Abstract
Although activation of one seven-transmembrane receptor can influence the response of a separate seven-transmembrane receptor, e.g., the phenomenon of synergism, the underlying mechanism(s) for this signaling process is unclear. The present study investigated communication between two receptors that exhibit classical synergism, e.g., human platelet thrombin and thromboxane A2 receptors. Activation of thrombin receptors caused an increase in ligand affinity of thromboxane A2 receptors. This effect (i) was shown to be specific, since a similar increase in ligand affinity was not caused by ADP or A23187; (ii) did not require cytosolic components, e.g., kinases, proteases, phosphatases, etc., because it occurred in isolated platelet membranes; (iii) was G protein-mediated because it was blocked by an Gαq C terminus antibody; and (iv) was associated with a net increase in Gαq coupling to thromboxane A2 receptors. Collectively, these data provide evidence that seven-transmembrane receptors that share a common Gα subunit can communicate with each other via a redistribution of their G proteins. Thus, activation of thrombin receptors increases Gαq association with thromboxane A2 receptors thereby shifting them to a higher affinity state. This signaling phenomenon, which modulates receptor-ligand affinity, may serve as a molecular mechanism for cellular adaptive processes such as synergism.
When an agonist binds to a receptor protein, a sequelae of events is initiated by which the biological signal is translocated to a specific cellular effector. Upon interaction of an agonist with a seven-transmembrane receptor, signaling begins with the activation of heterotrimeric guanine nucleotide-binding proteins (G protein), which are in close association with the receptor (1). The signal is then further propagated through G protein dissociation into a Gα subunit and a Gβγ dimer, each of which can activate specific effector molecules, e.g., adenylyl cyclase, phospholipase C, ion-channels, etc. (1–4). Although different seven-transmembrane receptors seem to function through distinct signal transduction pathways, it also is apparent that there are certain points along the activation cascade by which these pathways can communicate. Thus, the interaction of an agonist with a receptor can influence the cellular response to a separate agonist interacting with a different receptor. One example of such “crosstalk” between pathways is the phenomenon of synergism, in which the response caused by two agonists added together is greater than the arithmetic sum of the individual responses caused by each agonist. Although synergism is observed widely in pharmacological therapeutics, the molecular mechanism(s) leading to such disproportionate responses remains unclear.
In the present study, we investigated the mechanism for the synergistic interaction between two agonists, which are known to stimulate human blood platelet aggregation, i.e., thrombin (5, 6) and thromboxane A2 (TXA2) (7, 8). It was found that activation of platelet thrombin receptors (9–12) causes an increase in ligand affinity of TXA2 receptors (13–15). Furthermore, this increased ligand affinity was associated with enhanced coupling of TXA2 receptors to their Gαq subunits. These findings therefore provide evidence that G proteins can serve as communication vectors between different seven-transmembrane receptors that share a common Gα subunit.
MATERIALS AND METHODS
Materials.
[3H]U46619 and [3H]SQ29,548 were purchased from DuPont/NEN. U46619 and SQ29,548 were obtained from Cayman Chemicals (Ann Arbor, MI). The 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonic acid (CHAPS), thrombin, hirudin, plasmin, ADP, A23187, rabbit preimmune IgG, and GTPγS were purchased from Sigma. The thrombin-receptor-activating peptide (TRAP) refers to the first six amino acids of the new amino terminus revealed after thrombin cleavage, i.e., SFLLRN (TRAP42–47) and was purchased from Research Genetics (Huntsville, AL). Rabbit polyclonal antibodies against the C-terminal region of Gαq (G-QL) were produced as described (16).
Platelet Aggregation.
Platelet-rich plasma (PRP), purchased from University of Illinois Hospital Blood Bank (Chicago), was isolated from citrate-phosphate-dextrose-anticoagulated human blood (17).The PRP was then incubated for 3 min with 10 μM indomethacin to prevent endogenous TXA2 production. Aggregation was measured at 20°C by the turbidimetric method (18).
[3H]U46619 Binding to Intact-Washed Platelets.
PRP was isolated from citrate-phosphate-dextrose-anticoagulated human blood (17) and was treated with 1 mM aspirin. The PRP was then spun at 1,100 × g for 15 min to pellet the cells. The platelet-free plasma was discarded, and the platelets were gently resuspended in buffer (138 mM NaCl/5 mM KCl/5 mM MgCl2/5.5 mM glucose/25 mM Tris⋅HCl/540nM prostacyclin (PGI2), pH 6.5)(19) to a cell count of ≈1 × 109 platelets/ml. [3H]U46619 binding was determined by a modification of previously described methods (19, 20). In brief, resuspended platelets were filtered onto Whatman GS/C filters under gentle vacuum. Various agonists were added for 3 min followed by addition of 4 nM [3H]U46619, which was allowed to incubate for an additional 5 min at 20°C. A 1,000-fold molar excess of unlabeled U46619 (4 μM) was used to determine nonspecific binding. The filters were quickly washed with ice-cold buffer to terminate the binding reaction and were counted in a liquid scintillation spectrometer (Beckman LS 6800).
[3H]SQ29,548 Binding to Solubilized Platelet Membranes.
Solubilized platelet membranes were prepared as described (15) from out-dated platelet concentrates obtained from Heartland Blood Services (Aurora, IL). The CHAPS concentration was adjusted to 2 mM, and [3H]SQ29,548 binding was performed by using a filtration-binding assay procedure (15). Nonspecific binding was determined by using a 1,000-fold molar excess of the unlabeled SQ29,548 (2 μM).
Saturation binding was carried out as described (15) with various concentrations (1–25nM) of [3H]SQ29,548, and nonspecific binding was determined with 2 μM of unlabeled SQ29,548. The program prizm (Graphpad, SanDiego) was used to fit the saturation binding curves to a one-binding site hyperbola and to determine the dissociation constants (Kd) and the number of binding sites (Bmax). Comparisons of mean ± SEM were performed by using a two-sample Student’s t test (P < 0.05).
Immunoaffinity Chromatography Purification of the Thromboxane A2 Receptor-Gαq Complex.
Solubilized platelet membranes were prepared as described (15), and the CHAPS concentration was adjusted to 2 mM. The preparation was incubated with vehicle or TRAP (50 μM) (9–12, 21–23) for 5 min and subsequently incubated (1 hr at 20°C) with an immunoaffinity matrix coupled to anti-receptor antibody (24) [Previous findings have shown that immunopurification of the TXA2 receptor protein results in copurification of its associated G protein, Gq (25)]. Two to three micrograms of protein/ml of [125I]G-QL IgG (or the same protein concentration of [125I]-labeled preimmune IgG [PI IgG]) was added, and the reaction mixture was allowed to incubate for 1 additional hr at 20°C. The matrix was then loaded on a column and washed with buffer (20 mM Tris/10 mM CHAPS/0.2 mM EGTA/0.5 mg/ml azolectin/550 mM KCl/20% glycerol, pH 7.4) to elute unbound proteins. The column was then eluted with 100 mM glycine (pH 2.5), and the elution fractions were counted for [125I]-activity. Specific binding attributable to Gαq was defined as the difference between the counts eluted from the [125I]G-QL IgG column minus the counts eluted from the [125I]PI IgG column. Typically, the percentage of specific binding was in the range of 60%.
RESULTS AND DISCUSSION
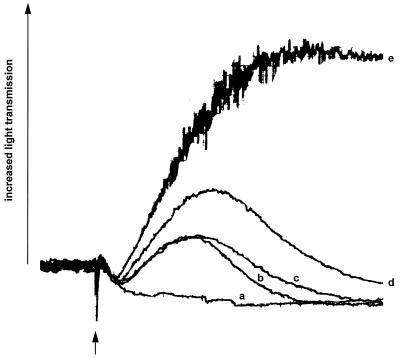
Fig. 1 demonstrates a synergistic aggregation response mediated by thrombin and TXA2 receptors. According to the rigorous definition of synergism, the biological effect caused by two agonists added together must exceed the response caused by twice the dose of either agonist added alone. As can be seen, platelet aggregation (17, 18) induced by combining 1 μM of the stable TXA2 analog, U46619 (20) with 3 μM TRAP (9–12, 21–23) exceeds the individual responses caused by either 2 μM U46619 or 6 μM TRAP. The magnitude of this synergistic effect is approximately a threefold increase in the extent of platelet aggregation.
Figure 1.
Synergistic effect of U46619 and TRAP on platelet aggregation. Aggregation was measured in PRP by the turbidimetric method (17, 18) by using a model 400 Lumi-aggregometer (Chronolog, Havertown, PA). PRP was stimulated (at arrow) with 3 μM or 6 μM TRAP (traces a and b, respectively), 1 μM or 2 μM U46619 (traces c and d, respectively), or 1 μM U46619 plus 3 μM TRAP (trace e). The aggregation traces are representative of three separate experiments.
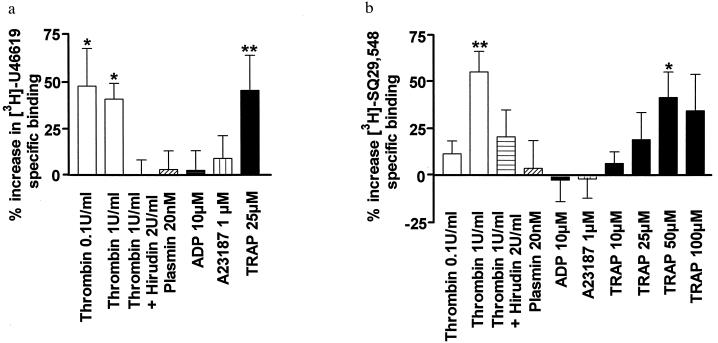
To investigate possible mechanisms for this phenomenon, we first determined whether radioligand binding to platelet TXA2 receptors (13–15) changes as a consequence of treatment with the native biological agonist, thrombin (5, 6). As shown in Fig. 2a, pretreatment of intact platelets with 0.1 unit/ml or 1 unit/ml thrombin produced a substantial (≈50%) increase in [3H]U46619-specific binding (19). Because this thrombin effect was blocked by hirudin (ref . 26; Fig. 2a), it can be concluded that the proteolytic activity of thrombin in some way alters the interaction of TXA2 receptors with their agonist. This could occur through at least two separate mechanisms: (i) proteolytic modification of TXA2 receptors; and/or (ii) thrombin stimulation of its own receptors (9–12). Regarding the first possibility, treatment of platelets with thrombin did not produce a measurable change in the apparent molecular weight of TXA2 receptors (data not shown). Consequently, proteolytic cleavage of the TXA2 receptor protein itself is unlikely. Furthermore, a separate serine protease, i.e., plasmin (27), did not produce a comparable effect on [3H]U46619 binding (Fig. 2a). Collectively, these results suggest that the ability of thrombin to increase TXA2 receptor binding is not produced either through proteolytic modification of the TXA2 receptor protein or through nonspecific proteolysis of platelet membrane proteins. On the other hand, evidence that thrombin produces the effect through stimulation of its own receptors is provided by experiments using TRAP (9–12, 21–23). Fig. 2a illustrates that TRAP caused an increase in [3H]U46619 binding comparable with that observed using thrombin (≈50%). This result therefore establishes that ligand binding to TXA2 receptors can be modulated by signal transduction through thrombin receptors.
Figure 2.
Ligand binding to platelet TXA2 receptors. (a) [3H]U46619 binding to intact washed platelets. Platelets, pretreated with aspirin (1 mM) to prevent endogenous TXA2 production, were incubated with thrombin, thrombin plus hirudin, ADP, A23187, plasmin, or TRAP at the concentrations indicated. Binding was performed as described (19), and results are expressed as percentage increase in [3H]U46619-specific binding relative to unstimulated platelets. All experiments were done in triplicate at least five times, and the values represent means ± SEM of all results. The average specific binding in the absence of agonist pretreatment was 18.2 ± 2.4 fmol/109 platelets. Statistical significance was evaluated using a Kruskal–Wallis analysis of variance followed by Dunn’s multiple comparison test (∗ P < 0.05; ∗∗ P < 0.01). (b) [3H]SQ29,548 binding to solubilized platelet membranes. Platelet membranes were solubilized in CHAPS (15) and pretreated with thrombin, thrombin plus hirudin, ADP, A23187, plasmin, or TRAP at the concentrations indicated. Binding was performed as described (15), and results are expressed as the percentage increase in [3H]SQ29,548-specific binding relative to unstimulated, solubilized platelet membranes. All experiments were done in triplicate at least four times, and the values represent means ± SEM of all results. The average specific binding in the absence of agonist pretreatment was 138 ± 15 fmol/mg protein. Statistical significance was evaluated by using a one-way analysis of variance followed by Bonferroni’s multiple comparison test (∗ P < 0.05; ∗∗ P < 0.01).
To examine the specificity of increased TXA2 receptor ligand binding, other platelet activating agents, ADP (28), and the calcium ionophore A23187 (29) were evaluated. However, neither agent induced a significant increase in [3H]U46619 binding, even at concentrations (10 μM and 1 μM, respectively, Fig. 2a), which produce maximal calcium mobilization, platelet aggregation, and exposure of intracellular membrane components (30, 31). These results therefore indicate that the “inter-receptor signaling” observed between thrombin and TXA2 receptors is not simply a consequence of generalized platelet activation, increased intracellular calcium, or exposure of cryptic TXA2 receptors.
We next attempted to define which platelet components (cytosolic and/or membrane) are required for thrombin-mediated inter-receptor signaling. In these experiments, we also tested whether the increase in ligand binding is limited to agonists, or whether antagonist binding, e.g., SQ29,548 (32), is elevated by thrombin treatment as well. It was found (Fig. 2b) that pretreatment of solubilized platelet membranes with 1 unit/ml thrombin resulted in a significant increase in [3H]SQ29,548 binding. Furthermore, as was observed in intact platelets: (i) preincubation with hirudin reduced inter-receptor signaling; (ii) neither proteolysis by plasmin nor addition of ADP or A23187 had an effect on TXA2 receptor–ligand interaction; and (iii) addition of TRAP also resulted in an increase in antagonist binding. These findings in a solubilized membrane preparation provide evidence that the communication pathway for inter-receptor signaling resides in the platelet membrane compartment and therefore occurs through a mechanism that does not require cytosolic kinases, phosphatases, or other soluble platelet components.
To assess whether thrombin induces an increase in TXA2 receptor affinity, we next performed saturation-binding experiments with [3H]SQ29,548 in solubilized platelet membranes. In the absence of TRAP, saturation binding revealed a Kd of 7.8 ± 0.1 nM (n = 4) and a Bmax of 1,938 ± 14 fmol/mg protein, consistent with results previously described by using SQ29,548 in solubilized platelet membranes (15, 33). On the other hand, after pretreatment with TRAP (50 μM), the Kd decreased by 23% (6 ± 0.4 nM; P < 0.05). Furthermore, because a similar increase in Bmax was not observed (1,744 ± 46 vs. 1,938 ± 14 fmol/mg protein), these findings suggest that an increase in TXA2 receptor affinity serves as the basis for inter-receptor signaling. Although this increase in affinity may appear to be modest, it should be noted that it represents an average of all TXA2 receptors whether they are in low or high affinity states. Because of this, the observed shift in Kd would underestimate the affinity of the TXA2 receptor population, which is altered by thrombin receptor activation. This notion is supported by the finding that when ligand binding is performed at a concentration below the Kd, a much larger percentage increase in specific binding is observed, i.e., 50% (Fig. 2). Consequently, we consider that the reported increase in TXA2 receptor affinity could be extremely important because it would produce its most pronounced effects at low agonist concentrations, which would presumably be encountered in the biological milieu.
The classical model of signal transduction through a G protein-coupled receptor predicts that the conformation of the receptor determines the conformational state of its associated G protein (1). Furthermore, recent evidence has suggested that the corollary to this also occurs, i.e., association of a G protein with its receptor can shift the receptor to a higher affinity state for its ligand (34, 35). The existence of such bidirectional signaling might therefore be responsible for the communication between thrombin and TXA2 receptors. Specifically, because both thrombin and TXA2 receptors share a common Gα subunit, i.e., Gαq (16, 25, 36), this subunit may serve as a vector for signaling between these two separate receptors. Thus, thrombin receptor stimulation may lead to a “redistribution” of activated Gαq such that more TXA2 receptors become G protein associated, and in so doing, shift to a higher affinity state for ligand binding.
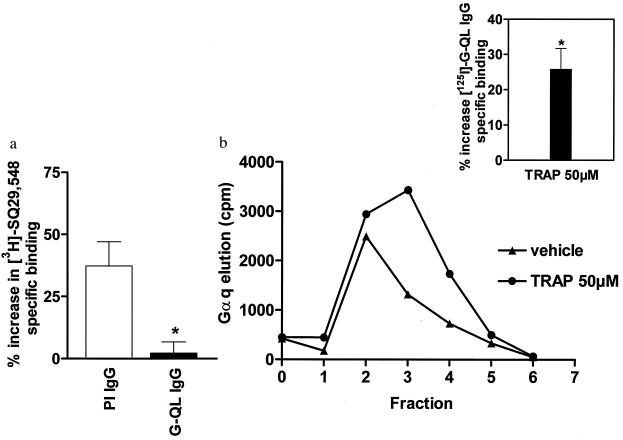
To assess whether G proteins are indeed involved in inter-receptor signaling, we first measured the effect of the nonhydrolyzable GTP analog, GTPγS on [3H]SQ29,548 binding. It was found that pretreatment of solubilized platelet membranes with GTPγS (100 μM) resulted in a 21 ± 3% (n = 13; P < 0.05) decrease in SQ29,548 binding. Because GTPγS causes dissociation of the Gα subunit from its receptor (1), these results suggest that the binding affinity of TXA2 receptors is in fact influenced by their G protein-binding status. To further test this hypothesis, [3H]SQ29,548 binding was performed in solubilized platelet membranes treated with an antibody (G-QL) directed against the C-terminal region of Gαq (16, 37). It can be seen that addition of G-QL IgG (150 μg/ml, Fig. 3a) resulted in almost complete inhibition of the thrombin-induced increase in SQ29,548 binding, whereas preimmune IgG (150 μg/ml) was without effect. Because previous studies have indicated that this antibody prevents TXA2 receptor-Gαq association (16, 36), these results provide evidence that thrombin-induced TXA2 receptor ligand binding is indeed due to an increased association of TXA2 receptors with Gαq.
Figure 3.
Involvement of Gαq in inter-receptor signaling. (a) Effect of G-QL IgG on [3H]SQ29,548 binding to solubilized platelet membranes. Platelet membranes were solubilized in CHAPS (15) and incubated with preimmune IgG (PI IgG; 150 μg/ml) or an antibody raised against the C terminus of Gαq (G-QL IgG; 150 μg/ml) in the presence of 1 unit/ml thrombin. [3H]SQ29,548 binding was performed as described (15), and results are expressed as the percentage increase in [3H]SQ29,548-specific binding relative to unstimulated solubilized platelet membranes. All experiments were done in triplicate at least four times, and the values represent means ± SEM of all results. The average specific binding in the absence of agonist pretreatment was 138 ± 15 fmol/mg protein. Statistical analysis measuring the effect of G-QL IgG on thrombin-induced ligand binding was performed by using a two-sample Student’s t test (∗, P < 0.05). Addition of G-QL IgG or PI IgG did not significantly affect baseline [3H]SQ29,548 binding (data not shown). For comparison with thrombin treatment alone see Fig. 2b. (b) Effect of TRAP treatment on Gαq association with TXA2 receptors. Solubilized platelet membranes were subjected to immunoaffinity chromatography purification (24) in the presence or absence of TRAP (fraction 1 = last wash; fractions 2–6 = elution fractions). Results of one representative experiment are expressed in counts per minute (cpm) of specifically eluted [125I]G-QL IgG. (Inset) Average TRAP-induced percentage increase in [125I]G-QL IgG counts relative to vehicle (n = 9). TRAP treatment had no effect on the amount of TXA2 receptor purified (data not shown). Statistical significance was evaluated by using a two-sample Student’s t test (∗, P < 0.05).
This notion was confirmed in the next series of experiments that directly measured the physical association of Gαq with TXA2 receptors. In these experiments, immunoaffinity chromatography purification of the receptor-G protein complex was performed (24, 25), and Gαq was quantitated by using [125I]G-QL antibody. Fig. 3b illustrates a representative Gαq elution profile in the presence or absence of TRAP (50 μM). It can be seen that TRAP caused a measurable increase in the specifically eluted [125I]G-QL counts. The average of nine such experiments revealed that the magnitude of this increased Gαq-receptor association was ≈25% (P < 0.05) (Fig. 3b Inset). These results therefore demonstrate that thrombin receptor activation causes a shift in the distribution of G proteins such that more Gαq becomes associated with TXA2 receptors.
Finally, preliminary experiments indicate that a separate Gαq-coupled platelet receptor, i.e., the platelet-activating factor (38–40) receptor also is capable of modulating TXA2 receptor ligand affinity. In these studies, platelet-activating factor (0.5 μg/ml) induced a 21 ± 7% (n = 5; P < 0.05) increase in [3H]SQ29,548 binding in solubilized platelet membranes. These results therefore suggest that inter-receptor signaling is not limited to thrombin and TXA2 receptors but also can occur between other receptor pairs sharing a common Gα subunit.
In summary, the above data identify a molecular mechanism for communication between seven-transmembrane receptors, such that G proteins cycle not only within a single signal transduction pathway but also between separate pathways. This signaling mechanism would allow the activation history of one class of receptors to modulate the sensitivity of a separate class of receptors and thereby serve as one molecular mechanism for cellular adaptive processes such as synergism.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL-24530) and North Atlantic Treaty Organization (CRG-940595) and was conducted under the auspices of the Association for U.S.–French Biomedical Cooperation.
ABBREVIATIONS
- TXA2
thromboxane A2
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- TRAP
thrombin-receptor-activating peptide
- PRP
platelet-rich plasma
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Gilman A G. Annu Rev Biochem. 1987;56:614–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Van Biesen T, Luttrell L M, Hawes B E, Lefkowitz R J. Endocr Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 3.Gudermann T, Schoneberg T, Schultz G. Annu Rev Neurosci. 1997;20:399–427. doi: 10.1146/annurev.neuro.20.1.399. [DOI] [PubMed] [Google Scholar]
- 4.Brass L F, Manning D R, Cichowski K, Abrams C S. Thromb Haemostasis. 1997;78:581–589. [PubMed] [Google Scholar]
- 5.Davey M G, Luscher E F. Nature (London) 1967;216:857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- 6.Di Cera E, Dang Q D, Ayala Y M. Cell Mol Life Sci. 1997;53:701–730. doi: 10.1007/s000180050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamberg M, Svensson J, Samuelsson B. Proc Natl Acad Sci USA. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needleman P, Minkes M, Raz A. Science. 1976;193:163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- 9.Vu T-K H, Hung D T, Whaeton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 10.Connolly A J, Ishihara H M, Kahn L, Farese R V, Jr, Coughlin S R. Nature (London) 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 12.Brass L F, Molino M. Thromb Haemostasis. 1997;78:234–241. [PubMed] [Google Scholar]
- 13.Ushikubi F, Nakajima M, Hirata M, Okuma M, Fujiwara M, Narumiya S. J Biol Chem. 1989;264:16496–16501. [PubMed] [Google Scholar]
- 14.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Nature (London) 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Lim C-T, Lam S C-T, Komiotis D, Venton D L, Le Breton G C. Biochem Pharmacol. 1992;43:313–322. doi: 10.1016/0006-2952(92)90294-s. [DOI] [PubMed] [Google Scholar]
- 16.Shenker A, Goldsmith P, Unson C G, Spiegel A M. J Biol Chem. 1991;266:9309–9313. [PubMed] [Google Scholar]
- 17.Hung S C, Ghali N I, Venton D L, Le Breton G C. Prostaglandins. 1982;24:195–206. doi: 10.1016/0090-6980(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 18.Born G, Cross M J. J Physiol (London) 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kattelman E J, Venton D L, Le Breton G C. Thromb Res. 1986;41:471–481. doi: 10.1016/0049-3848(86)91692-0. [DOI] [PubMed] [Google Scholar]
- 20.Coleman R A, Humphrey P P A, Kennedy I G, Levy P, Lumley P. Br J Pharmacol. 1979;68:127P–128P. [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia J G N, Patterson C, Bahler C, Aschner J, Hart C M, English D. J Cell Physiol. 1993;156:541–549. doi: 10.1002/jcp.1041560313. [DOI] [PubMed] [Google Scholar]
- 22.Bahou W F, Coller B S, Potter C L, Norton K J, Kutok J L, Goligorski M S. J Clin Invest. 1993;91:1405–1413. doi: 10.1172/JCI116344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau L-F, Pumiglia K Y, Cote P M, Feinstein B. Biochem J. 1994;303:391–400. doi: 10.1042/bj3030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borg C, Lim C T, Yeomans D C, Dieter J P, Komiotis D, Anderson E G, Le Breton G C. J Biol Chem. 1994;269:6109–6116. [PubMed] [Google Scholar]
- 25.Knezevic I, Borg C, Le Breton G C. J Biol Chem. 1993;268:26011–26017. [PubMed] [Google Scholar]
- 26.Ascenzi P, Amiconi G, Bode W, Bolognesi M, Coletta M, Menegatti E. Mol Aspects Med. 1995;16:215–313. doi: 10.1016/0098-2997(95)00002-x. [DOI] [PubMed] [Google Scholar]
- 27.Martin B M, Feinman R D, Detwiler T C. Biochemistry. 1975;14:1308–1314. doi: 10.1021/bi00677a032. [DOI] [PubMed] [Google Scholar]
- 28.Gachet C, Hechler B, Leon C, Vial C, Leray C, Ohlmann P, Cazenave J-P. Thromb Haemostasis. 1997;78:271–275. [PubMed] [Google Scholar]
- 29.Massini P, Luscher E F. Biochim Biophys Acta. 1974;372:109–121. doi: 10.1016/0304-4165(74)90077-4. [DOI] [PubMed] [Google Scholar]
- 30.Wencel-Drake J D, Dieter M G, Lam S C-T. Blood. 1993;82:1197–1203. [PubMed] [Google Scholar]
- 31.Hsu-Lin S-C, Berman C L, Furie B C, August D, Furie B. J Biol Chem. 1984;258:9121–9126. [PubMed] [Google Scholar]
- 32.Ogletree M L, Harris D N, Greenberg R M, Haslanger F, Nakane M. J Pharmacol Exp Ther. 1985;234:435–441. [PubMed] [Google Scholar]
- 33.Hedberg A, Hall S E, Ogletree M L, Harris D N, Liu E C-K. J Pharmacol Exp Ther. 1988;245:786–792. [PubMed] [Google Scholar]
- 34.Clawges H M, Depree K M, Parker E M, Graber S G. Biochemistry. 1997;36:12930–12938. doi: 10.1021/bi970112b. [DOI] [PubMed] [Google Scholar]
- 35.Green M A, Chidiac P, Wells J W. Biochemistry. 1997;36:7380–7394. doi: 10.1021/bi961940s. [DOI] [PubMed] [Google Scholar]
- 36.Benka L, Lee M, Wang G R, Buckman S, Burlacu A, Cole L, DePina A, Dias P, Granger A, Grant B, et al. FEBS Lett. 1995;363:49–52. doi: 10.1016/0014-5793(95)00278-h. [DOI] [PubMed] [Google Scholar]
- 37.Strathmann M, Simon M I. Proc Natl Acad Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chesney C M, Pifer D D, Byers L W, Muirhead E E. Blood. 1982;59:582–585. [PubMed] [Google Scholar]
- 39.Izumi T, Shimizu T. Biochim Biophys Acta. 1995;1259:317–333. doi: 10.1016/0005-2760(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 40.Carlson S A, Chatterjee T K, Fisher R A. J Biol Chem. 1996;271:23146–23153. doi: 10.1074/jbc.271.38.23146. [DOI] [PubMed] [Google Scholar]