Abstract
To determine the relations between post-stroke aphasia severity and aphasia type and lesion location, a retrospective review was undertaken using the medical records of 97 Korean patients, treated within 90 days of onset, for aphasia caused by unilateral left hemispheric stroke. Types of aphasia were classified according to the validated Korean version of the Western Aphasia Battery (K-WAB), and severities of aphasia were quantified using WAB Aphasia Quotients (AQ). Lesion locations were classified as cortical or subcortical, and were determined by magnetic resonance imaging. Two-step cluster analysis was performed using AQ values to classify aphasia severity by aphasia type and lesion location. Cluster analysis resulted in four severity clusters: 1) mild; anomic type, 2) moderate; Wernicke's, transcortical motor, transcortical sensory, conduction, and mixed transcortical types, 3) moderately severe; Broca's aphasia, and 4) severe; global aphasia, and also in three lesion location clusters: 1) mild; subcortical 2) moderate; cortical lesions involving Broca's and/or Wernicke's areas, and 3) severe; insular and cortical lesions not in Broca's or Wernicke's areas. These results revealed that within 3 months of stroke, global aphasia was the more severely affected type and cortical lesions were more likely to affect language function than subcortical lesions.
Keywords: Stroke, Aphasia, Classification
INTRODUCTION
Stroke is the leading cause of long-term disability among adults in developed countries, and aphasia is one of the most common and devastating cognitive impairments of stroke (1,2). About 21-38% of acute stroke patients experience aphasia (2, 3), and in the majority of cases the left perisylvian areas are damaged (4), though lesions are also found in the subcortical (5, 6) or right cortical regions in some cases (2).
The Western Aphasia Battery (WAB) is used routinely to evaluate adult language function, and has high internal consistency, test-retest reliability, and validity (7). WAB is also widely used to determine the presence, type, and severity of aphasia (8). In the WAB, by evaluating language profiles for fluency, comprehension, repetition, and naming, aphasia can be classified into global, Broca's, mixed transcortical, transcortical motor, Wernicke's, transcortical sensory, conduction, and anomic types (9). In addition, the WAB describes severities of aphasia as aphasia quotients (AQ) (10).
It has been reported that 20-40% of aphasias are of the global type, whereas the classic aphasia types, such as, Broca's orWernicke's aphasia, are found in a quarter of patients, and 10-15% of patients are unclassifiable according to traditional typologies during the acute stage of stroke (2). However, aphasia usually recovers to similar extents during the subacute to chronic stages (2, 11).
Relations between aphasia severity, aphasia type, and lesion location could be helpful for rehabilitation planning, but literature on this topic is limited, especially in the Korean population. Therefore, we undertook this study to determine the natures of relations between the severity of post-stroke aphasia and aphasia type and lesion location in Korean patients.
MATERIALS AND METHODS
Ninety-seven consecutive aphasic patients that were examined using the validated Korean version of the Western Aphasia Battery (K-WAB) after stroke from June 2003 to June 2007 at two university hospital speech therapy units in Korea were enrolled in the present study. Medical records were reviewed retrospectively. The K-WAB results of patients with aphasia were subjected to analysis only when aphasia was caused by first stroke and when results were obtained within 3 months of stroke onset. When K-WAB was performed twice or more, only the initial result was included in the analysis. K-WAB results of non-stroke conditions, such as, traumatic brain injury, a brain tumor, and neurodegenerative disease, were excluded.
Ninety-seven patients (37 females and 60 males; average age 61.0±13.8 yr, range 13-88 yr) with post-stroke aphasia were enrolled. Seventy-five (77.3%) had a cerebral infarction and 22 (22.7%) a cerebral hemorrhage. Seventy-eight had a cortical lesion and 19 a subcortical lesion. Ninety of the patients were right handed, one was left handed, one was ambidextrous, and 5 were not determined. Patients underwent K-WAB at a mean 26.0±20.9 days (range 2-90 days) after stroke. The institutional review boards at both institutions involved approved the data analysis. Two trained speech-language pathologists (SLP) undertook K-WAB (12) (one from each hospital). The same SLPs administered K-WAB to all patients at each hospital.
The WAB assessment is composed of four domains of fluency, comprehension, repetition, and naming. Aphasia types were categorized using Kertesz's classification (9), and aphasia severities were quantified using Aphasia Quotients (AQ; range 0-100), which were calculated using the formula proposed by Kertesz (10) (fluency score+comprehension score/20+repetition score /10+naming score/10)×2.
A single physician classified stroke lesion locations as cortical or subcortical, based on magnetic resonance imaging (MRI) findings. Twenty-six cortical lesions that extended into subcortical areas due to infarction of the middle cerebral artery territory were classified as cortical lesions. Using a brain atlas (13), locations of cortical lesions was classified further as the lesions involving Broca's area (Brodmann area 22), Wernicke's area (Brodmann area 44), Broca's and Wernicke's areas, or as insula or cortex not involving Broca's or Wernicke's areas. Lesions involving only basal ganglia, thalamus, periventricular white matter, or centrum semiovale were classified as subcortical lesions. To compare aphasia severities according to the timing of K-WAB examination after stroke, times after onset were classified as <30 or 30-90 days. Handedness was determined using the Edinburgh handedness scale (14).
Statistical analysis was performed using SPSS for Windows version 12.0. Differences between the AQ scores of cortical and subcortical lesions and between AQ scores at <30 and 30-90 days were compared using the independent samples t test. Statistical significance was accepted for P values of <0.05.
To categorize aphasia severity by aphasia type and lesion location, two-step cluster analysis was conducted using AQ scores and Schwarz's Bayesian criteria. Cluster analysis identifies groups of individuals that are similar to each other but different from individuals in other groups. Two step cluster analysis has two steps; it first pre-clusters patients into many small sub-clusters, and then clusters these sub-clusters into the desired number of clusters. It can also automatically select the number of clusters (15).
AQ scores, categorized by sex or stroke type, were compared using the independent samples t test. Pearson's correlation analysis was also performed to examine relations between AQ and age or time after onset.
RESULTS
Aphasia types and severities
Types and severities of aphasia in the 97 subjects are summarized in Table 1. In frequency order, aphasia types were; global 27 subjects (27.8%), anomic 21 (21.6%), Broca's 20 (20.6%), Wernicke's 12 (12.4%), transcortical sensory 7 (7.2 %), transcortical motor 6 (6.2%), mixed transcortical 3 (3.1 %), and conduction 1 (1.0%), respectively.
Table 1.
Aphasia type and severity after stroke
Values are means±SDs.
Mean severities expressed as AQ values were; total 34.7±29.1, global 7.6±8.2, mixed transcortical 19.5±12.0, Broca's 26.6±16.4, Wernicke's 32.1±11.9, transcortical sensory 46.7±12.2, transcortical motor 48.0±11.7, anomic 74.3±24.0, and conduction 89.2, respectively.
Aphasia severities by lesion location are shown in Table 2. Based on AQ values, aphasia with cortical lesions tended to be severer than aphasia with subcortical lesions (cortical 32.1 ±27.6 vs. subcortical 46.9±33.7; P=0.074 by the Student's t test). In terms of K-WAB sub-items, repetition was found to be significantly different for cortical and subcortical lesions (P=0.049). No statistical difference was found between cortical lesions (n=52) and cortico-subcortical lesions (n=26) in terms of AQ values (cortical 32.2±28.3 vs. cortico-subcortical 34.2±26.4; P=0.799 by the Student's t test).
Table 2.
Cortical and subcortical lesions and aphasia severity
Values are means±SDs.
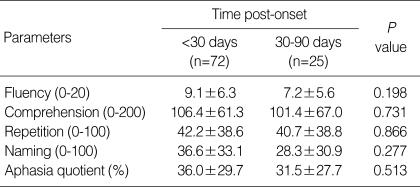
Aphasia severities, analyzed by time after onset, are shown in Table 3. No significant difference was found between AQ values examined at <30 days and 30-90 days after stroke (P=0.513 by Student's t test). Similarly, Pearson's correlation analysis revealed no significant correlation between AQ values and time after onset (r=-0.184, P=0.089).
Table 3.
Time post-onset and aphasia severity
Values are means±SDs.
When aphasia severities were examined by gender and by stroke type (infarction vs. hemorrhage), no significant differences were found (P=0.895 and P=0.323, respectively). Pearson's correlation analysis of the relation between age and AQ revealed no significant correlation (r=-0.049, P=0.653).
Cluster analysis
Two-step cluster analysis was conducted based on AQ values to assess whether the 4 distinct aphasic subgroups differed in terms of severity (Table 4). This analysis differentiated patients into four distinct subgroups based on AQ values. Cluster 1 (AQ=74.3±24.0) included less severe aphasic patients of the anomic type; Cluster 2 (AQ=39.7±17.5) included Wernicke's, transcortical motor, and transcortical sensory, conduction, and mixed transcortical types; Cluster 3 (AQ=26.6±16.4) included Broca's type aphasia; and Cluster 4 (AQ=7.6±8.2) included the more severe aphasic global type characterized by non-fluent speech with marked impairments in comprehension, repetition, and naming. Eleven patients could not be allocated to any cluster.
Table 4.
Profiles of aphasia severity clusters
*11 cases were excluded.
AQ, aphasia quotients.
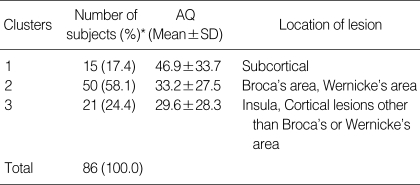
Cluster analysis, based on AQ values, was also performed to determine whether lesion location subgroups differed in terms of severity (Table 5). This analysis revealed three distinct subgroups. Cluster 1 (AQ=46.9±33.7) included mildly affected aphasic lesions in the subcortical area; Cluster 2 (AQ=33.2±27.5) included lesions in Broca's area or Wernicke's area, or in both; and Cluster 3 (AQ=29.6±28.3) included more severe cortical lesions involving the insula and cortical lesions other than those in Broca's or Wernicke's areas. Again, it was not possible to allocate 11 aphasic patients to any cluster.
Table 5.
Profiles of lesion location clusters
*11 cases were excluded.
AQ, aphasia quotients.
DISCUSSION
The main findings of this study was that global aphasia is the most severe form of aphasia and cortical lesions are more likely to affect language function than subcortical lesions within 3 months after stroke, regardless of age, sex, or type of stroke.
In the present study, the most frequent type of aphasia was global aphasia, followed by anomic and Broca's aphasia. This finding concurs with a previous report (2). Severities of aphasia, as represented by AQ values of aphasia types, also concurred with previous reports.
However, in previous studies, WAB measurements varied considerably between the acute and chronic stroke stages (2, 16). de Boissezon et al. (17) noted that subcortical aphasia improved over the first year after onset, and that this was accompanied by increases in bilateral regional cerebral blood flow. This implies that the WAB examination timing may markedly affect aphasia type and severity distributions. Bakheit et al. (18) also found in their retrospective study that time after stroke affected AQ scores, but that severity by aphasia type was maintained at 4, 8, 12, and 24 weeks after onset. Furthermore, these investigators also reported that rates of improvement depended on aphasia type.
In the present study, we did not follow up aphasia severity longitudinally, but included tests performed only within 90 days of stroke, which is a study limitation. However when we compared AQ scores obtained at <30 days and at 30-90 days after onset, no significant difference was found, and Pearson's correlation coefficients for AQ scores vs. time after onset revealed no significant relation. Furthermore, order of severity by aphasia type was identical for the two K-WAB examination periods.
Stroke severity might impact aphasia severity and type, but this issue was also not addressed during the present study. In a previous study, Pedersen et al. (2) concluded that outcome is dependent on initial stroke severity, but not on age or sex.
Although aphasia is traditionally associated with cortical deficit, several studies have reported that aphasia can also be caused by subcortical brain lesions, i.e., lesions of the thalamus, basal ganglia, or corona radiata (17, 19, 20). In the present study, 19 patients had a subcortical lesion, and of these, the anomic type was most frequent, which concurs with the findings of previous studies (2, 21, 22).
The specific roles of subcortical structures in language have yet to be elucidated. Subcortical aphasias are heterogenous in terms of type and lesion location, and usually differ from classical cortical aphasic syndromes. Furthermore, subcortical aphasias often have a good prognosis (23), and repetitive speech is usually preserved, which suggests a similarity with the transcortical type (24).
The thalamus, which is the main subcortical structure involved in speech, is extensively connected to the temporal, parietal, and frontal cortexes, and provides semantic-lexical associations and working memory for the language process. Therefore, lesions of the thalamus can cause dysfunctions at the prelinguistic level, for example, in concept generation or in the control, release, and inhibition of preformed speech patterns (25, 26). Striatocapsular lesions have also been reported to cause language dysfunctions by impairing executive language functions, such as, fluency. However, responsive language, such as, comprehension, repetition, and naming, are largely spared by these lesions (27).
Disconnections between language cortical areas or the total disruption off neural inputs to remote cortical areas by subcortical lesions have been suggested to underlie the development of aphasia (20, 28). In the present study, thalamic aphasia was found to be more severe than non-thalamic aphasia, but we could not analyze it statistically because only three cases of thalamic aphasia were enrolled. Cluster analysis based on the aphasia severities of 19 subcortical lesions revealed two clusters, and interestingly, all three cases with thalamic lesions were included in the severely affected cluster. Thalamic aphasia patients are known to develop partial fluency and good repetition, but highly variable listening comprehension (26).
Most lesions involving the left frontal or temporal lobes include specific language areas (such as, Broca's or Wernicke's area), although cortical lesions that do not involve these areas have been encountered. In the present study, 14 aphasic patients had cortical involvement of an area other than Broca's or Wernicke's areas, and seven patients had insular lesions. The insular cortex contains more primitive structures than the neocortex, and it is highly interconnected with the basal forebrain and hypothalamus. Furthermore, it has been suggested to participate in motor planning for speech production (29). In the present study, seven insular lesions were included in the most severely affected cluster.
In the present study, based on AQ values, cases with cortical lesions tended to have severer aphasia than those with subcortical lesions. However, this relation was not significant, presumably due to the small sample size. Furthermore, because only three cases of mixed transcortical and one case of conduction-type aphasia were enrolled, their severity data should be interpreted cautiously. Patients described as having the mixed non-fluent type in the literature exhibit poor fluency and repetition capacity, but sometimes better comprehension than patients with the global type (2, 16, 18).
The WAB classifies aphasia into classic subtype categories, but about 11% (11 of 97) of our patients could not be classified by cluster analysis, which suggests that traditional classification schemes are limited.
This study describes relations between post-stroke aphasia severity and lesion type and lesion location. We conclude that global aphasia is the most severe form of aphasia and that cortical lesions are more likely to affect language function than subcortical lesions within 3 months of stroke regardless of age, sex, or type of stroke.
References
- 1.Choi-Kwon S, Kim HS, Kwon SU, Kim JS. Factors affecting the burden on caregivers of stroke survivors in South Korea. Arch Phys Med Rehabil. 2005;86:1043–1048. doi: 10.1016/j.apmr.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17:35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- 3.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49:11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang Y, Wang C, Zhao X, Gong X, Sun X, Chen H, Wang Y. Study on the pathogenic mechanism of Broca's and Wernicke's aphasia. Neurol Res. 2006;28:59–65. doi: 10.1179/016164106X91889. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch BE. Subcortical brain mechanisms in speech and language. Folia Phoniatr Logop. 2001;53:233–251. doi: 10.1159/000052679. [DOI] [PubMed] [Google Scholar]
- 6.Hillis AE, Barker PB, Wityk RJ, Aldrich EM, Restrepo L, Breese EL, Work M. Variability in subcortical aphasia is due to variable sites of cortical hypoperfusion. Brain Lang. 2004;89:524–530. doi: 10.1016/j.bandl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Shewan CM, Kertesz A. Reliability and validity characteristics of the Western aphasia battery (WAB) J Speech Hear Disord. 1980;45:308–324. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- 8.Kertesz A. Western aphasia battery test manual. New York: Grune & Stratton; 1982. [Google Scholar]
- 9.Kertesz A. Aphasia and associated disorders: taxonomy, localization, and recovery. New York: Grune & Stratton; 1979. [Google Scholar]
- 10.Kertesz A, Poole E. The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can J Neurol Sci. 1974;1:7–16. [PubMed] [Google Scholar]
- 11.Thompson CK. The neurobiology of language recovery in aphasia. Brain Lang. 2000;71:245–248. doi: 10.1006/brln.1999.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Na DL. Paradise. Korean version-the Western Aphasia Battery (Paradise. K-WAB) Seoul: Paradise Welfare Foundation, Institute for Children with Disabilities; 2001. [Google Scholar]
- 13.Damasio H, Damasio A. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- 14.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 15.Martinez IN, Moran JM, Pena FJ. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J Androl. 2006;27:596–603. doi: 10.2164/jandrol.05153. [DOI] [PubMed] [Google Scholar]
- 16.Kauhanen ML, Korpelainen JT, Hiltunen P, Maatta R, Mononen H, Brusin E, Sotaniemi KA, Myllyla VV. Aphasia, depression, and nonverbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis. 2000;10:455–461. doi: 10.1159/000016107. [DOI] [PubMed] [Google Scholar]
- 17.de Boissezon X, Demonet JF, Puel M, Marie N, Raboyeau G, Albucher JF, Chollet F, Cardebat D. Subcortical aphasia: a longitudinal PET study. Stroke. 2005;36:1467–1473. doi: 10.1161/01.STR.0000169947.08972.4f. [DOI] [PubMed] [Google Scholar]
- 18.Bakheit AM, Shaw S, Carrington S, Griffiths S. The rate and extent of improvement with therapy from the different types of aphasia in the first year after stroke. Clin Rehabil. 2007;21:941–949. doi: 10.1177/0269215507078452. [DOI] [PubMed] [Google Scholar]
- 19.Jensen AM, Chenery HJ, Copland DA. A comparison of picture description abilities in individuals with vascular subcortical lesions and Huntington's disease. J Commun Disord. 2006;39:62–77. doi: 10.1016/j.jcomdis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997;58:355–402. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Na DL. Normative data on the Korean version of the Western Aphasia Battery. J Clin Exp Neuropsychol. 2004;26:1011–1020. doi: 10.1080/13803390490515397. [DOI] [PubMed] [Google Scholar]
- 22.Berthier ML. Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging. 2005;22:163–182. doi: 10.2165/00002512-200522020-00006. [DOI] [PubMed] [Google Scholar]
- 23.Devinsky O, D'Esposito M. Neurology of cognitive and behavioral disorders. Oxford, New York: Oxford University Press; 2004. [Google Scholar]
- 24.Kreisler A, Godefroy O, Delmaire C, Debachy B, Leclercq M, Pruvo JP, Leys D. The anatomy of aphasia revisited. Neurology. 2000;54:1117–1123. doi: 10.1212/wnl.54.5.1117. [DOI] [PubMed] [Google Scholar]
- 25.Asanuma C, Andersen RA, Cowan WM. The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: divergent cortical projections from cell clusters in the medial pulvinar nucleus. J Comp Neurol. 1985;241:357–381. doi: 10.1002/cne.902410309. [DOI] [PubMed] [Google Scholar]
- 26.Van Buren JM, Borke RC. Alterations in speech and the pulvinar. A serial section study of cerebrothalamic relationships in cases of acquired speech disorders. Brain. 1969;92:255–284. doi: 10.1093/brain/92.2.255. [DOI] [PubMed] [Google Scholar]
- 27.Mega MS, Alexander MP. Subcortical aphasia: the core profile of capsulostriatal infarction. Neurology. 1994;44:1824–1829. doi: 10.1212/wnl.44.10.1824. [DOI] [PubMed] [Google Scholar]
- 28.Perani D, Vallar G, Cappa S, Messa C, Fazio F. Aphasia and neglect after subcortical stroke. A clinical/cerebral perfusion correlation study. Brain. 1987;110(Pt 5):1211–1229. doi: 10.1093/brain/110.5.1211. [DOI] [PubMed] [Google Scholar]
- 29.Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]