Abstract
High-level gentamicin resistance (HLGR) in enterococci has increased since the 1980s, but the clinical significance of the resistance and its impact on outcome have not been established. One hundred and thirty-six patients with bacteremia caused by enterococci with HLGR (HLGR group) were compared with 79 patients with bacteremia caused by enterococci without HLGR (non-HLGR group). Hematologic malignancy, neutropenia, Enterococcus faecium infection, nosocomial infection and monomicrobial bacteremia were more common in the HLGR group than the non-HLGR group, and APACHE II scores were also higher (P<0.05, in each case). Neutropenia, monomicrobial infection, stay in intensive care at culture, and use of 3rd generation cephalosporin, were independent risk factors for acquisition of HLGR enterococcal bacteremia. Fourteen-day and 30-day mortalities were higher in the HLGR group than the non-HLGR group in univariate analysis (37% vs. 15%, P=0.001; 50% vs. 22%, P<0.001). However, HLGR was not an independent risk factor for mortality due to enterococcal bacteremia in multivariate analysis. Therefore, HLGR enterococcal bacteremia is associated with more severe comorbid conditions and higher mortality than non-HLGR enterococcal bacteremia but the HLGR itself does not contribute significantly to mortality.
Keywords: Enterococcus; Bacteremia; Gentamicins; Drug Therapy, Combination; HLGR; Mortality
INTRODUCTION
Enterococci are increasingly encountered as significant pathogens, and have become a major cause of nosocomial infections. Moreover, multi-drug resistant enterococci including vancomycin-resistant enterococci have emerged, and now represent a major clinical problem (1-3). Antibiotic treatment of diseases caused by these organisms is complicated by intrinsic and acquired resistance to various antibiotics. Resistances to penicillin, vancomycin and high-level aminoglycosides are clinically important because they limit antibiotic treatment options.
A combination of cell wall active agents and aminoglycosides is recommended to achieve a synergistic bactericidal effect in enterococcal endocarditis and meningitis (4). Moreover, many infectious disease specialists use combination therapy for non-endocarditis bacteremia and other serious enterococcal infections, especially in critically ill patients (5, 6). However, combination treatment is not applicable in cases of infection caused by enterococci with high-level aminoglycoside resistance. For this reason, it is important to establish the risk factors of infection caused by enterococci with high-level aminoglycoside resistance, and the influence of high-level aminoglycoside resistance on outcome. However there have been few clinical studies of these matters and no conclusive data are available (7-12).
We performed this study to compare the clinical features of bacteremia caused by enterococci with high-level gentamicin resistance (HLGR) with those of bacteremia caused by non-HLGR enterococci, to investigate the risk factors for bacteremia caused by enterococci with HLGR, and to determine whether HLGR influences outcome.
MATERIALS AND METHODS
Patients
All patients with positive blood cultures for Enterococcus faecalis and Enterococcus faecium between January 1999 and August 2003 were identified by a review of the computerized records of the Clinical Microbiology Laboratory of Seoul National University Hospital (Seoul, Republic of Korea), a 1500-bed tertiary care University Hospital and referral center. Patients of age 16 or older were included. Enterococcal bacteremia occurring 60 days or more after a previous episode in a patient that had already been registered was counted as a separate case and was included in the study (13). Medical records of the patients were retrospectively reviewed.
Microbiological tests
Enterococcus species were identified on the basis of 6.5% NaCl tolerance, bile-esculin hydrolysis, and growth rate at 45℃. Species were identified with the Vitek system (bioMérieux, Marcy l'Etoile, France), and by tests for motility, yellow pigmentation and methyl-α-D-glucopyranoside (14, 15). Antibiotic susceptibilities were determined by the disk diffusion method, following the recommendations of the Clinical and Laboratory Standards Institute (16). HRGR was determined by the disk diffusion method with 120 µg gentamicin discs (Oxoid Ltd., Basingstoke, UK) (16).
Definitions
Clinically significant bacteremia was defined as the isolation of enterococci from two or more separately obtained blood cultures, from a single blood culture and from a primary site, or from a single blood culture with a clinically apparent primary site.
HLGR was defined as a minimal inhibitory concentration of gentamicin exceeding 500 µg/mL (16).
Polymicrobial bacteremia was defined as the isolation from blood culture of one or more species of bacteria in addition to the enterococci (the same blood culture, or another blood culture within 24 hr of the initial culture that yielded enterococci). A single concomitant isolation of another bacterial species was deemed sufficient, except for coagulase-negative staphylococci, diphtheroids, α-hemolytic streptococci, and Bacillus species, which required isolation from two blood cultures.
Enterococcal bacteremia was considered to have been of community-onset if the enterococci were isolated from cultures of blood samples obtained within 48 hr of hospital admission (if the patient had not been transferred from another hospital), and if the patient had symptoms or signs suggestive of infection on admission. Otherwise, the enterococcal bacteremia was considered to be nosocomial.
Appropriate antibiotic treatment was defined as the use of one or more active antibiotic to which the organism was susceptible in vitro within five days of the date on which a positive blood culture was obtained (17). Antibiotics considered active included penicillin, ampicillin, piperacillin, vancomycin, teicoplanin, quinupristin-dalfopristin and linezolid.
Neutropenia was defined as a neutrophil count of <500 cells/µL or a count of ≤1,000 cells/µL with a predicted decrease to <500 cells/µL.
Statistical analyses
Categorical variables were compared using Fisher's exact test or Pearson chi-square test, as appropriate, and continuous variables were compared using the Student's t test. All tests of significance were 2-tailed, and P≤0.05 was considered significant. Logistic regression analysis was carried out to determine the risk factors for acquisition of bacteremia caused by enterococci with HLGR, and Cox-regression survival analysis was used to determine the independent risk factors for outcome of enterococcal bacteremia. Variables that were not significant in the univariate analysis (P>0.05) were excluded from the multivariate analysis. Multivariate analysis was performed in the backward stepwise conditional manner. Statistical analyses of the data were performed with SPSS for Windows (version 11.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Enterococcus species in blood isolates
We identified 215 cases of clinically significant E. faecalis and E. faecium bacteremia. One hundred and fifty (70%) were caused by E. faecium and 65 (30%) by E. faecalis. One hundred and thirty six (63%) were caused by enterococci with HLGR and 79 (37%) by enterococci without HLGR. The HLGR rate was 70% (105/150) in E. faecium and 48% (31/65) in E. faecalis.
Enterococci were isolated from two or more separately obtained blood cultures in 113 (53%) cases, from a single blood culture and from a primary site in 40 (19%) cases, and from a single blood culture with a clinically apparent primary site in 62 (29%) cases. Eight patients (two with E. faecalis, six with E. faecium) were re-enrolled because bacteremia caused by the same organism occurred again after 60 days.
Clinical features of patients with bacteremia caused by HLGR
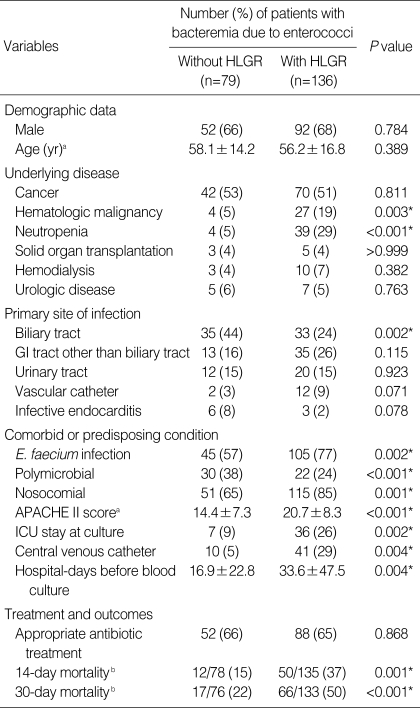
The demographic, clinical features and outcomes of each group are shown in Table 1. The most common underlying disease in both groups was cancer, accounting for approximately 50% of cases. Two hundred six of the cases (96%) were of bacteremia without endocarditis or meningitis. Nine cases (4%) of endocarditis and no cases of meningitis were detected.
Table 1.
Clinical features of 215 patients with bacteremia caused by enterococci with or without HLGR
*Statistically significant, P≤0.05.
aContinuous variables are expressed as means (±SD); bExpressed as number of deaths/number of patients followed up (%).
HLGR, high-level gentamicin resistance; GI, gastrointestinal; APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit.
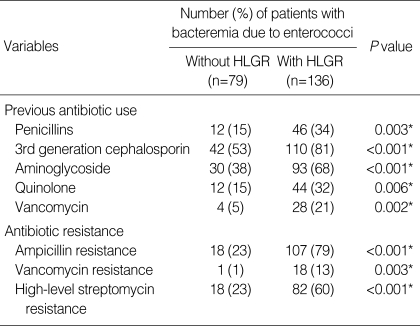
Hematologic malignancy, neutropenia, E. faecium infection, monomicrobial bacteremia and nosocomial infection were significantly more common in bacteremia caused by enterococci with HLGR than in bacteremia caused by enterococci without HLGR (P<0.05, in each case). On the other hand, biliary tract infection was less common in the former. Mean acute physiology and chronic health evaluation (APACHE) II scores were higher in bacteremia caused by enterococci with HLGR. Rates of resistance to ampicillin, vancomycin and high-level streptomycin were also higher in isolates of enterococci with HLGR (Table 2).
Table 2.
Previous antibiotic use and antibiotic resistance in 215 patients with bacteremia caused by enterococci with or without HLGR
*Statistically significant, P≤0.05.
HLGR, high-level gentamicin resistance.
Risk factors for the acquisition of bacteremia caused by enterococci with HLGR
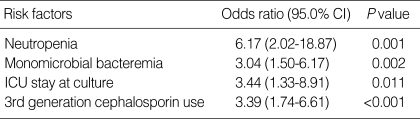
We performed multivariate analysis with a logistic regression model to identify the independent risk factors for the acquisition of bacteremia caused by enterococci with HLGR. The multivariate analysis included the following variables; hematologic malignancy, neutropenia, biliary tract infection, central venous catheter, intensive care unit (ICU) stay at time of culture, nosocomial infection, duration of hospital stay before bacteremia, monomicrobial infection, E. faecium infection, and previous use of penicillins, vancomycin, 3rd generation cephalosporin, aminoglycoside and quinolone. Multivariate analysis identified neutropenia, monomicrobial bacteremia, ICU stay at time of culture and use of 3rd generation cephalosporin as independent risk factors for bacteremia caused by enterococci with HLGR (Table 3).
Table 3.
Associated factors for acquisition of bacteremia due to enterococci with HLGR determined by multivariate analysis using a logistic regression model
HLGR, High-level gentamicin resistance; ICU, Intensive care unit.
Influence of HLGR on mortality in patients with enterococcal bacteremia
In univariate analysis, fourteen-day and 30-day mortalities were significantly higher in patients with bacteremia caused by enterococci with HLGR than in patients with bacteremia caused by enterococci without HLGR (37% vs. 15%, P=0.001; 50% vs. 22%, P<0.001; Table 1).
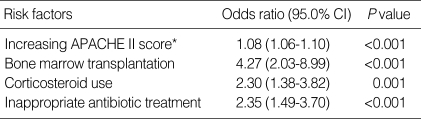
We performed multivariate analysis with the Cox-regression model to determine whether the difference in mortality was due to HLGR. The multivariate analysis included the following variables; biliary tract infection, hematologic malignancy, bone marrow transplantation, neutropenia, cancer chemotherapy, corticosteroid use, immunosuppressant use, E. faecium infection, APACHE II score, ampicillin resistance, vancomycin resistance, high-level gentamicin resistance and inappropriate antibiotic treatment. The analysis identified APACHE II score, bone marrow transplantation, corticosteroid use and inappropriate antibiotic treatment as independent risk factors for 30-day mortality (Table 4). When we controlled for these factors, the odds ratio for mortality due to bacteremia caused by enterococci with HLGR compared with bacteremia caused by enterococci without HLGR was 1.36, which was not statistically significant (95% confidence interval: 0.75-2.44).
Table 4.
Independent risk factors for 30-day mortality in 209 episodes of enterococcal bacteremia, as determined by survival analysis using the Cox-regression model
*Per 1 point increase in score.
APACHE, Acute physiology and chronic health evaluation.
DISCUSSION
Enterococci with HLGR comprised 63% of the enterococcal isolates that caused clinically significant bacteremia in our institute. In previous studies, the proportion of HLGR in blood isolates was 33-62% (7, 8, 10-12). Most isolates with HLGR were E. faecalis in the 1980s and early 1990s (10, 11). However, a recent study found almost the same rate of HLGR in E. faecalis and E. faecium blood isolates (12). HLGR was prevalent among enterococcal blood isolates in our study, as in previous studies. However, in our case the frequency of HLGR was significantly higher in E. faecium than in E. faecalis. Our data thus suggest that HLGR is now also prevalent in E. faecium. This may be due to the increase of multi-drug resistant E. faecium as the cause of nosocomial bacteremia (1, 18-20).
Some investigators have reported no connection between HLGR and the severity of the underlying disease or adverse outcome in enterococcal bacteremia (10, 11). On the other hand, others have reported higher mortality in patients with bacteremia caused by enterococci with HLGR (12). In the present study, we found that bacteremia caused by enterococci with HLGR was associated with more severe underlying disease and higher mortality than bacteremia caused by enterococci without HLGR. There are two possible explanations for the discrepancy between the studies. First, the predominant isolate differed; most of the blood isolates in the previous studies were E. faecalis, whereas the predominant blood isolate in our study was E. faecium. Although we observed the effects of HLGR on clinical features and outcomes in both E. faecium and E. faecalis, the differences were more prominent in E. faecium (data not shown). Second, it is possible that the sample sizes in the previous studies were too small to reveal the differences.
In previous studies, independent risk factors for the acquisition of bacteremia due to enterococci with HLGR were intensive care unit stay, previous use of antibiotics, especially broad-spectrum cephalosporin, chronic renal failure and E. faecalis species (8, 11). The results of our study are consistent with these findings; however, E. faecalis species was not a risk factor in our study. Two kinds of patients were at especially high risk of acquiring infections with HLGR enterococci in our study: patients with neutropenia and patients with intensive care unit stay. Our data suggest that the judicious use of 3rd generation cephalosporin is important to reduce infections caused by enterococci with HLGR, especially in patients belonging to these two groups.
There has been controversy over whether HLGR influences the prognosis of patients with enterococcal bacteremia. Watanakunakorn et al. and Caballero-Granado et al. reported that HLGR did not influence crude mortality from enterococcal bacteremia in 178 and 93 cases, respectively (10, 11). Shaked et al., however, recently reported that HLGR was an independent risk factor for mortality in 117 patients with enterococcal bacteremia (12). However, HLGR was associated with more severe underlying disease, resistance to other antibiotics and many comorbid conditions. Therefore confounding factors would need to be corrected for by an appropriate statistical model and an adequate sample size. It is possible that the sample size in the latter study was too small for the confounding factors to have been adequately corrected for in the multivariate analysis. In any event, in our study, which involved more cases than the previous studies, we found that HLGR did not significantly influence the outcome of enterococcal bacteremia.
The anatomical site of infection should be taken into account in treating enterococcal infection, because the appropriate treatment strategy differs for different sites. In cases of endocarditis or meningitis, combination therapy with a cell-wall active agent plus an aminoglycoside should be employed (4, 21). Antibiotic use in enterococcal infections without endocarditis or meningitis has been questioned because enterococci are traditionally considered to be pathogens of low virulence (22). However, there is some evidence that appropriate antibiotic use is associated with improved outcome in patients with enterococcal bacteremia (13). Combination therapy with a cell-wall active agent plus an aminoglycoside has also been considered in enterococcal bacteremia without endocarditis (5, 6), but in a number of studies there was no statistically significant difference in outcome between monotherapy and combination therapy (10, 23-25).
In our study, most of the cases were of enterococcal bacteremia without endocarditis or meningitis, and appropriate antibiotic treatment was associated with improved outcome. However, we also failed to obtain evidence that combination therapy was more effective. When we performed a multivariate analysis on 131 patients with enterococcal bacteremia without endocarditis who had received appropriate antibiotic treatment, monotherapy did not emerge as an independent risk factor for 30-day mortality (95% confidence interval: 0.72-5.79; P=0.180). It appears that prognosis of enterococcal bacteremia may be improved by appropriate antibiotic treatment rather than by giving combination antibiotic therapy.
Our study has some limitations. First, since it was retrospective in design, confounding factors which can affect mortality were not uniformly controlled. Because the factors influencing the physicians' choice of antibiotics were not determined, they may have influenced our results as unmeasured confounding factors in the analysis. Second, we did not perform molecular epidemiologic studies such as pulsed-field gel electrophoresis to exclude outbreaks of specific strains or common sources of infection. Third, this was a single-center study and factors such as the rate of referrals or surgery for complex disorders could have impacted the results.
In conclusion, patients with HLGR enterococcal bacteremia were more likely to have severe underlying diseases and were associated with higher mortality than patients with non-HLGR enterococcal bacteremia. However, HLGR itself did not influence mortality in enterococcal bacteremia.
ACKNOWLEDGEMENT
We express our gratitude to the Medical Research Collaborating Center (MRCC) of Seoul National University Hospital for statistical consultation and review.
Footnotes
This study was presented in part at the 17th ECCMID (European Congress of Clinical Microbiology and Infectious Diseases), Munich, Germany, 31st March-3rd April, 2007 (Abstract No. 939).
This study was supported by a grant from the Seoul National University College of Medicine Research Fund (800-2004-0360).
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Lee K, Jang SJ, Lee HJ, Ryoo N, Kim M, Hong SG, Chong Y. Increasing prevalence of vancomycin-resistant enterococcus faecium, expanded-spectrum cephalosporin-resistant klebsiella pneumoniae, and imipenem-resistant pseudomonas aeruginosa in Korea: KONSAR study in 2001. J Korean Med Sci. 2004;19:8–14. doi: 10.3346/jkms.2004.19.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K, Lee HS, Jang SJ, Park AJ, Lee MH, Song WK, Chong Y Members of Korean Nationwide Surveillance of Antimicrobial Resistance Group. Antimicrobial resistance surveillance of bacteria in 1999 in Korea with a special reference to resistance of enterococci to vancomycin and gram-negative bacilli to third generation cephalosporin, imipenem, and fluoroquinolone. J Korean Med Sci. 2001;16:262–270. doi: 10.3346/jkms.2001.16.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray BE. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessels MR. Streptococcal and enterococcal infections. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 17th ed. New York: McGraw-Hill; 2008. pp. 881–890. [Google Scholar]
- 6.Moellering RC., Jr . Enterococcus species, Streptococcus bovis, and Leuconostoc species. In: Mandell GL, Bennet JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and practice of infectious diseases. Philadelphia: Churchill Livingstone; 2005. pp. 2411–2421. [Google Scholar]
- 7.Noskin GA, Till M, Patterson BK, Clarke JT, Warren JR. High-level gentamicin resistance in Enterococcus faecalis bacteremia. J Infect Dis. 1991;164:1212–1215. doi: 10.1093/infdis/164.6.1212. [DOI] [PubMed] [Google Scholar]
- 8.Huycke MM, Spiegel CA, Gilmore MS. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells VD, Wong ES, Murray BE, Coudron PE, Williams DS, Markowitz SM. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992;116:285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- 10.Watanakunakorn C, Patel R. Comparison of patients with enterococcal bacteremia due to strains with and without high-level resistance to gentamicin. Clin Infect Dis. 1993;17:74–78. doi: 10.1093/clinids/17.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Caballero-Granado FJ, Cisneros JM, Luque R, Torres-Tortosa M, Gamboa F, Diez F, Villanueva JL, Pérez-Cano R, Pasquau J, Merino D, Menchero A, Mora D, López-Ruz MA, Vergara A The Grupo Andaluz para el estudio de las Enfermedades Infecciosas. Comparative study of bacteremias caused by Enterococcus spp. with and without high-level resistance to gentamicin. J Clin Microbiol. 1998;36:520–525. doi: 10.1128/jcm.36.2.520-525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaked H, Carmeli Y, Schwartz D, Siegman-Igra Y. Enterococcal bacteraemia: epidemiological, microbiological, clinical and prognostic characteristics, and the impact of high level gentamicin resistance. Scand J Infect Dis. 2006;38:995–1000. doi: 10.1080/00365540600868321. [DOI] [PubMed] [Google Scholar]
- 13.Vergis EN, Hayden MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, Wagener MM, Schmitt B, Muder RR. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Ann Intern Med. 2001;135:484–492. doi: 10.7326/0003-4819-135-7-200110020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Facklam RR, Collins MD. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devriese LA, Pot B, Kersters K, Lauwers S, Haesebrouck F. Acidification of methyl-alpha-D-glucopyranoside: a useful test to differentiate Enterococcus casseliflavus and Enterococcus gallinarum from Enterococcus faecium species group and from Enterococcus faecalis. J Clin Microbiol. 1996;34:2607–2608. doi: 10.1128/jcm.34.10.2607-2608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2008. p. M100-S18. [Google Scholar]
- 17.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, Ramphal R, Wagener MM, Miyashiro DK, Yu VL. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 18.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh KV, Murray BE, Wolff J, Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 19.Boyle JF, Soumakis SA, Rendo A, Herrington JA, Gianarkis DG, Thurberg BE, Painter BG. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J Clin Microbiol. 1993;31:1280–1285. doi: 10.1128/jcm.31.5.1280-1285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyce JM, Opal SM, Chow JW, Zervos MJ, Potter-Bynoe G, Sherman CB, Romulo RL, Fortna S, Medeiros AA. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–1153. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott TS, Foweraker J, Gould FK, Perry JD, Sandoe JA. Guidelines for the antibiotic treatment of endocarditis in adults: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2004;54:971–981. doi: 10.1093/jac/dkh474. [DOI] [PubMed] [Google Scholar]
- 22.Hoge CW, Adams J, Buchanan B, Sears SD. Enterococcal bacteremia: to treat or not to treat, a reappraisal. Rev Infect Dis. 1991;13:600–605. doi: 10.1093/clinids/13.4.600. [DOI] [PubMed] [Google Scholar]
- 23.Graninger W, Ragette R. Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin Infect Dis. 1992;15:49–57. doi: 10.1093/clinids/15.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Gullberg RM, Homann SR, Phair JP. Enterococcal bacteremia: analysis of 75 episodes. Rev Infect Dis. 1989;11:74–85. doi: 10.1093/clinids/11.1.74. [DOI] [PubMed] [Google Scholar]
- 25.Maki DG, Agger WA. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine (Baltimore) 1988;67:248–269. [PubMed] [Google Scholar]