diabetes during a 10-year follow-up period, but sleep apnea was determined by a self-report of snoring as a surrogate for a confirmed diagnosis.29, 30 In addition, a longitudinal analysis of the Wisconsin Cohort found an association between sleep apnea and diabetes, but the impact of sleep apnea was not statistically significant when adjusted for BMI.24 Finally, a recent longitudinal analysis of the Busselton Health Study detected an association between moderate-severe sleep apnea and diabetes using portable monitoring, but the small sample size, limited distribution of sleep apnea severity, and few incident cases of diabetes resulted in a wide confidence interval.31 Our study is novel in that it used a longitudinal design that was powered to demonstrate an independent association between confirmed sleep apnea and diabetes, used the gold standard test for diagnosis, assessed a broad range of sleep apnea severity, and examined the impact of sleep apnea treatment.
Several potential mechanistic pathways may explain how sleep apnea and its physiologic sequelae (intermittent hypoxemia and recurrent arousals) ultimately lead to metabolic abnormalities. Previous studies found that severity of hypoxemia is related to the degree of glucose intolerance and insulin resiatnce.8, 32, 33 Although insulin levels were not available in the current study, hypoxemia (T90, defined as a greater than 2% of the night spent with an oxygen saturation less than 90%) was significantly associated with the outcome of diabetes (data not shown); intermittent hypoxemia may act through mechanisms of oxidative stress to mediate alterations in glucose metabolism.34-35 We also observed that the arousal index was associated with development of diabetes. Recurrent arousals (and sleep loss) may act through sympathetic activation and subsequent alterations in hypothalamic-pituitary-adrenal axis, leading to altered cortisol levels, decreased pancreatic beta-cell activity, elevated growth hormone levels, and alterations in neuroendocrine control of appetite.35-39
Our findings suggest an increased risk of diabetes among patients with sleep apnea, despite “usual care” administration of various therapies, primarily positive airway pressure. A few explanations might account for this finding. First, it is likely that our population had untreated sleep apnea for years before seeking diagnosis and treatment, resulting in prolonged exposure to an associated metabolic risk. Second, diabetes may develop even if patients receive effective therapy for sleep apnea. Finally, reduced compliance with positive airway pressure and limited efficacy of other treatments may have played a role in reducing the potential benefit of therapy.
We observed that regular use of positive airway pressure among patients in the upper two quartiles of severity was associated with a significant attenuation of incident diabetes, even after adjusting for subsequent weight loss. These findings are consistent with several treatment studies that have demonstrated improvements in insulin sensitivity and postprandial glucose with airway pressurization therapy. 40-42 In contrast, others studies, including 2 short-term randomized controlled trials, have not shown a benefit.23, 25 Future longitudinal and randomized studies examining the impact of treatment in various patient populations (e.g. sleepy and non-sleepy patients) are needed.
Several methodologic issues should be considered in the interpretation of our results. First, as with any observational study, it is possible that residual confounding affected our adjusted hazard ratios, despite our attempts to control for major risk factors. For example, family history of diabetes could not be reliably ascertained through electronic medical record extraction and was not included in our analysis. In addition, BMI may not fully adjust for visceral obesity, known to be associated with components of the metabolic syndrome.47,48 Our comparison group also consisted of mainly obese males referred for suspected sleep apnea, however, thereby reducing the potential for residual confounding, given that similar risk factors were prominent in the comparison group. Change in BMI was also accounted for, to exclude the possibility that differential weight gain in one group may account for the difference in incident diabetes during the study.
Second, tracking of positive airway pressure treatment status was done through documentation in the electronic record, based on clinical assessment by treating physicians in conjunction with documentation of equipment and supply orders. Although the possibility of misclassification of treatment status exists (with a bias towards the null hypothesis), this scenario would not explain our observed findings of the attenuation of incident diabetes conferred by positive airway pressure use among our patients in the upper 2 quartiles of sleep apnea severity. In addition, our moderate long-term rates (~60%) of regular CPAP use are similar to those described using direct data card measures that track hours of use.43
Third, treatment status in this observational cohort study was not randomly assigned. Patients who complied with positive airway pressure may have also been leading healthier lifestyles and complying with other therapies for the prevention of diabetes (e.g., diet and exercise). Yet, this possibility would not explain the attenuation of incident diabetes among patients in the upper 2 quartiles of severity that was observed even after adjustment for weight loss.
Finally, the relatively low observed values for the Epworth Sleepiness Scale (mean <9; see Table 1) may prompt questions regarding thresholds for initiating CPAP in the context of risk of future diabetes. Virtually all patients in our study were symptomatic, however, with episodes such as gasping during sleep, loud habitual snoring, or a bed-partner noting witnessed apneas; many of these patients also had coexistent cardiovascular risk factors and some had prevalent cardiovascular disease. Thus, caution is warranted in extrapolating our findings to healthier populations.
In summary, sleep apnea is significantly associated with the risk of type II diabetes, independently of other risk factors, including age, race, gender, baseline fasting glucose, and BMI, and changes in BMI. Increased severity of sleep apnea is associated with an increased risk of diabetes, and the risk may be partially explained by hypoxemia and arousals. As a treatable condition, sleep apnea may represent a modifiable risk factor for development of diabetes.
Acknowledgments
Funding Sources: VA Health Services Research and Development/Clinical Science, Career Development Awards to Dr. Yaggi. National Research Service Award Institutional Research Training Grant (5T32HL07778) to Dr. Botros.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors had access to the data and a role in writing the manuscript. They have no relevant conflicts of interest to disclose.
CITATIONS
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005 Jan;28(Suppl 1):S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007 Oct 1;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005 Mar 19–25;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Peppard P, Young T, Palta M, Skatrud JB. Prospective study of the association between sleep-disordered breathing and hypertension. NEJM. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Yaggi H, Concato J, Kernan W, Lichtman J, Brass L, Mohsenin V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. NEJM. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Finn L, Peppard P, et al. Sleep DIsordered Breathing and Mortality: Eighteen-Year Follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004 Sep 15;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 9.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006 Mar;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 10.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004 May;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002 Mar 1;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 12.Meslier N, Gagnadoux F, Giraud P, et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J. 2003 Jul;22(1):156–160. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 13.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002 Mar 1;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 14.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003 Mar;26(3):702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 15.Tiihonen M, Partinen M, Narvanen S. The severity of obstructive sleep apnoea is associated with insulin resistance. J Sleep Res. 1993 Mar;2(1):56–61. doi: 10.1111/j.1365-2869.1993.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999 Aug 1;22(5):667–689. [PubMed] [Google Scholar]
- 18.Meoli AL, Casey KR, Clark RW, et al. Hypopnea in sleep-disordered breathing in adults. Sleep. 2001 Jun 15;24(4):469–470. [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A, UCLA . A Manual of Standardized Terminology, Techniques and scoring System for Sleep Stages of Human Subjects. Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- 20.ADA Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2008;31(Supplement 1):s55–s60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 21.Loube DI, Gay PC, Strohl KP, Pack AI, White DP, Collop NA. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115(3):863–866. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld P. Sample-size Formula for the Proportional-Hazards Regression Model. Biometrics. 1983 June;39(2):499–503. 1983. [PubMed] [Google Scholar]
- 23.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007 Apr;29(4):720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 24.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005 Dec 15;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007 Nov;62(11):969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West S. Prevalence of Obstructive Sleep Apnea in Men with Type 2 Diabetes. Thorax. 2006 November;61(11):945–950. doi: 10.1136/thx.2005.057745. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ficker JH, Dertinger SH, Siegfried W, et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur Respir J. 1998 Jan;11(1):14–19. doi: 10.1183/09031936.98.11010014. [DOI] [PubMed] [Google Scholar]
- 28.Bottini P. Sleep-Disordered Breathing in Nonobese diabetic subjects with Autonomic Neuropathy. Eur Respir J. 2003 October;22(4):654–660. doi: 10.1183/09031936.03.00070402. 2003. [DOI] [PubMed] [Google Scholar]
- 29.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002 Mar 1;155(5):387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 30.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med. 2000 Jul;248(1):13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 31.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009 Feb 15;5(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- 32.Redline S, Storfer-Isser A, Rosen CL, et al. Association between Metabolic Syndrome and Sleep-disordered Breathing in Adolescents. Am J Respir Crit Care Med. 2007 Aug 15;176(4):401–408. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006 Jun 1;29(6):777–783. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 34.Polotsky V. Intermittent Hypoxia Increases Insulin Resistance in Genetically Obese Mice. Journal of Physiology. 2003 July 23;(5521):253–264. doi: 10.1113/jphysiol.2003.048173. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005 May;127(5):1674–1679. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 36.Punjabi N. Disorders of Glucose Metabolism in Sleep Apnea. J Appl Physiol. 2005 November;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. 2005. [DOI] [PubMed] [Google Scholar]
- 37.Chasens ER, Weaver TE, Umlauf MG. Insulin resistance and obstructive sleep apnea: is increased sympathetic stimulation the link? Biol Res Nurs. 2003 Oct;5(2):87–96. doi: 10.1177/1099800403257088. [DOI] [PubMed] [Google Scholar]
- 38.Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia--implications for sleep apnea. Clin Exp Hypertens A. 1988;10(Suppl 1):413–422. doi: 10.3109/10641968809075998. [DOI] [PubMed] [Google Scholar]
- 39.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995 Oct;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Archives of Internal Medicine. 2005;165(4):447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 41.Harsch IA, Schahin SP, Bruckner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004 May-Jun;71(3):252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 42.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004 Jan 15;169(2):156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 43.Hirshkowitz M, Littner M, Kuna S, Berry RB, Norris M, Almenoff P. Sleep-related Breathing Disorders: Sourcebook. 2nd Edition Healthcare Analysis & Information Group (HAIG), VHA; Milwaukee, WI: 2003. [Google Scholar]


