Abstract
Immunosuppressive therapy can improve clinical, biochemical and histological features and considerably prolong survival in patients with autoimmune hepatitis. Although ethnicity may affect disease severity and presentation, the long-term outcome of immunosuppression in Korean populations is unknown. This study was aimed to assess the efficacy of immunosuppressive therapy and determine the prognosis of autoimmune hepatitis in Korean populations. We reviewed the medical records of 86 patients diagnosed as having autoimmune hepatitis at the Samsung Medical Center between 1994 and 2008. Seventy-two (83.7%) patients reached remission after a median treatment duration of 3.5 months (range 1 to 44 months). Attempts to withdraw medications were made in 24 cases after the median treatment duration of 36 months (median 6 to 125 months). Thirteen of 24 (54.1%) patients relapsed after treatment withdrawal. Of the 86 patients, 6 (7.2%) experienced disease progression and the overall 5-and 10-yr progression-free survival rates were 91.2% and 85.5%, respectively. In conclusion, immunosuppressive therapy for autoimmune hepatitis results in a favorable rate of remission and excellent progression-free survival, but the relapse rate after treatment withdrawal is high. This suggests that long-term immunosuppressive therapy may be particularly important for treatment of Korean patients.
Keywords: Hepatitis, Autoimmune; Immunosuppression; Recurrence; Survival
INTRODUCTION
Autoimmune hepatitis is a chronic inflammatory disease of the liver characterized by increased transaminase levels, hypergammaglobulinemia, presence of circulating autoantibodies and interface hepatitis on liver biopsy (1, 2). The pathogenesis of autoimmune hepatitis is postulated to be an aberrant autoreactivity to liver cells in genetically predisposed individuals (2). In untreated autoimmune hepatitis, the mortality rates have been reported to be as high as 80% (3). Prednisolone alone or in combination with azathioprine is the standard treatment for autoimmune hepatitis, and this treatment improves clinical, biochemical and histological features and prolongs survival (4). Autoimmune hepatitis is a relatively common disease in Western countries and its clinical features and prognosis are mostly described in Caucasians. Ethnicity may affect disease severity and presentation (5, 6), but the result of immunosuppressive treatment in Asian countries is not well known and has not been reported in Korea. Although relapse after withdrawal of immunosuppressive therapy is a common and challenging problem (7), there have been no reports describing the clinical course of autoimmune hepatitis after cessation of therapy in Korea.
We examined the clinical manifestations of 86 patients treated with immunosuppressive therapy. Here, we describe the long-term clinical outcome and prognosis of autoimmune hepatitis in Koreans.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the medical records of 86 consecutive patients with autoimmune hepatitis who were treated with immunosuppressive therapy between August 1994 and January 2008 at the Samsung Medical Center, Seoul, Korea. All patients were seronegative for hepatitis B surface antigen and anti-hepatitis C virus (HCV). Patients who had excessive alcohol consumption or exposure to a hepatotoxic drug or herbal medication were excluded from the study. Based on pretreatment features, all patients satisfied "probable" or "definite" criteria proposed by the International Autoimmune Hepatitis Group (8). This study was examined the institutional review board (IRB) of Samsung Medical Center and was granted an exemption from IRB because it was retrospective study (IRB number 2009-09-121).
Definition
Remission was defined as follows: 1) disappearance of symptoms; 2) normal serum bilirubin and globulin levels; and 3) decrease in serum aminotransferase levels to less than twice normal. Treatment failure was defined as clinical or laboratory deterioration despite compliance with conventional therapy, including the development of jaundice, ascites or hepatic encephalopathy. Incomplete response was defined as some or no improvement in clinical or laboratory findings during therapy, but with no worsening in the condition. Failure to achieve remission after 3 yr of treatment was included in this definition. A relapse was indicated by an increase in the serum aminotransferase level to more than three-fold the upper limit of normal. The absence of symptoms and serum aspartate transaminase (AST) levels less than three fold normal at least 6 months after drug withdrawal during the entire period of observation constituted a sustained remission. Patients with acute-onset liver dysfunction (serum aminotransferase levels higher than ten-fold the upper normal limit and/or serum bilirubin levels higher than five-fold the upper normal limit) were diagnosed with acute presentation. Disease progression was defined as the occurrence of any of the following: 1) progression to cirrhosis among chronic active hepatitis patients or an increase of at least 2 points in the Child-Pugh score; 2) occurrence of esophageal and/or gastric variceal bleeding, spontaneous bacterial peritonitis or hepatic encephalopathy; 3) death related to liver disease; or 4) occurrence of hepatocellular carcinoma (HCC).
Treatment
As an initial medical treatment, all patients received prednisolone monotherapy (20-60 mg/day) or a combination therapy of prednisolone (20-40 mg/day) and azathioprine (50 mg/day). Most patients received combination treatment but those patients with leukopenia and/or thrombocytopenia due to liver cirrhosis and women in child-bearing age who wanted pregnancy in a near future were treated with prednisolone alone as the initial treatment. After the initial induction, the dose of prednisolone was gradually tapered and then reduced to the lowest dose necessary to maintain remission. Treatment withdrawal was attempted in 24 patients (for 11 of 24 patients, withdrawal attempts occurred after follow-up liver biopsy) after a 2-yr remission period. The decision to withdraw therapy was made by the attending physician on an individual basis based on either histological (disappearance of interface hepatitis) or biochemical criteria. Liver function tests were obtained every 1-3 months during induction of remission, at 3-6 month intervals during maintenance and every 1-3 months after discontinuation of treatment.
Histological examinations
Percutaneous needle biopsy of the liver was performed in 61 (70.9%) patients immediately before initial treatment. Follow-up liver biopsies were performed in 21 patients to plan the withdrawal of treatment, which was not essential. Histological scores were assessed in accordance with the Knodell histology activity index (HAI).
Statistical analysis
All analysis was performed using the SPSS statistical program (release 13.0, SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test was used to compare the differences in continuous variables, and the chi-square test was used to compare the dichotomous variables. A P value <0.05 was considered statistically significant. The progression free survival and relapse rates were calculated and plotted using the Kaplan-Meier method.
RESULTS
Patient characteristics
The clinical and laboratory findings of the 86 patients are summarized in Table 1.
Table 1.
Baseline clinical characteristics of patients
Data are expressed as median (range) without specific notation.
IAHG, International Autoimmune Hepatitis Group; AST, aspartate transaminase; ALT, alanine aminotransferase; PT, prothrombin time; INR, international normalized ration; ANA, antinuclear antibodies; SMA, smooth muscle antibodies; AMA, anti-mitochondrial antibodies.
At presentation, the median age of the patients was 51 yr (range 17 to 79 yr) and the peak incidence was between 40 and 50 yr. Seventy-two (83.7%) patients were women. The median duration of follow-up was 43 months (range 1 to 152 months). All the patients met pretreatment criteria for the diagnosis of definite (18 patients) or probable (68 patients) autoimmune hepatitis. Eighty-three (96.5%) patients had circulating antinuclear (ANA) and/or anti-smooth muscle (SMA) autoantibodies. Three patients (3.4%) had both ANA and anti-mitochondrial antibodies (AMA). The most common symptoms were jaundice (45.3%) and fatigue (16.2%), but 32 patients (37.2%) were asymptomatic and had only an abnormal liver function test. Twenty (23.2%) patients had symptomatic concurrent autoimmune disease; the most frequent disease being rheumatoid arthritis in 7 (8.1%) patients, followed by thyroid disease in 6 (7.0%) patients.
Response to therapy
Twelve (14.0%) patients were treated with prednisolone monotherapy (20-60 mg/day) and 74 (86.0%) were treated with a combination of prednisolone (20-40 mg/day) and azathioprine (50 mg/day). Seventy-two (83.7%) patients reached remission after a median treatment duration of 3.5 months (range 1 to 44 months). Eleven (12.8%) patients achieved an incomplete response. Maintenance therapy with low dose prednisolone and azathioprine was continued in all incomplete responders to reduce and stabilize disease activity. Of these patients, 2 patients stopped medication during follow-up and after aggravation of laboratory findings, treatment had to be re-started. One patient died of sepsis during the low dose immunosuppressive therapy. Three (3.5%) patients met the criteria for treatment failure. Two of three patients died of liver decompensation within 2 month despite of compliance with therapy and one patient discharged the hospital hopelessly due to liver failure.
Outcomes after treatment withdrawal
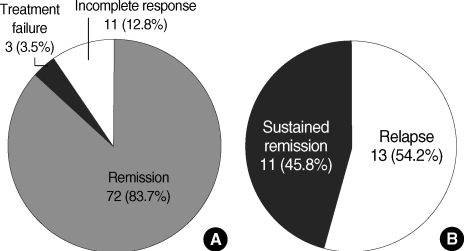
In 24 (27.9%) of the 86 patients, immunosuppressive therapy was discontinued after long-term remission (median 33 months, range 24 to 90 months). Eleven (45.8%) of these patients maintained remission for a median of 28 months (range 7 to 64 months). Thirteen patients (54.2%) experienced a relapse within a median of 4 months (range 1.2 to 96 months) following termination of treatment (Fig. 1). The relapse rates in the first and second year were 41.7% and 45.8%, respectively (Fig. 2). Previous regimens were re-administered for the relapsers and all patients showed response to the second therapy.
Fig. 1.
Induction of remission in autoimmune hepatitis patients after immunosuppressive treatment (A) and after withdrawal of immunosuppressive treatment (B). The median duration of treatment was 36 months (6-125 months) and the median time to remission was 3.5 months (1-44 months). Treatment withdrawal was attempted in 24 of 86 patients.
Fig. 2.
The Kaplan-Meier plot of relapse rate in patients with autoimmune hepatitis. Thirteen patients (54.2%) experienced a relapse within a median of 4 months (range 1.2 to 96 months) following termination of treatment. The relapse rates in the first and second year are 41.7% and 45.8%, respectively.
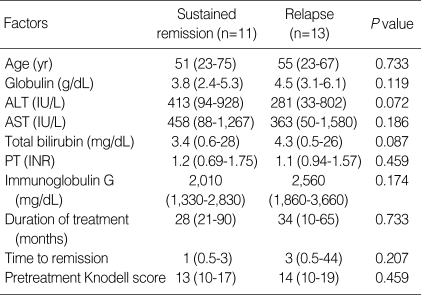
Factors associated with relapse
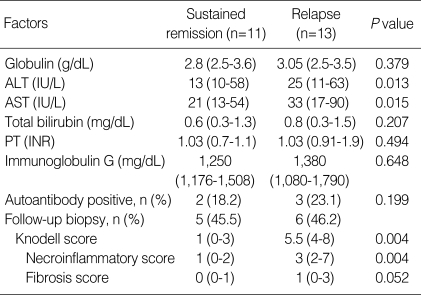
The clinical and laboratory features of patients with sustained remission were compared to those of patients who relapsed (Table 2, 3). The pretreatment clinical and laboratory findings revealed no statistical differences between the two groups. Prior to drug termination, the duration of treatment (median 28 months, range 21 to 90 months vs. median 34 months, range 10 to 65 months) and the time to remission (median 1 month, range 0.5 to 3 months vs. median 3 months, range 0.5 to 44 months) were statistically insignificant between the two groups. Patients who relapsed after treatment withdrawal had higher levels of AST and alanine aminotransferase (ALT) at the end of treatment than those who had sustained remission (P<0.05). Globulin and immunoglobulin G levels at the time of drug withdrawal were similar between the two groups. The Knodell HAI score after follow-up biopsy was higher in the relapsed group (median 5, range 4 to 8 vs. median 1, range 0-3; P=0.004), and in particular, the necroinflammatory score (median 3, range 2 to 7 vs. median 1, range 0-2; P=0.004) was higher in the relapsed patients than in patients with sustained remission. Of the 11 patients who discontinued treatment after follow-up liver biopsy, interface hepatitis disappeared in six patients. Of these patients, five maintained a sustained remission and only one patient experienced a relapse after treatment withdrawal. All five patients who had residual interface hepatitis relapsed after discontinuation of therapy. Among the 13 patients who withdrew treatment without follow-up liver biopsy, six patients maintained sustained remission and seven patients relapsed.
Table 2.
Comparison of features upon admission of patients who maintained remission and patients who relapsed after treatment withdrawal
Data are expressed as median (range) without specific notation.
ALT, alanine aminotransferase; AST, aspartate transaminase; PT, prothrombin time; INR, international normalized ration.
Table 3.
Comparison of features at the end of therapy of patients who sustained remission and patients who relapsed after treatment withdrawal
Data are expressed as median (range) without specific notation.
ALT, alanine aminotransferase; AST, aspartate transaminase; PT, prothrombin time; INR, international normalized ration.
Histological findings
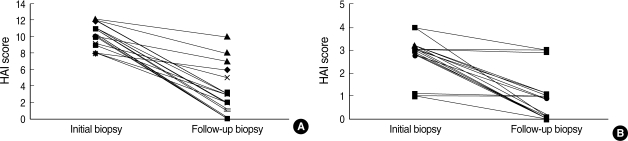
At presentation, the median HAI score according to the Knodell scoring system was 13 (range 4 to 15), with a median necroinflammatory HAI score of 10 (range 3 to 12) and a median fibrosis score of 3 (range 1 to 4). A diagnosis of liver cirrhosis was confirmed in 11 (12.8%) patients. Follow-up liver biopsy was obtained after a median of 26 months (range 24 to 126 months) of follow-up in 21 patients. At the follow-up biopsy, the majority of patients showed histological improvement in terms of the necroinflammatory score (median 3, range 1 to 10) and fibrosis score (median 1, range 1 to 3) (Table 4, Fig. 3).
Table 4.
Liver histology before treatment and at follow-up biopsy
Data are expressed as median (range) without specific notation.
Fig. 3.
Changes in necroinflammatory (A) and fibrosis score (B) at follow-up biopsy. Follow-up liver biopsy was obtained after a median of 26 months (range 24 to 126 months) of follow-up in 21 patients. The necroinflammatory score improve from a median of 10 (range 3 to 12) to 3 (range 1 to 10) and the fibrosis scores improve from 3 (median 1, range 1 to 3) to 1 (range 1 to 3).
HAI, histological activity score (according to knodell's scoring system).
Progression free survival
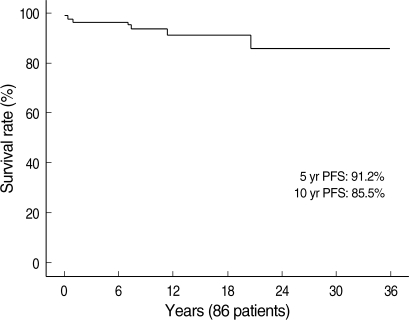
During the follow-up period (median 43 months, range 1-152 months), disease progression was noted in six patients. The causes of disease progression included decompensated liver cirrhosis (two patients), hepatocelluar carcinoma (one patient) and liver-related death (three patients). The five-year and 10-yr progression-free survivals were 91.2% and 85.5%, respectively (Fig. 4). The overall 10-yr survival for the 86 patients was 96.2%.
Fig. 4.
The Kaplan-Meier plots of progression-free survival (PFS) for patients with autoimmune hepatitis. The five-year and 10-yr progression-free survivals are 91.2% and 85.5%, respectively.
DISCUSSION
Autoimmune hepatitis is a common disease in Western countries, with a prevalence of 8 to 19 per 100,000 and an incidence of 0.6 to 1.9 per 100,000 (9). The prevalence of autoimmune hepatitis shows geographical variation with higher prevalence in Caucasian populations: Australia 62%, Germany 34%, USA 11-23%, Brazil 5-10% and Hong Kong 1% of all cases of chronic active hepatitis (9, 10). Autoimmune hepatitis is a relatively uncommon disease in Asian countries such as Japan, Hong Kong and Korea (9, 10). Hence, the clinical features of autoimmune hepatitis including response to therapy and prognosis after treatment are well studied in Caucasians. In Korea, the frequency is estimated to be less than 1% of chronic hepatitis cases and below 5% in non-B and non-C hepatitis cases (11). This is the first study to assess the clinical features, response to therapy and long-term prognosis in a large series of Korean patients with autoimmune hepatitis.
We were able to identify several differences in clinical features between our data and the Caucasian data reported in the literature. In our series, the mean age was 51 yr, the age distribution showed a peak incidence at middle age and there were only a few young patients. These findings are similar to those from a Japanese series and are different from those from a Caucasian series where the peak age was in the 4th decade. The observed differences between Caucasian and Asian data may be explained by the lower frequency of HLA-DR3 in Asian patients, which is reported to affect the early onset of disease (6, 12). Cirrhosis was present at accession in only 13% of our patients, which is less frequent than in previous Western studies that reported cirrhosis in 85% of African American patients and 38% in Caucasian American patients (13). These differences in clinical features may be explained in part by genetic differences between Asian and Caucasian populations. In Caucasians, type 1 autoimmune hepatitis is associated with human leukocyte antigen (HLA) serotype B1, B8, DR3 and DR4 (5, 6, 12). In contrast, a recent Korean study reported that type 1 autoimmune hepatitis is associated with HLA DRB1*0405, which is very similar to findings in previous Japanese reports (14, 15). We were not able to include the HLA data in this present study since HLA typing is not routinely performed in our clinics. Other clinical parameters such as the male to female ratio, degree of liver function test abnormality, detection rate of autoantibody and rate of concurrent autoimmune disease were similar to those reported in Western studies and those reported in another small series in Korea (11, 16).
Previous studies have reported that prednisolone alone or in combination with azathioprine can induce a clinical, biochemical and histological remission in 65 to 85% of cases, depending on the definition of response (3, 4, 17). It has been reported that 90% of adults show improvements in serum aminotransferase, bilirubin and g-globulin levels within 2 weeks and rarely achieve remission in less than 12 months, with the probability of remission during therapy diminishing after 2 yr (4, 18). In our study, 73 (83.7%) patients achieved remission within 4 months (median 3.5 month, range 1 to 44 months), which is comparable to findings from recent Caucasian studies. Although a substantial percentage of patients achieved successful induction of remission, relapse after withdrawal of therapy remains a common problem (7, 19-21). The frequency of relapse was 50% at 6 months and 70% at 3 yr after cessation of the immunosuppressive treatment. Only 21% achieved sustained remission after drug withdrawal in a previous study (7). In our series, more than half of the patients (54%) relapsed within a median of 4 months (range 1-96 months) and the 1-yr and 2-yr relapse rates were 41.7% and 45.8%, respectively, after the withdrawal of immunosuppressive therapy, which is comparable to previous Western data. The sustained remission rate (45.8%) may seem to be slightly higher than that reported in previous studies due to a difference in the observation period after treatment withdrawal. Since the duration of follow-up in our patients with sustained remission was 28 months (range, 7 to 64 months), which is shorter than that of previous studies, there is a possibility that the sustained remission rate in this study may have been overestimated.
There have been several reports on factors that can predict the relapse of autoimmune hepatitis after withdrawal of immunosuppressive therapy. Patients who were treated to normal serum AST, γ-globulin or immunoglobulin G levels were reported to have a lower frequency of relapse following drug cessation than patients treated to near-normal levels, in spite of the disappearance of interface hepatitis after liver biopsy (22). The duration of treatment is a predicting factor of relapse: sustained remission after drug cessation is reported to be significantly more likely (67% probability) in patients who received a total duration of therapy of 4 yr (23). In our study, although we were not able to disclose any significant pretreatment clinical factor at presentation that could predict relapse after treatment withdrawal, higher levels of serum aminotransferases (ALT and AST) at the end of treatment was a predictor of relapse. However, this finding could not be clarified since the serum levels of AST and ALT were within the normal range in both groups, and because it is critical not to withdraw treatment until biochemical remission achieves complete normalization (20, 24, 25). Similar to previous studies, those patients who relapsed had a tendency to have higher (but not statistically significant) levels of γ-globulin and immunoglobulin G. Follow-up biopsies were obtained for 11 patients after medication withdrawal. Patients with sustained remission had a lower HAI score than those who relapsed. Five of six patients who showed complete resolution of interface hepatitis at follow-up biopsy maintained sustained remission. Ideally, immunosuppressive therapy should not be withdrawn without histological resolution, but treatment withdrawal was attempted in five patients without histological remission because they had received treatment for long periods (mean 74 months, range 38 to 113 months) with clinical and biochemical remission. As a result, all patients with residual interface hepatitis at follow-up biopsy relapsed. Overall, relapse (13 patients, 54.2%) appeared within a median of 4 months in our study in spite of biochemical remission. These findings suggest that histological remission, especially normalization of the necroinflammatory score, as well as clinical and biochemical remission, should be achieved before cessation of treatment.
Our results from follow-up biopsies demonstrate that histological improvement in both the necroinflammatory score and fibrosis grade were achieved after long-term immunosuppressive therapy. Two patients who had liver cirrhosis at initial biopsy experienced complete resolution of fibrosis to grade 0 after immunosuppressive therapy. This result is in concordance with previous studies which reported significant regression of fibrosis with steroid therapy (16, 23, 26). Also, none of our patients experienced histological progression after immunosuppressive therapy.
Of the six patients who experienced disease progression in our study, one patient developed HCC during the follow-up period. Although it has been known for a long time that HCC is rare in autoimmune hepatitis, several reports describing the association between HCC and autoimmune hepatitis have recently been published (27, 28). The prognosis of autoimmune hepatitis has been reported to be excellent in previous studies, with a 10-yr life expectancy among treated patients being greater than 90%, even in patients with cirrhosis at the accession of therapy (26, 29). The prognosis of autoimmune hepatitis in our study was comparable to those in previous studies with an estimated 10-yr survival of 96.2% and a 10-yr progression-free survival of over 85%, which suggests that autoimmune hepatitis can be well managed with immunosuppressive therapy without disease progression.
In conclusion, immunosuppressive therapy for autoimmune hepatitis results in a favorable rate of remission and excellent progression-free survival, but the relapse rate after treatment withdrawal was high in our study. This suggests that long-term immunosuppressive therapy may be particularly important for treatment of patients from Korean populations.
References
- 1.Czaja AJ, Ammon HV, Summerskill WH. Clinical features and prognosis of severe chronic active liver disease (CALD) after corticosteroid-induced remission. Gastroenterology. 1980;78:518–523. [PubMed] [Google Scholar]
- 2.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 3.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnick GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 4.Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479–497. doi: 10.1053/jhep.2002.34944. [DOI] [PubMed] [Google Scholar]
- 5.Czaja AJ, Doherty DG, Donaldson PT. Genetic bases of autoimmune hepatitis. Dig Dis Sci. 2002;47:2139–2150. doi: 10.1023/a:1020166605016. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson PT, Czaja AJ. Genetic effects on susceptibility, clinical expression, and treatment outcome of type 1 autoimmune hepatitis. Clin Liver Dis. 2002;6:707–725. doi: 10.1016/s1089-3261(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 7.Hegarty JE, Nouri Aria KT, Portmann B, Eddleston AL, Williams R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology. 1983;3:685–689. doi: 10.1002/hep.1840030510. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Buschenfelde KH, Mieli-Vergani G, Nakanuma Y, Nishioka M, Penner E, Porta G, Portmann BC, Reed WD, Rodes J, Schalm SW, Scheuer PJ, Schrumpf E, Seki T, Toda G, Tsuji T, Tygstrup N, Vergani D, Zeniya M. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 9.Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635–647. doi: 10.1016/s1089-3261(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 10.Toda G, Zeniya M, Watanabe F, Imawari M, Kiyosawa K, Nishioka M, Tsuji T, Omata M. Present status of autoimmune hepatitis in Japan--correlating the characteristics with international criteria in an area with a high rate of HCV infection. Japanese National Study Group of Autoimmune Hepatitis. J Hepatol. 1997;26:1207–1212. doi: 10.1016/s0168-8278(97)80453-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS. Autoimmune hepatitis: recent update on diagnosis and treatment. Korean J Hepatol. 2006;12:318–332. [PubMed] [Google Scholar]
- 12.Czaja AJ, Donaldson PT. Genetic susceptibilities for immune expression and liver cell injury in autoimmune hepatitis. Immunol Rev. 2000;174:250–259. doi: 10.1034/j.1600-0528.2002.017401.x. [DOI] [PubMed] [Google Scholar]
- 13.Lim KN, Casanova RL, Boyer TD, Bruno CJ. Autoimmune hepatitis in African Americans: presenting features and response to therapy. Am J Gastroenterol. 2001;96:3390–3394. doi: 10.1111/j.1572-0241.2001.05272.x. [DOI] [PubMed] [Google Scholar]
- 14.Lim YS, Oh HB, Choi SE, Kwon OJ, Heo YS, Lee HC, Suh DJ. Susceptibility to type 1 autoimmune hepatitis is associated with shared amino acid sequences at positions 70-74 of the HLA-DRB1 molecule. J Hepatol. 2008;48:133–139. doi: 10.1016/j.jhep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Omagari K, Kinoshita H, Kato Y, Nakata K, Kanematsu T, Kusumoto Y, Mori I, Furukawa R, Tanioka H, Tajima H, Koga M, Yano M, Kohno S. Clinical features of 89 patients with autoimmune hepatitis in Nagasaki Prefecture, Japan. J Gastroenterol. 1999;34:221–226. doi: 10.1007/s005350050247. [DOI] [PubMed] [Google Scholar]
- 16.Jung SH, Kim BH, Dong SH, Kim HJ, Chang YW, Lee JI, Chang R. Clinical features of Korean patients with autoimmune hepatitis diagnosed since 1991. Korean J Gastroenterol. 2001;37:362–369. [Google Scholar]
- 17.Czaja AJ, Davis GL, Ludwig J, Taswell HF. Complete resolution of inflammatory activity following corticosteroid treatment of HBsAg-negative chronic active hepatitis. Hepatology. 1984;4:622–627. doi: 10.1002/hep.1840040409. [DOI] [PubMed] [Google Scholar]
- 18.Czaja AJ, Rakela J, Ludwig J. Features reflective of early prognosis in corticosteroid-treated severe autoimmune chronic active hepatitis. Gastroenterology. 1988;95:448–453. doi: 10.1016/0016-5085(88)90503-3. [DOI] [PubMed] [Google Scholar]
- 19.Czaja AJ, Menon KV, Carpenter HA. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: a retrospective analysis. Hepatology. 2002;35:890–897. doi: 10.1053/jhep.2002.32485. [DOI] [PubMed] [Google Scholar]
- 20.Montano-Loza AJ, Carpenter HA, Czaja AJ. Consequences of treatment withdrawal in type 1 autoimmune hepatitis. Liver Int. 2007;27:507–515. doi: 10.1111/j.1478-3231.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- 21.Seo S, Toutounjian R, Conrad A, Blatt L, Tong MJ. Favorable outcomes of autoimmune hepatitis in a community clinic setting. J Gastroenterol Hepatol. 2008;23:1410–1414. doi: 10.1111/j.1440-1746.2008.05365.x. [DOI] [PubMed] [Google Scholar]
- 22.Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol. 2007;102:1005–1012. doi: 10.1111/j.1572-0241.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 23.Kanzler S, Gerken G, Lohr H, Galle PR, Meyer zum Buschenfelde KH, Lohse AW. Duration of immunosuppressive therapy in autoimmune hepatitis. J Hepatol. 2001;34:354–355. doi: 10.1016/s0168-8278(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 24.Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99:1510–1516. doi: 10.1111/j.1572-0241.2004.30457.x. [DOI] [PubMed] [Google Scholar]
- 25.Miyake Y, Iwasaki Y, Terada R, Takagi S, Okamaoto R, Ikeda H, Sakai N, Makino Y, Kobashi H, Takaguchi K, Sakaguchi K, Shiratori Y. Persistent normalization of serum alanine aminotransferase levels improves the prognosis of type 1 autoimmune hepatitis. J Hepatol. 2005;43:951–957. doi: 10.1016/j.jhep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Schvarcz R, Glaumann H, Weiland O. Survival and histological resolution of fibrosis in patients with autoimmune chronic active hepatitis. J Hepatol. 1993;18:15–23. doi: 10.1016/s0168-8278(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 27.Masaki N, Hayashi S. Hepatocellular carcinoma complicating autoimmune hepatitis. Nippon Rinsho. 2001;59(Suppl 6):455–464. [PubMed] [Google Scholar]
- 28.Hardee JT, Breth GF, El-Serag HB. Hepatocellular carcinoma associated with autoimmune hepatitis. J Clin Gastroenterol. 2003;37:271–272. doi: 10.1097/00004836-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Kanzler S, Lohr H, Gerken G, Galle PR, Lohse AW. Long-term management and prognosis of autoimmune hepatitis (AIH): a single center experience. Z Gastroenterol. 2001;39:339–341. doi: 10.1055/s-2001-13708. [DOI] [PubMed] [Google Scholar]