Abstract
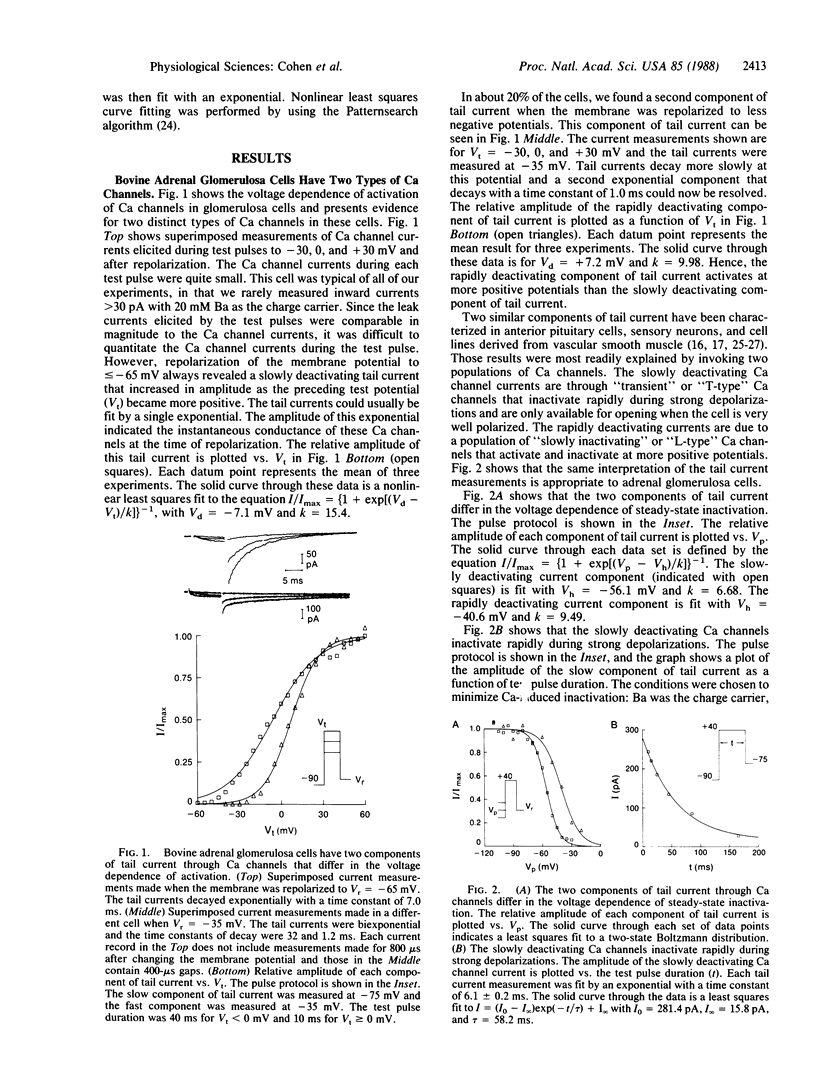
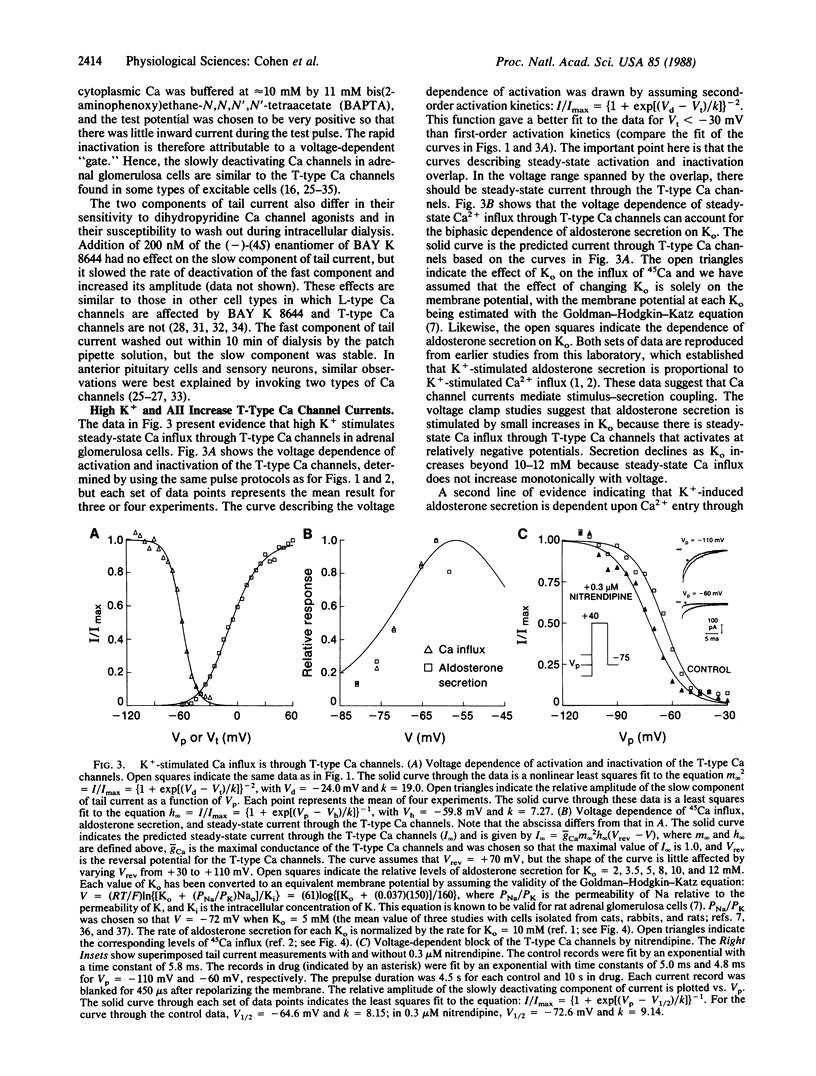
Ca channel currents were studied in freshly dispersed bovine adrenal glomerulosa cells to better understand the control of aldosterone secretion by extracellular K concentration (Ko) and angiotensin II (AII). The whole-cell variation of the patch voltage clamp technique was used. Two types of Ca channels were found. One type is similar to the "T-type" Ca channels found in many excitable cells. These channels deactivate slowly (tau approximately equal to 7 ms at -75 mV) and inactivate rapidly during strong depolarizations. The second channel type activates and inactivates at more positive potentials than the T-type Ca channels and deactivates rapidly. These channels are similar to the "L-type" Ca channels found in muscle and nerve. Our studies provide three reasons for concluding that T-type Ca channels have an important role in mediating stimulus-secretion coupling in response to high K+ or AII: (i) aldosterone secretion and steady-state current through T-type Ca channels are biphasic functions of Ko and both increase in parallel for Ko = 2-10 mM; (ii) nitrendipine blocks the T-type Ca channels and the stimulation of aldosterone secretion by high K+ or AII with similar potency; (iii) AII increases Ca entry through the T-type Ca channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Catt K. J. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986 Jan;118(1):112–118. doi: 10.1210/endo-118-1-112. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Braley L. M., Menachery A. I., Brown E. M., Williams G. H. Comparative effect of angiotensin II, potassium, adrenocorticotropin, and cyclic adenosine 3',5'-monophosphate on cytosolic calcium in rat adrenal cells. Endocrinology. 1986 Sep;119(3):1010–1019. doi: 10.1210/endo-119-3-1010. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Jornot L., Vallotton M. B. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984 Jul 25;259(14):8863–8869. [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Single low-voltage-activated calcium channels in chick and rat sensory neurones. J Physiol. 1987 May;386:571–601. doi: 10.1113/jphysiol.1987.sp016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G. Calcium channel currents in pars intermedia cells of the rat pituitary gland. Kinetic properties and washout during intracellular dialysis. J Gen Physiol. 1986 Jul;88(1):83–105. doi: 10.1085/jgp.88.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Büki B., Muscsi I., Spät A. Polyphosphoinositide metabolism in adrenal glomerulosa cells. Mol Cell Endocrinol. 1985 Jun;41(1):105–112. doi: 10.1016/0303-7207(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Fakunding J. L., Chow R., Catt K. J. The role of calcium in the stimulation of aldosterone production by adrenocorticotropin, angiotensin II, and potassium in isolated glomerulosa cells. Endocrinology. 1979 Aug;105(2):327–333. doi: 10.1210/endo-105-2-327. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R., Lobo M. V., Rasmussen H., Marusic E. T. Calcium: its role in the mechanism of action of angiotensin II and potassium in aldosterone production. Endocrinology. 1981 Dec;109(6):2196–2201. doi: 10.1210/endo-109-6-2196. [DOI] [PubMed] [Google Scholar]
- Foster R., Lobo M. V., Rasmussen H., Marusic E. T. The effect of calcium on potassium-induced depolarization of adrenal glomerulosa cells. FEBS Lett. 1982 Nov 29;149(2):253–256. doi: 10.1016/0014-5793(82)81111-3. [DOI] [PubMed] [Google Scholar]
- Foster R., Rasmussen H. Angiotensin-mediated calcium efflux from adrenal glomerulosa cells. Am J Physiol. 1983 Sep;245(3):E281–E287. doi: 10.1152/ajpendo.1983.245.3.E281. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Baukal A. J., Balla T., Catt K. J. Angiotensin-induced formation and metabolism of inositol polyphosphates in bovine adrenal glomerulosa cells. Biochem Biophys Res Commun. 1987 Jan 15;142(1):15–22. doi: 10.1016/0006-291x(87)90445-1. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hyatt P. J., Tait J. F., Tait S. A. The mechanism of the effect of K+ on the steroidogenesis of rat zona glomerulosa cells of the adrenal cortex: role of cyclic AMP. Proc R Soc Lond B Biol Sci. 1986 Feb 22;227(1246):21–42. doi: 10.1098/rspb.1986.0007. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Blair M. L. Effects of angiotensin II on membrane current in cardiac Purkinje fibers. J Mol Cell Cardiol. 1981 Sep;13(9):797–809. doi: 10.1016/0022-2828(81)90237-6. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Intracellular calcium and adenosine 3',5'-cyclic monophosphate as mediators of potassium-induced aldosterone secretion. Biochem J. 1985 May 15;228(1):69–76. doi: 10.1042/bj2280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima K., Kojima I., Rasmussen H. Dihydropyridine calcium agonist and antagonist effects on aldosterone secretion. Am J Physiol. 1984 Nov;247(5 Pt 1):E645–E650. doi: 10.1152/ajpendo.1984.247.5.E645. [DOI] [PubMed] [Google Scholar]
- Kunze D. L., Hamilton S. L., Hawkes M. J., Brown A. M. Dihydropyridine binding and calcium channel function in clonal rat adrenal medullary tumor cells. Mol Pharmacol. 1987 Apr;31(4):401–409. [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Matsunaga H., Maruyama Y., Kojima I., Hoshi T. Transient Ca2+-channel current characterized by a low-threshold voltage in zona glomerulosa cells of rat adrenal cortex. Pflugers Arch. 1987 Apr;408(4):351–355. doi: 10.1007/BF00581128. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Saffran M. Ionic dependence of adrenal steroidogenesis and ACTH-induced changes in the membrane potential of adrenocortical cells. J Physiol. 1973 Oct;234(1):43–64. doi: 10.1113/jphysiol.1973.sp010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Mironneau C., Grosset A., Hamon G., Savineau J. P. Action of angiotensin II on the electrical and mechanical activity of rat uterine smooth muscle. Eur J Pharmacol. 1980 Dec 5;68(3):275–285. doi: 10.1016/0014-2999(80)90525-7. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natke E., Jr, Kabela E. Electrical responses in cat adrenal cortex: possible relation to aldosterone secretion. Am J Physiol. 1979 Aug;237(2):E158–E162. doi: 10.1152/ajpendo.1979.237.2.E158. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- Quinn S. J., Cornwall M. C., Williams G. H. Electrical properties of isolated rat adrenal glomerulosa and fasciculata cells. Endocrinology. 1987 Mar;120(3):903–914. doi: 10.1210/endo-120-3-903. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schiebinger R. J., Braley L. M., Menachery A., Williams G. H. Unique calcium dependencies of the activating mechanism of the early and late aldosterone biosynthetic pathways in the rat. J Endocrinol. 1986 Aug;110(2):315–325. doi: 10.1677/joe.0.1100315. [DOI] [PubMed] [Google Scholar]
- Simon B. J., Beam K. G. Slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1985 Jan;85(1):1–19. doi: 10.1085/jgp.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B. Angiotensin-receptor signaling in cultured vascular smooth muscle cells. Am J Physiol. 1986 May;250(5 Pt 2):F759–F769. doi: 10.1152/ajprenal.1986.250.5.F759. [DOI] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]


