Abstract
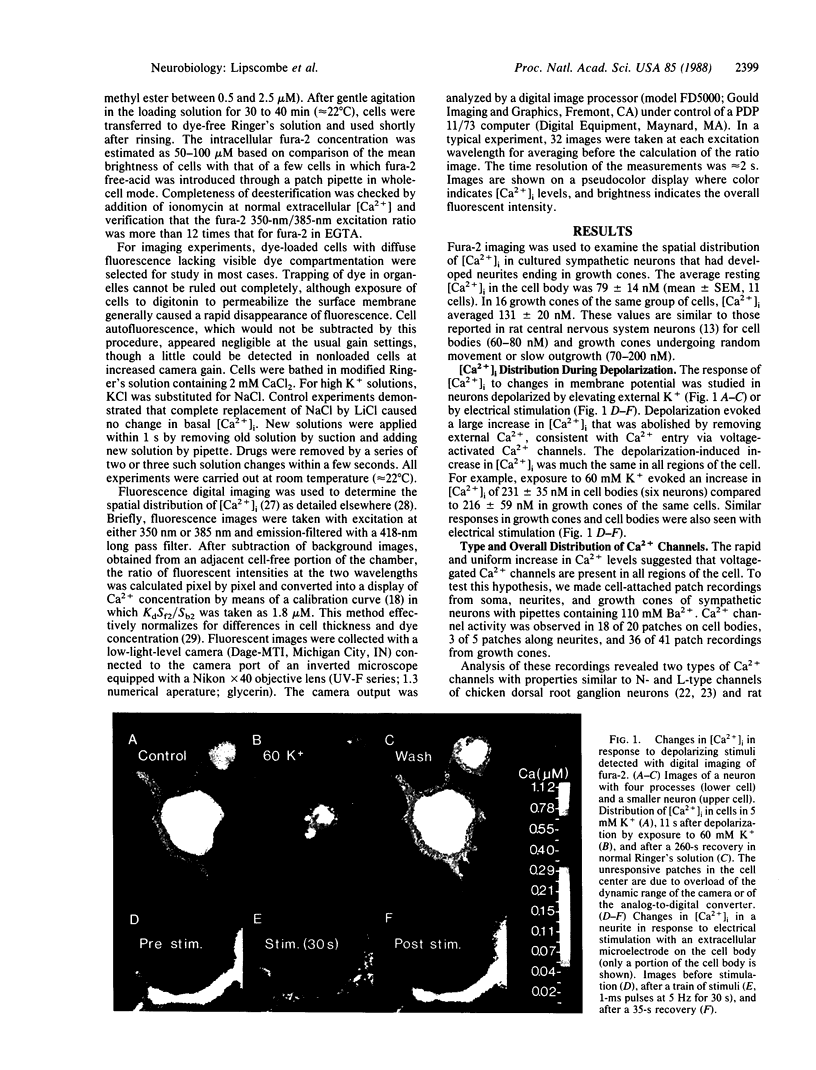
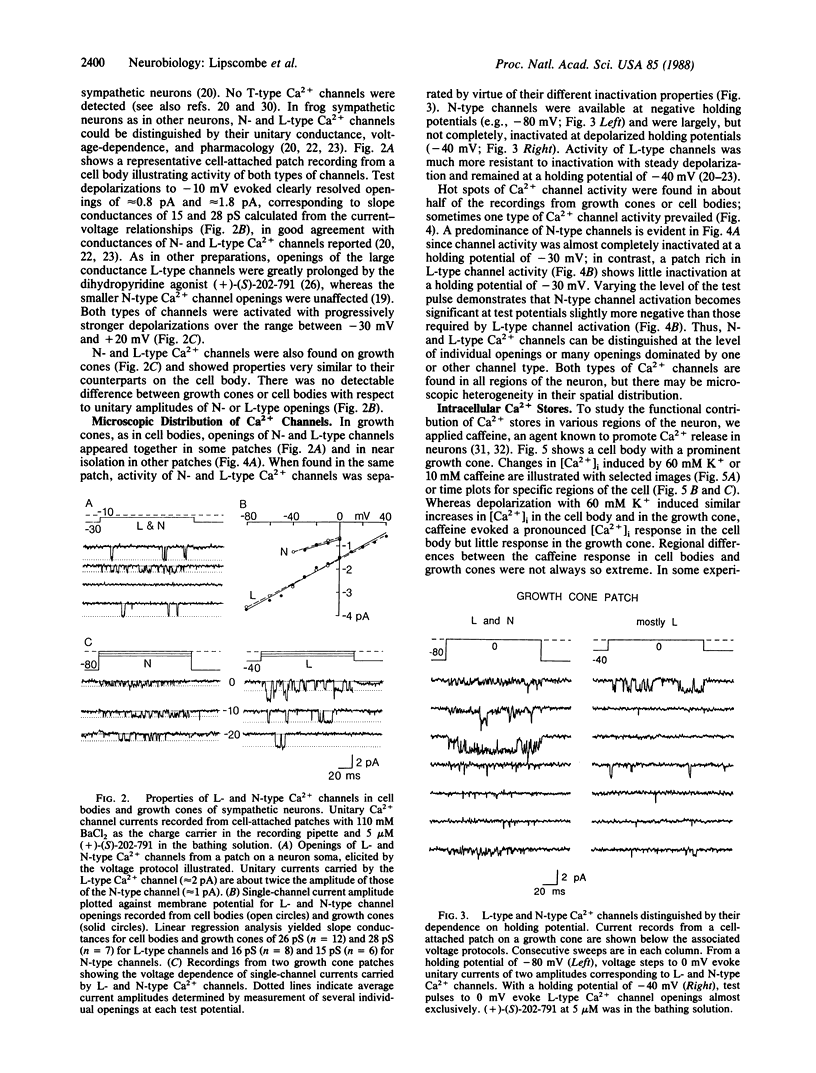
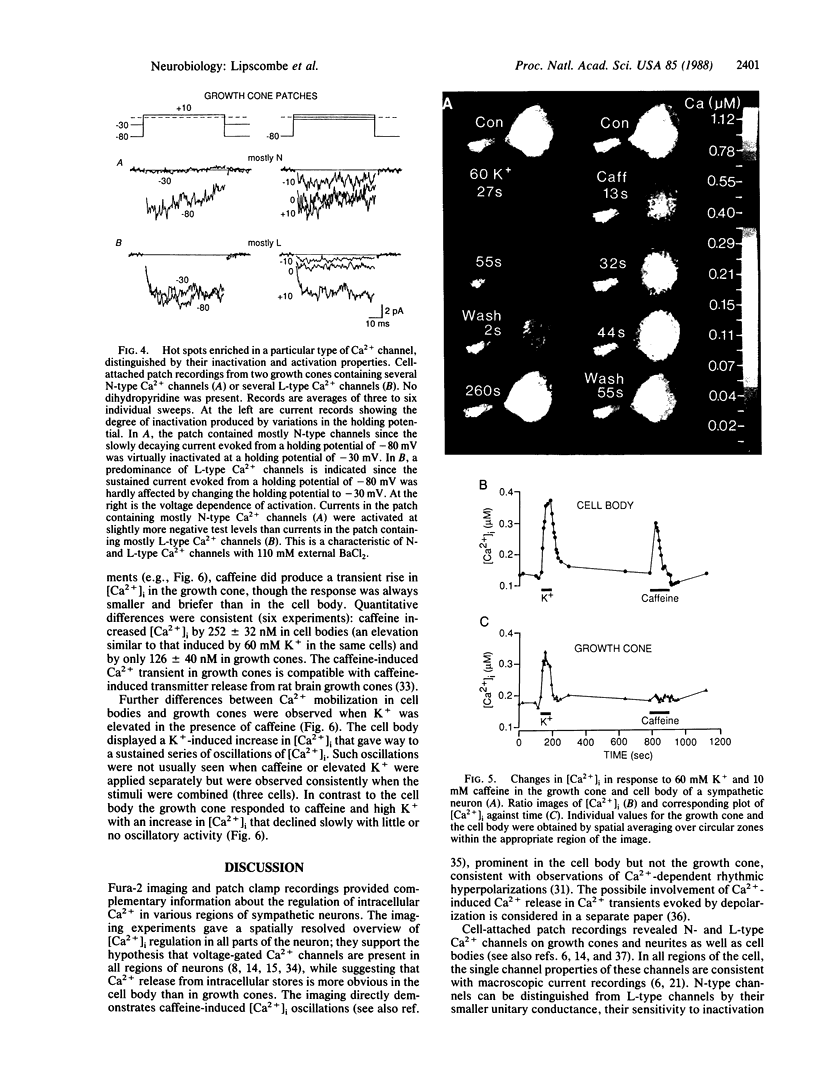
Ca2+ imaging and single-channel recording were used to study the regulation of cytosolic free Ca2+ ([Ca2+]i) in local regions of frog sympathetic neurons. Digital imaging with the fluorescent Ca2+ indicator fura-2 demonstrated: (i) resting [Ca2+]i of 70-100 nM; (ii) significant increases in [Ca2+]i in growth cones and cell bodies following depolarization induced by extracellular electrical stimulation or increased external K+; (iii) in cell bodies, large transient increases in [Ca2+]i following exposure to caffeine and sustained oscillations in [Ca2+]i in the presence of elevated K+ and caffeine; and (iv) in growth cones, smaller and briefer changes in [Ca2+]i in response to caffeine. The nature of the depolarization-induced Ca2+ entry was studied with cell-attached patch recordings (110 mM Ba2+ in recording pipette). Ca2+ channel activity was observed in 18 of 20 patches on cell bodies, 3 of 5 patches along neurites, and 36 of 41 patch recordings from growth cones. We observed two types of Ca2+ channels: L-type channels, characterized by a 28-pS slope conductance, sensitivity to dihydropyridine Ca2+ channel agonist, and availability even with depolarizing holding potentials; and N-type channels, characterized by a 15-pS slope conductance, resistance to dihydropyridines, and inactivation with depolarized holding potentials. Both types of channels were found on growth cones and along neurites as well as on cell bodies; channels often appeared concentrated in local hot spots, sometimes dominated by one channel type.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Farber I. C., Shahar A., Grinvald A. Localization of voltage-sensitive calcium channels along developing neurites: their possible role in regulating neurite elongation. Dev Biol. 1982 Dec;94(2):351–365. doi: 10.1016/0012-1606(82)90353-0. [DOI] [PubMed] [Google Scholar]
- Atchison W. D., O'Leary S. M. BAY K 8644 increases release of acetylcholine at the murine neuromuscular junction. Brain Res. 1987 Sep 1;419(1-2):315–319. doi: 10.1016/0006-8993(87)90599-3. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Schacher S., Siegelbaum S. A. Action potentials, macroscopic and single channel currents recorded from growth cones of Aplysia neurones in culture. J Physiol. 1986 May;374:289–313. doi: 10.1113/jphysiol.1986.sp016080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsover S. R., Spector I. Measurements of calcium transients in the soma, neurite, and growth cone of single cultured neurons. J Neurosci. 1986 Jul;6(7):1934–1940. doi: 10.1523/JNEUROSCI.06-07-01934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. P., Reese T. S. Recycling of plasmalemma in chick tectal growth cones. J Neurosci. 1987 Jun;7(6):1752–1759. doi: 10.1523/JNEUROSCI.07-06-01752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan C. S., Kater S. B. Suppression of neurite elongation and growth cone motility by electrical activity. Science. 1986 Jun 27;232(4758):1638–1640. doi: 10.1126/science.3715470. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Yamamoto K., Kuba K., Morita K., Kato E. Calcium localization in the sympathetic ganglion of the bullfrog and effects of caffeine. Brain Res. 1980 Nov 24;202(1):21–32. [PubMed] [Google Scholar]
- Goodman C. S., Bastiani M. J., Doe C. Q., du Lac S., Helfand S. L., Kuwada J. Y., Thomas J. B. Cell recognition during neuronal development. Science. 1984 Sep 21;225(4668):1271–1279. doi: 10.1126/science.6474176. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Farber I. C. Optical recording of calcium action potentials from growth cones of cultured neurons with a laser microbeam. Science. 1981 Jun 5;212(4499):1164–1167. doi: 10.1126/science.7233210. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henkart M., Landis D. M., Reese T. S. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol. 1976 Aug;70(2 Pt 1):338–347. doi: 10.1083/jcb.70.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Role L. W., Fischbach G. D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983 Oct 13;305(5935):632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of prolonged depolarization on synaptic transfer in the stellate ganglion of the squid. J Physiol. 1971 Jul;216(2):503–512. doi: 10.1113/jphysiol.1971.sp009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubun S., Prod'hom B., Becker C., Porzig H., Reuter H. Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers. Mol Pharmacol. 1986 Dec;30(6):571–584. [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockerbie R. O., Gordon-Weeks P. R. Further characterization of [3H]gamma-aminobutyric acid release from isolated neuronal growth cones: role of intracellular Ca2+ stores. Neuroscience. 1986 Apr;17(4):1257–1266. doi: 10.1016/0306-4522(86)90092-8. [DOI] [PubMed] [Google Scholar]
- Lockerbie R. O. The neuronal growth cone: a review of its locomotory, navigational and target recognition capabilities. Neuroscience. 1987 Mar;20(3):719–729. doi: 10.1016/0306-4522(87)90235-1. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- ROSENBLUTH J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962 Jun;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S. G., Holz G. G., 4th, Dunlap K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987 Aug;409(4-5):361–366. doi: 10.1007/BF00583789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I. J., Wagner J. A., Snyder S. H., Thayer S. A., Olivera B. M., Miller R. J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Stockbridge L. L., Stockbridge N. L. Regional properties of calcium entry in barnacle neurons determined with Arsenazo III and a photodiode array. J Neurosci. 1986 Apr;6(4):1148–1159. doi: 10.1523/JNEUROSCI.06-04-01148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., MacDermott A. B., Weight F. F. Detection of intracellular Ca2+ transients in sympathetic neurones using arsenazo III. 1983 Jul 28-Aug 3Nature. 304(5924):350–352. doi: 10.1038/304350a0. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Voltage dependent calcium currents in PC12 growth cones and cells during NGF-induced cell growth. Pflugers Arch. 1987 May;408(6):634–641. doi: 10.1007/BF00581167. [DOI] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Pelto D. J., Thompson J. M., Rapoport S. I. Blockers of calcium permeability inhibit neurite extension and formation of neuromuscular synapses in cell culture. Brain Res. 1984 Jun;316(2):263–270. doi: 10.1016/0165-3806(84)90311-0. [DOI] [PubMed] [Google Scholar]
- Sun Y. A., Poo M. M. Evoked release of acetylcholine from the growing embryonic neuron. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2540–2544. doi: 10.1073/pnas.84.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. L., Gorman A. L. Localization of neuronal Ca2+ buffering near plasma membrane studied with different divalent cations. Cell Mol Neurobiol. 1983 Dec;3(4):297–310. doi: 10.1007/BF00734712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Goldin S. M. Calcium channels in rat brain synaptosomes: identification and pharmacological characterization. High affinity blockade by organic Ca2+ channel blockers. J Neurosci. 1985 Mar;5(3):841–849. doi: 10.1523/JNEUROSCI.05-03-00841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Burnstock G. Junctional subsurface organs in frog sympathetic ganglion cells. J Neurocytol. 1976 Feb;5(1):125–136. doi: 10.1007/BF01176186. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983 Oct 13;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]