Abstract
Serum creatinine level is the most commonly used indices for assessment of glomerular filtration rate (GFR), even though these indices have been shown to have some limitations in clinical practice. We investigated the diagnostic efficacy of serum cystatin C compared to that of serum creatinine levels and identified the relating factors associated with changes in serum cystatin C levels in gout patients with renal impairment. A total of 68 gouty patients with renal impairment were enrolled in this study. Diagnostic efficacy of serum cystatin C levels was evaluated through non-parametric receiver operating characteristic (ROC) analysis. The risk factors for changes in serum cystatin C levels were confirmed using multivariate regression analysis. With 24-hr urine creatinine clearance (Ccr) as the reference for GFR, 1/cystatin C (r=0.702, P<0.001) showed a significantly higher correlation with Ccr than 1/creatinine (r=0.665, P<0.001). Multivariate correlation analysis demonstrated that the clinical parameters for increased serum cystatin C are a higher stage of chronic kidney disease, older age, use of allopurinol, and lower high density lipoprotein-cholesterol. The area under the curve (AUC) at ROC plots identified that of serum cystatin C was significantly greater than that of serum creatinine (AUC 0.804 of cystatin C and AUC 0.745 of creatinine). The study suggests that serum cystatin C is a reliable endogenous marker for the assessment of renal function or GFR in gout patients with renal impairment.
Keywords: Cystatin C, Gout, Creatinine, Kidney Failure, Glomerular Filtration Rate
INTRODUCTION
Until now, the glomerular filtration rate (GFR) was considered the best overall method of estimating the renal function of subjects (1). The determination of urinary clearance of exogenous substances, such as inulin, 99mTc-DTPA, and 51Cr-EDTA, has traditionally been considered the ideal standard method for estimating accurate GFR (2). Even though methods using exogenous substances are accurate, these procedures cannot be easily applied in clinical practice because of the need for continuous exogenous substance infusions, frequent urine samplings, and high cost. To overcome these limitations, formulas to estimate or predict 'true GFR' using serum creatinine levels were developed. Two commonly used representative formulas for the assessment of GFR in adults are the Modification of Diet in Renal Disease Study (MDRD) equation (3) and the Cockcroft-Gault (C&G) equation (4).
Cystatin C is a member of the family of cysteine protease inhibitors. It has a low molecular weight of only 13 kDa and is produced by all nucleated cells at a stable rate. Cystatin C was first discovered as a promising endogenous marker for GFR in 1985 (5, 6). It is wholly filtered through the glomerulus and then reabsorbed, being successively metabolized without secretion in the proximal tubule. Dharnidharka et al. (7) performed a meta-analysis of 46 cystatin C-related studies to evaluate the superiority of cystatin C levels over serum creatinine levels. These authors determined that serum cystatin C is a more potent marker of GFR than serum creatinine. Recently, Herget-Rosenthal and his colleagues found that determining cystatin C levels is a more sensitive test for the early detection of renal function impairment or reduced GFR (1). It has been recognized that an increase in serum cystatin C might well reflect changes in renal function or GFR for renal injury in diverse clinical conditions or diseases, including nephropathy associated with type 2 diabetes mellitus (8), hypertensive nephropathy (9), solid organ transplantation (10), and coronary angiopathy (11).
Gout is an inflammatory rheumatic disease characterized by deposition of crystallized monosodium urate, and uric acid, within the joints. It is frequently associated with hyperuricemia (12). The spectrum of renal diseases in gout patients includes urate stones, acute uric acid nephropathy, and chronic urate nephropathy. Chronic urate nephropathy may be associated with a series of complicating factors, such as hormonal changes, insulin resistance, therapeutic drug management for gout, and microvascular damage from untreated hypertension, rather than simply from excessive depositions of uric acid within renal tissues. Whatever the underlying of mechanism of renal disease in gout patients, individuals with gout have renal problems.
Currently, there are no clinical studies regarding the potential of using serum cystatin C to predict renal impairment in gout patients. Therefore, our study was designed 1) to compare diagnostic accuracy between serum cystatin C and serum creatinine and 2) to identify non-renal determinants associated with serum cystatin C in patients with gout.
MATERIALS AND METHODS
Subjects
For this study, 68 male Korean gout patients with renal impairment from the outpatient clinic of the Department of Rheumatology at Daegu Catholic University Medical Center were enrolled who fulfilled the preliminary criteria for the classification of primary gout put forth by the American College of Rheumatology (13). Patients who participated in this study gave informed consent for review of their medical records and use of urine and blood samples. This study was approved by the Institutional Review Board Committee of the Daegu Catholic University Medical Center
Exclusion criteria were as follows; patients on dialysis treatment, those with chronic renal disease at either stage IV or V according to guidelines proposed by the National Kidney Foundation of the United States through its Kidney Disease Outcomes Quality Initiative (K/DOQI) program (14), and individuals with diabetic nephropathy or hypertensive nephropathy. Based on the K/DOQI guidelines, patients enrolled in this study were assigned to one of three groups; normal GFR (≥90 mL/min/1.73 m2 and persistent albuminuria; stage 1 of chronic kidney disease [CKD]), mildly decreased GFR (60-89 mL/min/1.73 m2 and persistent albuminuria; stage 2 of CKD), and moderately decreased GFR (30-59 mL/min/1.73 m2; stage 3 of CKD).
Methods
Blood was sampled from a forearm vein after midnight fasting of 12-hr and stored at -70℃ until laboratory tests were performed. And spot urine collection was simultaneously acquired. Twenty four-hours urine collection was performed before the visit to the outpatient clinic for 1 day. Body mass index (BMI, kg/m2) was assessed. Disease duration in patients was estimated by examining medical records and interviewing individual patients.
Serum creatinine, serum cystatin C, blood urea nitrogen, serum uric acid, fasting glucose, lipid profiles such as total cholesterol, high density lipoprotein cholesterol (HDL-cholesterol), low density lipoprotein cholesterol (LDL-cholesterol), and triglyceride, erythrocyte sediment rate (ESR), and C-reactive protein (CRP) were assessed from blood samples at the time of enrollment in this study. Serum creatinine concentration was determined by the kinetic Jaffe method according to the manufacturer's instructions (Cobas Intergra, Roche, Switzerland). The quantitative measurement of cystatin C in human serum was performed using the HiSense™ latex-enhanced turbidimetric immunoassay cystatin C kit (HBI Co, Anyang, Korea). The degree of agglutination between latex particles coated with antibodies specific for human cystatin C and serum cystatin C levels is closely correlated with the concentration of cystatin C in the sample. An optical density reading at 660 nm of the aggregates was used to calculate the concentration of serum cystatin C.
GFR was estimated from creatinine clearance (Ccr) using a 24-hr urine collection in the subjects. The propriety of the urine collection was referred from the guideline that daily creatinine excretion should be 20 to 25 mg/kg of lean body weight in male adults. Estimates of GFR were corrected for a body surface area of 1.73 m2 using the method of DuBois and DuBois. Ccr was used as the reference GFR to assess the diagnostic efficacy of the test.
Statistic analysis
The data are presented as mean±standard deviation (SD) or number (n) and proportion (%). The differences in continuous variables among the three stages of renal function were compared by ANOVA test. Linear correlations were verified using the Pearson correlation test between continuous variables or using Spearman's correlation analysis between continuous and non-continuous variables. After simple linear regression analysis, confounding factors related to serum cystatin C concentration were reanalyzed using multivariate regression analysis.
Non-parametric receiver operating characteristic (ROC) analyses were performed to evaluate diagnostic values of individual parameters generated by graphically plotting sensitivity versus 1-specificity. The diagnostic accuracy of the test is measured by the area under the curve (AUC). Statistical significance is considered a value of P<0.05. All statistical analyses were performed using SPSS version 12.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Basic clinical characteristics of enrolled patients
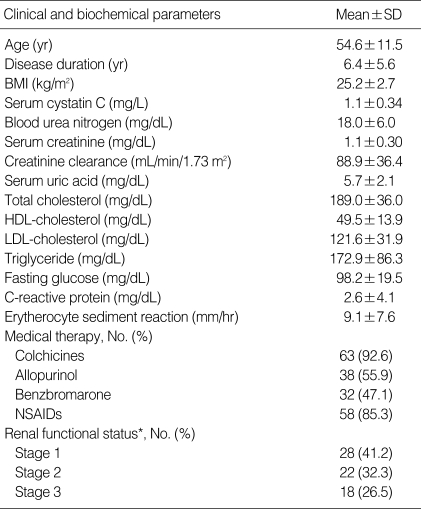
A total of 68 male gouty patients with renal impairment (54.6±11.5 yr of mean age and 6.4±5.6 yr of mean disease duration) were enrolled in this study. Clinical parameters related to the characteristics of patients and renal function were assessed; these included age at study, disease duration, body mass index (BMI), serum uric acid, uses of medications for gout such as colchicine, allopurinol, benzbromarone, and non-steroidal anti-inflammatory drugs (NSAIDs), lipid profiles, fasting glucose, ESR, CRP, serum cystatin C levels, serum creatinine levels, and Ccr (Table 1). In this study, we assigned enrolled patients to one of three groups; 28 patients were in the stage 1 of CKD, 22 patients in stage 2 of CKD, and 18 patients in stage 3 of CKD.
Table 1.
Characteristics of clinical and biochemical parameters in gout patients with renal impairment (n=68)
*Estimated according to K/DOQI guideline.
SD, standard deviation; BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; NSAID, non-steroidal anti-inflammatory drugs; GFR, glomerular filtration rate; Ccr, Creatinine clearance.
The differences in clinical parameters between the three groups were analyzed by ANOVA test. The results showed significant differences in age at study (49.1±10.6 vs. 56.1±9.7 vs. 61.1±11.3, P=0.001), serum cystatin C (0.9±0.2 vs. 1.1±0.2 vs. 1.4±0.4, P<0.001), serum creatinine (1.0±0.2 vs. 1.1±0.2 vs. 1.4±0.3, P<0.001), and blood urea nitrogen (17.2±5.9 vs. 15.5±4.3 vs. 22.1±5.8, P=0.001) among three groups. However, clinical parameters such as disease duration, BMI, serum uric acid, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglyceride, fasting glucose, CRP, and ESR did not reveal significant differences among the three groups (data not shown).
Association of serum cystatin C and serum creatinine for GFR with Ccr
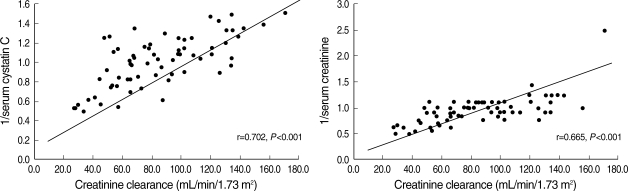
The measured renal parameters, both serum cystatin C and serum creatinine, were significantly correlated with GFR of Ccr (Fig. 1). The correlation coefficient between 1/cystatin C and Ccr (r=0.702, P<0.001) was higher than that between 1/creatinine (r=0.665, P<0.001) and Ccr (Fig. 1). A significant correlation between serum creatinine and serum cystatin C was also identified (r=0.815, P<0.001).
Fig. 1.
Correlation between the 1/serum cystatin C and 1/serum creatinine for GFR estimated by Ccr.
Clinical and laboratory parameters related with serum cystatin C
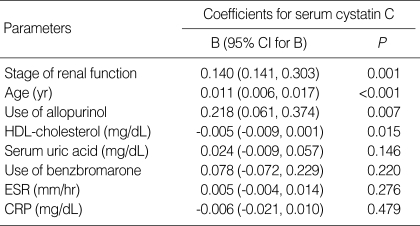
We also identified non-renal determinants associated with serum cystatin C in this study using simple linear correlation analysis. Among all patients, serum cystatin C was closely related with stages of renal function (r=0.576, P<0.001), age at study (r=0.586, P<0.001), the use of allopurinol (r=0.406, P=0.001), the use of benzbromaron (r=-0.329, P=0.006), serum uric acid (r=0.379, P=0.001), ESR (r=0.364, P=0.002), CRP (r=0.279, P=0.021), and HDL-cholesterol (r=-0.265, P=0.033). Multivariate regression analysis identified that more advanced renal stage (B=0.140, P=0.001), older age (B=0.011, P<0.005), use of allopurinol (B=0.218, P=0.007), and lower HDL-cholesterol (B=-0.005, P=0.015) were associated with higher serum cystatin C in gout patients (Table 2).
Table 2.
Multivariate regression analysis for clinical and laboratory parameters associated with serum cystatin C
CI, confidence interval.
Correlation for clinical and biochemical parameters according to renal function was assessed in each three groups. Some clinical parameters, such as the use of allopurinol (r=0.447, P=0.017), serum uric acid (r=0.419, P=0.027), and CRP (r=0.477, P=0.010) for stage 1 of CKD, only age at study (r=0.530, P=0.011) for stage 2, and age at study (r=0.517, P=0.028), the use of allopurinol (r=0.497, P=0.036), BUN (r=0.474, P=0.047), and ESR (r=0.677, P=0.002) were significantly associated with serum cystatin C. However, Multivariate regression analysis for each renal function group showed only age of study was significantly correlated with serum cystatin C at both stage 2 and stage 3 of CKD (P=0.011 and P=0.018, respectively), although parameters related with serum cystatin C could not be identified.
Diagnostic accuracy of serum cystatin C in gout
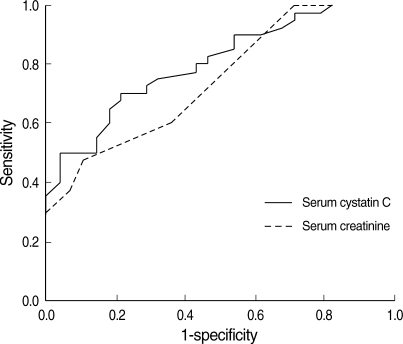
The diagnostic accuracy of serum cystatin C was evaluated using a non-parametric ROC plot (Fig. 2). The results showed that the AUC for serum cystatin C had greater areas compared with that of serum creatinine (AUC 0.804, P<0.001 vs. AUC 0.745, P=0.001). This findings demonstrated serum cystatin C in gout patients has more diagnostic accuracy compared with serum creatinine.
Fig. 2.
Comparison between serum cystatin C (solid line) and serum creatinine (dotted line) in gout patients by nonparametric ROC plot analysis. The AUC for serum cystatin C and creatinine were 0.804 and 0.745, respectively.
DISCUSSION
Serum cystatin C has been recognized as a reliable endogenous index of GFR, since its introduction in 1985 (5, 6). In addition, more sensitive indicators of GFR or renal function have been identified in a variety of diverse systemic diseases including nephropathy in type 2 diabetes mellitus (8), hypertensive nephropathy (9), solid organ transplantation (10), and coronary angiopathy (11). Few studies have investigated serum cystatin C as an endogenous marker of renal function in rheumatic diseases other than rheumatoid arthritis (15, 16). This is the first study to investigate the clinical application of serum cystatin C to assess renal function in gout patients with renal impairment. We found that serum cystatin C is closely associated with serum creatinine and a good potential marker of GFR in gout patients.
Gout is a common, manageable medical disease related to other systemic rheumatic diseases and has a wide spectrum of clinical features, ranging from acute gouty arthritis to chronic tophaceous gout. This disease is frequently associated with hyperuricemia (defined as a serum urate concentration over 7 mg/dL) due to overproduction or underexcretion of urate, or a combination of both. The physician should pay careful attention to the development of associated comorbidities, such as chronic renal diseases, cardiovascular diseases, or metabolic syndrome. Of special clinical interest is the association between gout and chronic renal diseases. However, some debate remains as to whether impairment of renal function is related to complicating factors such as hormonal changes, insulin resistance, therapeutic drugs, and untreated hypertension, or to excessive depositions within renal tissue leading to renal impairment, cell death and tissue hypoxia by hyperuricemia (17). Talbott and Terplan (18) presented diverse histopathological findings in the biopsies and autopsy from 279 gout patients, and identified both clinicolaboratory and pathologic findings. Murray and Goldberg (19) showed that hyperuricemia was a primary cause of chronic interstitial nephritis in a retrospective study of 101 patients. Recent study determined that hyperuricemia may contribute to the development of chronic gouty nephropathy as well as play a crucial role in the progression of renal pathology (17).
Until now, traditional measures for assessing renal function such as measuring serum creatinine have been widely used in gout patients, although they have some limitations for accurate estimation of GFR. Here, we evaluated the diagnostic efficacy and accuracy of using serum cystatin C levels to estimate GFR and compared these results to those obtained using traditional renal function indicators such as serum creatinine and Ccr. Analysis for linear associations showed that reciprocal cystatin C levels correlate well with Ccr of 24 hr urine (r=0.702). In addition, the level of cystatin C is better correlated with Ccr than the reciprocal creatinine level (r=0.665) (Fig. 1). Our results from 68 male gout patients indicate that serum cystatin C is a novel and reliable marker of GFR. Zahran et al. (20) reviewed a number of studies to compare the diagnostic accuracy of serum cystatin C levels and serum creatinine levels in many clinical situations, including transplant patients, patients with native kidney disease, adults with native kidney disease, and pediatric patients with native kidney disease (20). Their finding that serum cystatin C was superior to serum creatinine remains controversial; many investigators prefer to use serum cystatin C rather than serum creatinine as an index of GFR (1, 7, 20). Our study found that the diagnostic accuracy of serum cystatin C levels were superior to that of serum creatinine using a ROC curve.
The weaknesses of traditional measures of GFR including serum creatinine have been recognized (1). Although serum creatinine has became the most popularly used serum marker of renal function, serum creatinine may be unreliable because they are frequently affected by muscle mass, age, gender, and aberrant renal tubular regulation of serum creatinine resulting in an overestimation of GFR. Using serum cystatin C levels has some advantages over serum creatinine and creatinine-based calculated GFR formulas, in that serum cystatin C levels are independent of age, gender, muscle mass, and renal tubular secretion (1). Tenstad et al. (21) found that the renal plasma clearance of cystatin C correlated well with GFR using 51Cr-EDTA with a linear correlation coefficient of 0.99. Given these characteristics, cystatin C can be considered to be an ideal indicator of GFR. The present study also found that serum cystatin C showed a good inverse correlation to Ccr in gout patients (r=0.702, P<0.001).
It has long been known that cystatin C levels are not highly influenced by confounding factors, such as diet, nutrition, or inflammatory status, whereas measurements for GFR based on creatinine levels, including the MDRD or C&G formulas, have substantial limitations such as age, sex, muscle mass, ethnicity, and methodology of determining serum creatinine level (1) However, cystatin C production was not thought to be affected by any factors until several authors demonstrated that serum cystatin C could be affected by both rheumatoid factor (22) and high doses of glucocorticoid (23). Additionally, indirect evidence, including decreasing cystatin C in asthmathic patients after cyclosporine therapy (24) and the positive correlation between CRP and cystatin C levels in large populations indicates that inflammatory status may also contribute to changes in serum cystatin C levels (25). In the present study, we assessed whether certain demographic and clinical factors were correlated with serum cystatin C levels among all enrolled patients. The data from our analyses indicates that the stage of renal disease, the age of patients at the time of study, serum uric acid, and HDL-cholesterol all influence the serum cystatin C level. In addition, allopurinol-treated patients have a tendency to have higher serum cystatin C levels. It may be considered that gout patients with renal impairment have allopurinol rather than benzbromarone as a uric acid lowering agent. Interestingly, our data illustrated a weakly positive association between ESR and serum cystatin C levels. This finding suggests that serum cystatin C can be influenced by inflammatory changes, which is in agreement with results of some previous studies (24, 25), but in contrast to the data of others (15). These conflicting results necessitate further investigation into the effects of acute phase reactants such as ESR and CRP on changes in the levels of serum cystatin C. In addition, only age at study was significantly correlated with serum cystatin C at subgroup analysis according to renal function status. This discrepancy between whole enrolled patients and each group patients may be resulted from smallsized populations.
Our study evaluated the diagnostic performance of serum cystatin C using Ccr as a reference GFR, even though the limitations of this technique were highlighted in previous studies (26, 27). However, Herget-Rosenthal et al. (28) discussed creatinine clearance for GFR as an equivalent marker of true GFR, likening it to different "gold standard" methods of GFR including inulin, 125I-iothalamate, and 99mTc-DTPA. In their study, cystatin C was found to be an accurate index of GFR in 110 renal transplantation patients. Several studies regarding cystatin C as diagnostic index of renal function in renal transplant patients using Ccr as a reference GFR have been performed, although the accuracy of serum cystatin C and serum creatinine for evaluating GFR differed between the various studies (28). Finally, some studies have demonstrated similar diagnostic performances of serum cystatin C and serum creatinine levels using different GFR references such as inulin (29) and 51Cr-EDTA (30).
Data derived from our cross-sectional study in 68 male gout patients with renal impairment illustrates that the serum cystatin C level is a novel and reliable marker for accurately predicting GFR that is superior to serum creatinine. This is the first study to demonstrate the clinical application of serum cystatin C levels in the field of gout, one of the inflammatory rheumatic diseases. In addition to renal parameters in gout patients, the results of this study revealed an association between non-renal components such as allopurinol use and HDL-cholesterol level and serum cystatin C. In the realm of inflammatory rheumatic diseases such as rheumatoid arthritis and gout, renal issues are continuous and prominent. There are some limitations for this study. The characteristics or patterns of changes of serum cystatin C level were not observed in this study because of limitation of cross-sectional study. And this study could not reveal relationship between serum cystatin C and inflammatory status of acute or interval stage of gout or serologic markers, such as CRP, ESR, or serum amyloid. To overcome these limitations, well-designed longitudinal study for serum cystatin C will be necessary in larger population.
In conclusion, serum cystatin C is a reliable endogenous marker for detection of renal impairment and estimation of true GFR. To conclusively determine the diagnostic value of serum cystatin C levels for monitoring renal function of gouty patients, further prospective studies in a larger study population are needed.
References
- 1.Herget-Rosenthal S, Bökenkamp A, Hofmann W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem. 2007;40:153–161. doi: 10.1016/j.clinbiochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Perrone RD, Steinman TI, Beck GJ, Skibinski CI, Royal HD, Lawlor M, Hunsicker LG. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1990;16:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 5.Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H. Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218:499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gammatrace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45:97–101. doi: 10.3109/00365518509160980. [DOI] [PubMed] [Google Scholar]
- 7.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 8.Mussap M, Dalla Vestra M, Fioretto P, Saller A, Varagnolo M, Nosadini R, Plebani M. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int. 2002;61:1453–1461. doi: 10.1046/j.1523-1755.2002.00253.x. [DOI] [PubMed] [Google Scholar]
- 9.Ozer BA, Dursun B, Baykal A, Gultekin M, Suleymanlar G. Can cystatin C be a better marker for the early detection of renal damage in primary hypertensive patients? Ren Fail. 2005;27:247–253. [PubMed] [Google Scholar]
- 10.Ling Q, Xu X, Li J, Wu J, Chen J, Xie H, Zheng S. A new serum cystatin C-based equation for assessing glomerular filtration rate in liver transplantation. Clin Chem Lab Med. 2008;46:405–410. doi: 10.1515/CCLM.2008.052. [DOI] [PubMed] [Google Scholar]
- 11.Artunc FH, Fischer IU, Risler T, Erley CM. Improved estimation of GFR by serum cystatin C in patients undergoing cardiac catheterization. Int J Cardiol. 2005;102:173–178. doi: 10.1016/j.ijcard.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 13.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 2):S1–S266. [PubMed] [Google Scholar]
- 15.Karstila K, Harmoinen AP, Lehtimäki TJ, Korpela MM, Mustonen JT, Saha HH. Measurement of the kidney function in patients with rheumatoid arthritis: plasma cystatin C versus 51Cr-EDTA clearance. Nephron Clin Pract. 2008;108:c284–c290. doi: 10.1159/000127362. [DOI] [PubMed] [Google Scholar]
- 16.Bokarewa M, Abrahamson M, Levshin N, Egesten A, Grubb A, Dahlberg L, Tarkowski A. Cystatin C binds serum amyloid A, downregulating its cytokine-generating properties. J Rheumatol. 2007;34:1293–1301. [PubMed] [Google Scholar]
- 17.Kang DH, Nakagawa T. Uric acid and chronic renal disease: possible implication of hyperuricemia on progression of renal disease. Semin Nephrol. 2005;25:43–49. doi: 10.1016/j.semnephrol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Talbott JH, Terplan KL. The kidney in gout. Medicine (Baltimore) 1960;39:405–467. [PubMed] [Google Scholar]
- 19.Murray T, Goldberg M. Chronic interstitial nephritis, Etiologic factors. Ann Intern Med. 1967;82:453–459. doi: 10.7326/0003-4819-82-4-453. [DOI] [PubMed] [Google Scholar]
- 20.Zahran A, El-Husseini A, Shoker A. Can cystatin C replace creatinine to estimate glomerular filtration rate? A literature review. Am J Nephrol. 2007;27:197–205. doi: 10.1159/000100907. [DOI] [PubMed] [Google Scholar]
- 21.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–414. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 22.Lamb E, Stowe H. Rheumatoid factor can interfere with cystatin C measurement. Ann Clin Biochem. 2003;40:195–196. [PubMed] [Google Scholar]
- 23.Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059. [PubMed] [Google Scholar]
- 24.Cimerman N, Brguljan PM, Krasovec M, Suskovic S, Kos J. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clin Chim Acta. 2000;300:83–95. doi: 10.1016/s0009-8981(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 25.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 26.Toto RD. Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens. 1995;4:505–509. doi: 10.1097/00041552-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 28.Herget-Rosenthal S, Trabold S, Huesing J, Heemann U, Philipp T, Kribben A. Cystatin C-an accurate marker of glomerular filtration rate after renal transplantation? Transpl Int. 2000;13:285–289. doi: 10.1007/s001470050703. [DOI] [PubMed] [Google Scholar]
- 29.Schück O, Teplan V, Jabor A, Stollova M, Skibova J. Glomerular filtration rate estimation in patients with advanced chronic renal insufficiency based on serum cystatin C levels. Nephron Clin Pract. 2003;93:c146–c151. doi: 10.1159/000070234. [DOI] [PubMed] [Google Scholar]
- 30.Donadio C, Lucchesi A, Ardini M, Giordani R. Cystatin C, beta 2-microglobulin, and retinol-binding protein as indicators of glomerular filtration rate: comparison with plasma creatinine. J Pharm Biomed Anal. 2001;24:835–842. doi: 10.1016/s0731-7085(00)00550-1. [DOI] [PubMed] [Google Scholar]