Abstract
The distribution of hypothalamic neurons expressing the peptides melanin-concentrating hormone (MCH; 'MCH neurons') or hypocretin/orexin (H/O; 'H/O neurons') was assessed with immunocytochemistry in male rats at high spatial resolution. Data were plotted on a rat brain atlas that includes a recently revised parcellation scheme for the lateral hypothalamic zone. Quantitative analysis revealed approximately three times more MCH neurons than H/O neurons in the hypothalamus, and approximately twice as many within the parcellations of the lateral hypothalamic area (LHA). The LHA contained 60% of MCH neurons and 81% of H/O neurons, and the same five LHA regions contained the vast majority of MCH (87%) or H/O (93%) neurons present within the LHA: namely the LHA dorsal region (LHAd: 31% of H/O; 38% of MCH), suprafornical region (LHAs: 28% of H/O; 11% of MCH), ventral region medial zone (LHAvm: 15% of H/O; 16% of MCH), juxtadorsomedial region (LHAjd: 14% of H/O and MCH) and magnocellular nucleus (LHAm: 5% of H/O; 7% of MCH). The zona incerta (ZI) contained 18% of MCH neurons. A high co-abundance of MCH and H/O neurons outside of the LHA was present in the posterior hypothalamic nucleus (PH: 11% of H/O; 9% of MCH). Morphological analysis revealed MCH and H/O neurons as typically tri-polar with irregularly shaped somata. These data provide a quantitative analysis of neurons expressing either MCH or H/O peptides within the rat hypothalamus, and they clarify differences in the distribution pattern for different subsets of these neuron types, especially within the LHA.
Keywords: melanin-concentrating hormone, hypocretin/orexin, lateral hypothalamic area
Introduction
Recent studies of LHA neural projections [1] have enhanced our understanding of the organization of this highly differentiated region. These ongoing studies give purpose to a reevaluation of neuron types present within the LHA; foremost among these are two neuron populations that are mostly restricted to the LHA -- namely neurons expressing either MCH [2–9] or H/O [2;3;10–14]. The distribution of MCH and H/O mRNA expression was reevaluated recently with reference to a revised LHA parcellation scheme [15]; it was found to be in general agreement with previous studies, with some exceptions. Notably, several earlier studies indicated the presence of either the peptide or mRNA for H/O or MCH in several extra-LHA nuclei that in the study of Swanson et al. [15] were found to express neither mRNA. Specifically, the presence of H/O-expressing neurons was reported previously in subthalamic- [16] and arcuate [17] nuclei; MCH-expressing neurons were previously reported in the paraventricular hypothalamic nucleus (PVH) [7;18], dorsal tuberomammillary nucleus (TMd) [19] and reticular thalamic nucleus (RT) [6]. These differences were attributed to a less rigorous cytoarchitectonic analysis in the earlier studies.
One aim of this study was to provide a rigorous cytoarchitectonic analysis of neurons expressing MCH or H/O peptides, as a comparison work to the previous analysis of H/O and MCH mRNA distribution [15], using the same cytoarchitectonic criteria [20]. A second aim was to perform a quantitative analysis, complimented by a basic morphological analysis, of hypothalamic neurons expressing either MCH or H/O peptides. The goal was to provide a clearer basis from which to examine possible differences between different subsets of neurons in the lateral hypothalamic zone, and in particular the LHA, that express either MCH or H/O.
Methods
Five adult male rats (300 – 350 g; Sprague Dawley, Harlan Laboratories) were used in these experiments, in accord with NIH Guidelines for the Care and Use of Laboratory Animals; protocols were approved by the University of Southern California Institutional Animals Care and Use Committee. The following methods have fuller previous description, see [1]. Transverse sections were obtained from 4% paraformaldehyde fixed tissue on a freezing microtome at a thickness of 25 µm, and divided sequentially into 5 series. One series of sections from each brain was processed for immunocytochemical detection of MCH (rabbit anti-MCH; 'PBL-234', gift of P. Sawchenko)[4]; a second series was processed for detection of H/O (rabbit anti-preproH/O; '2050', gift of L. de Lecea)[10]; a third series was stained with thionin. It is noteworthy that two hypocretin peptides, designated A and B, have been identified -- derived from the common precursor molecule preproH/O [10]. By selecting an antibody directed at the latter, the aim was to identify all hypocretin neurons. In contrast, the MCH precursor molecule, preproMCH, is also a precursor for neuropeptide-glutamic acid-isoleucine (NEI) and neuropeptide-glycine-glutamic acid (NGE) see [21] -- hence the selection of an antibody capable of recognizing MCH but not its precursor molecule, see [4]. Primary antibodies were followed by biotinylated donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch Labs) and then an avidin-biotin-horseradish peroxidase complex (Vector ABC Elite kit; Vector Labs); 3,3'-Diaminobenzidine (Sigma) was used to visualize the labeling. Sections processed for detection of MCH or H/O were matched with adjacent thionin stained sections and camera lucida drawings were made with a 10x objective. In these drawings labeled cells and boundaries between regions evident from the Nissl cytoarchitecture were identified and marked, with reference to a rat brain atlas [20]; a 100x objective was used to produce drawings of individual neurons. A labeled cell was marked as present when its nucleus was visible within a given section; analysis was restricted to one side (right) of the brain. The total counted MCH and H/O neurons was the sum of counted neurons in 2 series (i.e. 1 series each for MCH and H/O) out of 5 series of sections processed from each of 5 brains. The data so obtained was transferred to template levels of Swanson's rat brain atlas, in which further details of the strategy for mapping results from multiple animals to a reference atlas are described [20].
Results
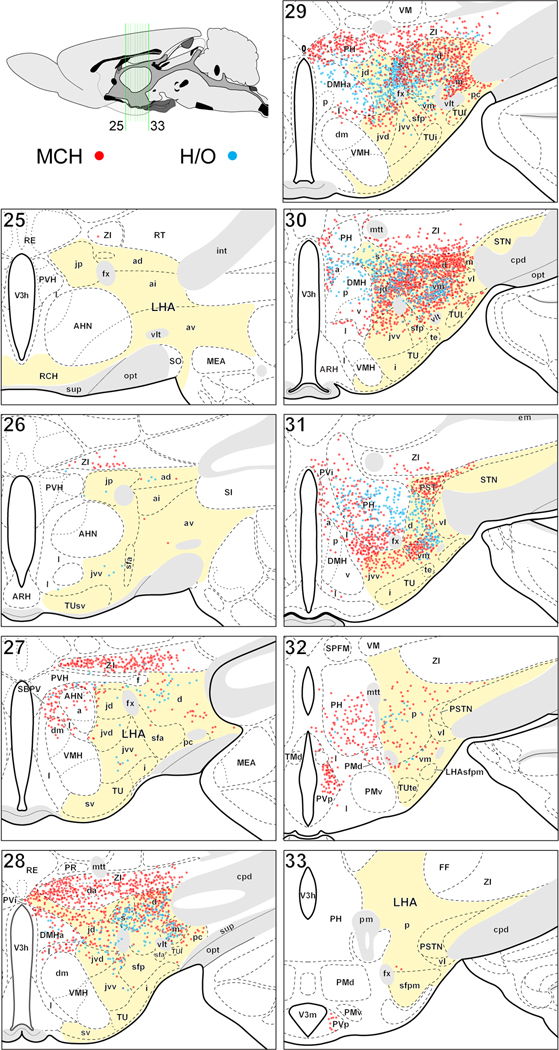
The spatial distribution results are presented on a reference map series (Fig. 1). The number of counted cells per region in the lateral hypothalamic zone on the right side of the brain for the five experiments (summed) is shown in tabular form (Table 1). Two additional figures show the morphology representative of individual MCH (Fig. 2) or H/O (Fig. 3) neurons in regions where they were most abundant. The present results are in close general agreement with those of Swanson et. al. [15] and both studies confirm the absence of previously reported MCH neurons in the PVH [7;18], TMd [19] and RT [6]; and H/O neurons in subthalamic [16] and arcuate [17] nuclei. The present study confirms the previous finding that the caudal half of the hypothalamus contains the vast majority of MCH and H/O neurons [15]. More specifically, in the present study 91% of MCH neurons and 97% of H/O neurons were caudal to the level of the PVH; furthermore, about half (52%) of all MCH neurons and about two-thirds (67%) of all H/O neurons were mapped to just two atlas levels -- levels 29 and 30 that also contain the major part of the dorsomedial hypothalamic nucleus (DMH) [20]. Also in agreement, the present and earlier study [15] indentify the LHAjd, LHAs, LHAd, LHAm and LHAvm as the LHA regions containing a preponderance of MCH and H/O neurons; the present study also confirms that MCH neurons have substantially wider extent than H/O neurons.
Figure 1.
Distribution of MCH- and ppH/O-immunoreactive somata from 5 adult male rats plotted on a reference atlas of the rat hypothalamus; the lateral hypothalamic zone is colored yellow. Each colored dot represents a single MCH (red) or H/O (blue) neuron; the dots are semi-transparent such that overlapping MCH or H/O neurons are indicated by greater color saturation or shades of purple. The number of plotted cells equals that counted in 2 series (one each for MCH and one for H/O) from 5 series of 25-µm-thick transverse sections obtained from 5 different rats. Immunoreactive somata were mapped with camera lucida from adjacent series of sections and with reference to an additional series from the same rats stained with thionin. The diagram upper left indicates in midsagittal view the location and extent of the reference atlas levels on to which data were plotted. For lateral zone abbreviations see Table 1, other: AHN, anterior hypothalamic nucleus; ARH, arcuate nucleus; cpd, cerebral peduncle; DMHa,p,v, dorsomedial nucleus anterior, posterior, ventral parts; FF, fields of Forel; fx, fornix; I, internuclear area; int, internal capsule; MEA, medial amygdalar nucleus; mtt, mammillothalamic tract; opt, optic tract; PH, posterior hypothalamic nucleus; pm, principal mammillary tract; PMd,v, dorsal and ventral premammillary nuclei; PR, perireuniens nucleus; PVH paraventricular nucleus; PVHf, PVH forniceal part; PVi, intermediate periventricular nucleus; PVp, posterior periventricular nucleus; RE, nucleus reuniens; RT, reticular nucleus; SBPV, subparaventricular region; SI, substantia innominata; SO, supraoptic nucleus; sup, supraoptic commissures; TMd, dorsal tuberomammillary nucleus; V3h, third ventricle hypothalamic part; V3m, third ventricle mammillary recess; vlt, ventrolateral hypothalamic tract; VM, ventral medial nucleus; VMHdm, ventromedial nucleus dorsomedial part; ZI, zona incerta; ZIda, ZI dopaminergic group.
Table 1.
Number (and relative percentage) of MCH- and ppH/O immunoreactive neurons counted within regions of the lateral hypothalamic zone. The values equate to the sum of neurons counted in 2 series (i.e. 1 series for each peptide) from 5 series of 25-µm-thick transverse sections obtained from 5 rats.
| Counts of MCH and H/O neurons | ||||
|---|---|---|---|---|
| MCH | H/O | |||
| Region of lateral hypothalamic zone | count | % | count | % |
| LPO (lateral preoptic area) | 0 | 0 | 0 | |
| LHAag (anterior group) | -- | -- | -- | -- |
| LHAad (dorsal zone) | 2 | <1 | 3 | <1 |
| LHAai (intermediate zone) | 0 | 0 | 0 | 0 |
| LHAav (ventral zone) | 2 | <1 | 0 | 0 |
| RCH (retrochiasmatic area) | 0 | 0 | 0 | 0 |
| LHAmg (middle group) | -- | -- | -- | -- |
| LHAmt (medial tier) | -- | -- | -- | -- |
| LHAjp (juxtaparaventricular region) | 0 | 0 | 2 | <1 |
| LHAjd (juxtadorsomedial region) | 379 | 14 | 184 | 14 |
| LHAjv (juxtaventromedial region) | -- | -- | -- | -- |
| LHAjvd (dorsal zone) | 67 | 2 | 33 | 3 |
| LHAjvv (ventral zone) | 127 | 5 | 27 | 2 |
| LHApf (perifornical tier) | -- | -- | -- | -- |
| LHAs (suprafornical region) | 304 | 11 | 365 | 28 |
| LHAsf (subfornical region) | -- | -- | -- | -- |
| LHAsfa (anterior zone) | 1 | <1 | 0 | 0 |
| LHAsfp (posterior zone) | 30 | 1 | 5 | <1 |
| LHAsfpm (premammillary zone) | -- | -- | -- | -- |
| LHAl (lateral tier) | -- | -- | -- | -- |
| LHAd (dorsal region) | 1019 | 37 | 393 | 31 |
| LHAv (ventral region) | -- | -- | -- | -- |
| LHAvm (medial zone) | 435 | 16 | 196 | 15 |
| LHAvl (lateral zone) | 14 | 1 | 0 | 0 |
| LHApc (parvicellular region) | 32 | 1 | 0 | 0 |
| LHAm (magnocellular nucleus) | 177 | 6 | 61 | 5 |
| TU (tuberal nucleus) | -- | -- | -- | -- |
| TUsv (subventromedial part) | 0 | 0 | 0 | 0 |
| TUi (intermediate part) | 0 | 0 | 0 | 0 |
| TUte (terete subnucleus) | 0 | 0 | 0 | 0 |
| TUl (lateral part) | 17 | 1 | 0 | 0 |
| LHApg (posterior group) | -- | -- | -- | -- |
| LHAp (posterior group) | 62 | 2 | 15 | 1 |
| PST (preparasubthalamic nucleus) | 86 | 3 | 0 | 0 |
| PSTN (parasubthalamic nucleus) | 3 | <1 | 0 | 0 |
| STN (subthalamic nucleus) | 0 | 0 | 0 | 0 |
| Total (n = 5) | 2757 | 1284 | ||
Figure 2.
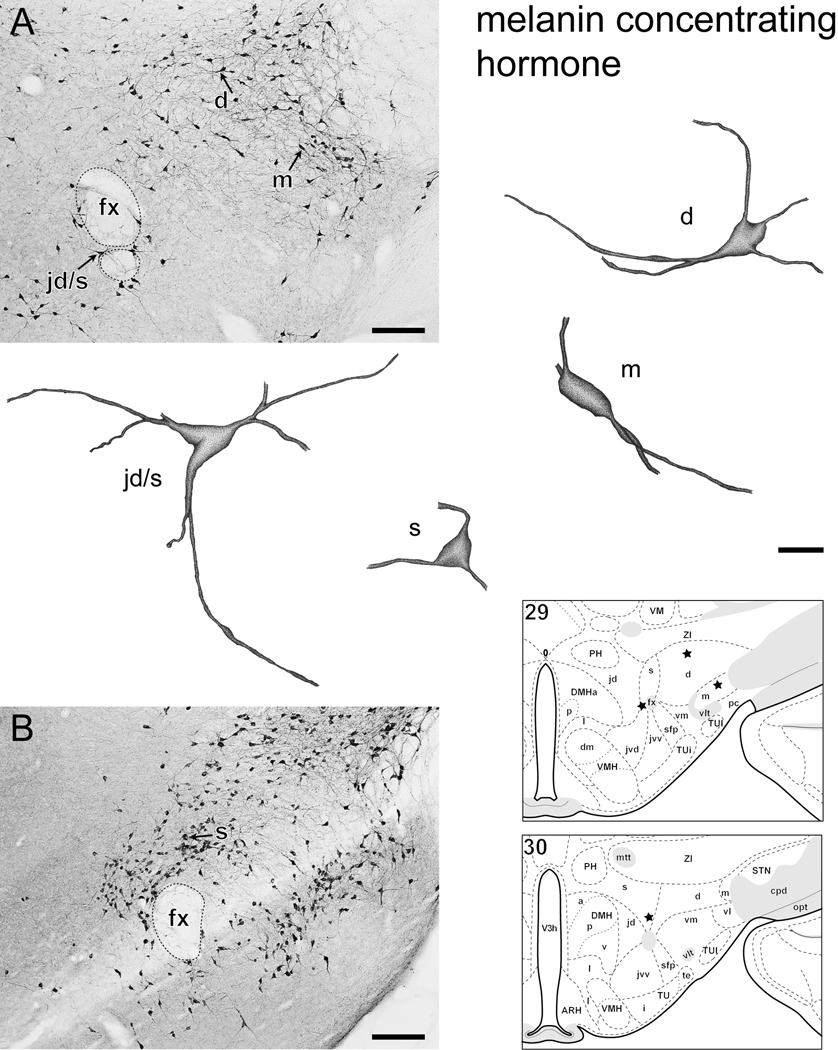
Examples of individual neurons immunoreactive for MCH (Fig. 2) or ppH/O (Fig. 3) within the LHA. 2A, B and 3A-C, photomicrographs showing MCH (2A, B) and ppH/O (3A-C) immunoreactive (ir) neurons within the lateral hypothalamic zone. The soma and dendrites of selected neurons within subregions of the LHA (identified with reference to corresponding thionin stained sections) were drawn with camera lucida. Individual neurons were traced as far as visible within 25-µm-thick transverse sections. Drawings are stippled and shaded to enhance the appearance of 3-dimensions. The location of each drawn neuron is indicated by an arrow on the photomicrograph with a corresponding letter(s) indicating LHA subdivision. For reference, approximate neuron location is shown on a corresponding rat brain atlas -- asterisks indicate approximate location of drawn neurons in each figure. Abbreviations: d, jd, m, s, vm, (respectively) LHA -dorsal region, -juxtadorsomedial region, -magnocellular nucleus, -suprafornical region, -ventral region medial zone; fx = fornix; for other atlas abbreviations see Fig. 1. Bars = 200 µm photomicrographs, 20 µm drawings.
Figure 3.
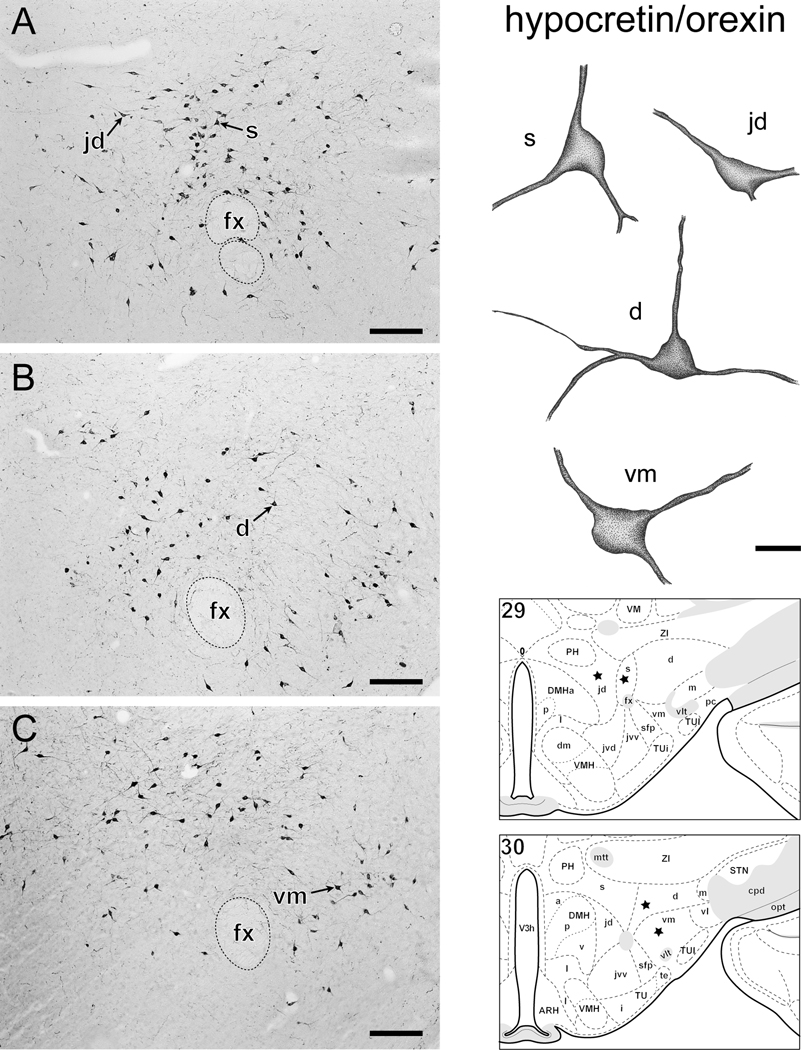
Examples of individual neurons immunoreactive for MCH (Fig. 2) or ppH/O (Fig. 3) within the LHA. 2A, B and 3A-C, photomicrographs showing MCH (2A, B) and ppH/O (3A-C) immunoreactive (ir) neurons within the lateral hypothalamic zone. The soma and dendrites of selected neurons within subregions of the LHA (identified with reference to corresponding thionin stained sections) were drawn with camera lucida. Individual neurons were traced as far as visible within 25-µm-thick transverse sections. Drawings are stippled and shaded to enhance the appearance of 3-dimensions. The location of each drawn neuron is indicated by an arrow on the photomicrograph with a corresponding letter(s) indicating LHA subdivision. For reference, approximate neuron location is shown on a corresponding rat brain atlas -- asterisks indicate approximate location of drawn neurons in each figure. Abbreviations: d, jd, m, s, vm, (respectively) LHA -dorsal region, -juxtadorsomedial region, -magnocellular nucleus, -suprafornical region, -ventral region medial zone; fx = fornix; for other atlas abbreviations see Fig. 1. Bars = 200 µm photomicrographs, 20 µm drawings.
However, there are also several apparent (though essentially minor) differences between the two studies. First, in the study of Swanson et al. [15], MCH neurons were mapped farther rostral than in the present study. Whereas in the present study the most rostrally mapped MCH neuron was present in the ZI at a midrostrocaudal level of the PVH (atlas level 25; Fig. 1), the earlier study mapped MCH neurons through ventral regions of the lateral hypothalamic zone to a level rostral to the PVH (atlas level 20); however, the more rostrally located MCH neurons were notably sparse (see Fig. 1 of [15]). Nevertheless they were mapped to four regions in which we observed no MCH neuron, namely: lateral preoptic area, magnocellular nucleus (the part of the pallidum ventral to the substantia innominata), nucleus of the diagonal band, and layer 3 of the olfactory tubercle. Also in the previous study [15], isolated MCH and H/O neurons were mapped to several additional regions in which we observed neither neuron; for MCH these included the LHAjp and a region dorsal to the posterodorsal part of the medial amygdala; for H/O they included LHA intermediate- ventral- and ventral region lateral zones, lateral- and medial parvicellular parts of the PVH, substantia innominata, and the subventricular part of the tuberal nucleus; in addition, a small cluster of H/O neurons were mapped in the earlier study to the LHA parvicellular region (see Fig 1., atlas level 28 of [15]). Furthermore, in the present study relatively few (<1% of total counted) MCH and H/O neurons were mapped to several regions that in the earlier work were reported to contain none; for MCH these included the anterior hypothalamic nucleus (anterior part), DMH (posterior and ventral parts; DMHp/v) and the LHA anterior region, dorsal zone; for H/O they also included the DMHp and DMHv, as well as the LHAjp, and the hypothalamic subparaventricular zone. In addition to these differences between the studies, the relative abundance and distribution of either neuron type appears to differ substantially in certain regions. Thus, in comparison to the earlier work (see Fig. 1 and Fig. 2 of [15]), in the present study a dense cluster of MCH neurons is apparent in the posterior part of the hypothalamic periventricular nucleus (PVp; Fig. 1, level 32); in addition, the density and relative abundance of MCH and H/O neurons in the present study is evidently higher in the LHAs, LHAvm and PH (compare Fig. 1 levels 28–32 of the present study to Fig 1. of [15]).
Discussion
Overall, the present results are in broad agreement with those of Swanson et al. [15]. However differences, relatively minor, are also apparent; some of the more likely possibilities to account for these are discussed here. Firstly, there are a host of possible confounds inherent to the use of immunocytochemistry [22;23]. Thus, although the antibodies used in the current study were previously characterized for their ability to recognize either MCH or ppH/O peptides to the exclusion of other molecules, see [4;10], the possibility of false positive or negative results cannot be eliminated entirely [22;23]. Secondly, there could be differences between the levels of MCH and/or ppH/O mRNA and that of the peptides, such that one or other was not detected, depending on the sensitivity of the techniques. For example, certain neuropeptides may only be detected in the soma when an artificial manipulation is employed to raise their concentration -- use of colchicine being a classic example [24]. However, the opposite may also be true, such that the level of a neuropeptide may under certain conditions increase relative to the level of its mRNA, e.g. [25]. Thirdly, in the present study the number of neurons plotted for each atlas level equals the number counted in a 25-µm-thick transverse section for 5 brains; whereas in the previous study the number approximates to that seen in a single 15-µm-thick section from a single brain -- although it should be noted that the latter was compared to the results of several experiments and was qualitatively representative of them all [15] -- this representational difference could account for apparent differences in distribution abundance (for example in the PH). A fourth source of possible differences is mapping errors, which are bound to occur to some degree; however, the latter is likely to have been negated somewhat by use of the same rat brain atlas and approach to mapping [20] -- the overall similarity of the data sets suggests further that mapping errors were not a major factor.
The present findings provide a general confirmation that the distribution of neurons expressing H/O and MCH peptides is very similar to their distribution as indicated by the presence of MCH and H/O neuron mRNA [15]. The morphological analysis revealed a typical tri-polar dendritic structure emerging from the somata of MCH and H/O LHA neurons (Fig. 2, Fig. 3); qualitatively, no obvious pattern of structural differences could be discerned for different subsets of either neuron type. These findings identify the MCH and H/O neurons as a type described previously as iso-dendritic [26]; the latter description aligns with a traditional view of the LHA as a rostral extension of the brainstem reticular formation [27;28]. However, the spatial analysis shows clear differences between different subsets of MCH and H/O neurons, and these differences do correlate to a growing body of evidence indicating multiple different properties for subsets of either neuron type. For example, differential projections have been described for different subsets of MCH [5–7;19;29–34] and H/O [34–36] neurons. Similarly, different functional properties have been suggested for, or attributed to, subsets of MCH [37] and H/O [38–40] neurons. In addition, different coexpression by subsets of MCH and H/O neurons of various neurochemicals has also been shown [5;41–43]. Furthermore, it is also noteworthy that sexually dimorphic extra-hypothalamic [44] and extra-LHA [45] expression of MCH has been reported; it remains to be determined whether or not sexually dimorphic expression of MCH might also occur in the LHA. Clearly it is also critical to compare these finding across species. Finally, although it was not within the scope of this study to provide an estimate of MCH and H/O cell number in the rat -- such analysis is not simple, see [46;47] -- a future study to address this could be a useful addition . The present study, together with the study of Swanson et al. [15] provide a reference atlas that may be of use to the investigator who seeks to study differential properties between different subpopulations of hypothalamic MCH and H/O neurons.
Acknowledgment
I thank Dr Larry Swanson for helpful comments; Mr David Nykin for diligent technical assistance. This work was supported by NIH grant RO1-NS16686
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- 1.Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol. 2005;493:412–438. doi: 10.1002/cne.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 3.Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- 4.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 5.Cvetkovic V, Brischoux F, Jacquemard C, Fellmann D, Griffond B, Risold PY. Characterization of subpopulations of neurons producing melanin-concentrating hormone in the rat ventral diencephalon. J. Neurochem. 2004;91:911–919. doi: 10.1111/j.1471-4159.2004.02776.x. [DOI] [PubMed] [Google Scholar]
- 6.Kohler C, Haglund L, Swanson LW. A diffuse alpha MSH-immunoreactive projection to the hippocampus and spinal cord from individual neurons in the lateral hypothalamic area and zona incerta. J. Comp Neurol. 1984;223:501–514. doi: 10.1002/cne.902230404. [DOI] [PubMed] [Google Scholar]
- 7.Saper CB, Akil H, Watson SJ. Lateral hypothalamic innervation of the cerebral cortex: immunoreactive staining for a peptide resembling but immunochemically distinct from pituitary/arcuate alpha-melanocyte stimulating hormone. Brain Res. Bull. 1986;16:107–120. doi: 10.1016/0361-9230(86)90018-3. [DOI] [PubMed] [Google Scholar]
- 8.Watson SJ, Akil H. The presence of two alpha-MSH positive cell groups in rat hypothalamus. Eur. J Pharmacol. 1979;58:101–103. doi: 10.1016/0014-2999(79)90351-0. [DOI] [PubMed] [Google Scholar]
- 9.Torterolo P, Sampogna S, Morales FR, Chase MH. MCH-containing neurons in the hypothalamus of the cat: searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–114. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. U. S. A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffond B, Deray A, Fellmann D, Ciofi P, Croix D, Bugnon C. Colocalization of prolactin- and dynorphin-like substances in a neuronal population of the rat lateral hypothalamus. Neurosci. Lett. 1993;156:91–95. doi: 10.1016/0304-3940(93)90447-s. [DOI] [PubMed] [Google Scholar]
- 13.Risold PY, Griffond B, Kilduff TS, Sutcliffe JG, Fellmann D. Preprohypocretin (orexin) and prolactin-like immunoreactivity are coexpressed by neurons of the rat lateral hypothalamic area. Neurosci. Lett. 1999;259:153–156. doi: 10.1016/s0304-3940(98)00906-9. [DOI] [PubMed] [Google Scholar]
- 14.Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci. Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci. Lett. 1999;260:161–164. doi: 10.1016/s0304-3940(98)00977-x. [DOI] [PubMed] [Google Scholar]
- 18.Broberger C, de Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 19.Casatti CA, Elias CF, Sita LV, Frigo L, Furlani VC, Bauer JA, Bittencourt JC. Distribution of melanin-concentrating hormone neurons projecting to the medial mammillary nucleus. Neuroscience. 2002;115:899–915. doi: 10.1016/s0306-4522(02)00508-0. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LW. Brain Maps: Structure of the Rat Brain. 3rd edition. Academic Press; 2004. pp. 1–215. [Google Scholar]
- 21.Bittencourt J, Celis ME. Anatomy, function and regulation of neuropeptide EI (NEI) Peptides. 2008;29:1441–1450. doi: 10.1016/j.peptides.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- 23.Saper CB. A guide to the perplexed on the specificity of antibodies. J Histochem. Cytochem. 2009;57:1–5. doi: 10.1369/jhc.2008.952770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropinreleasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 25.Wayne NL, Lee W, Michel S, Dyer J, Sossin WS. Activity-dependent regulation of neurohormone synthesis and its impact on reproductive behavior in aplysia. Biol. Reprod. 2004;70:277–281. doi: 10.1095/biolreprod.103.022491. [DOI] [PubMed] [Google Scholar]
- 26.Ramon-Moliner E, Nauta WJ. The isodendritic core of the brain stem. J Comp Neurol. 1966;126:311–335. doi: 10.1002/cne.901260301. [DOI] [PubMed] [Google Scholar]
- 27.Nauta WJH, Haymaker W. Hypothalamic Nuclei and Fiber Connections. In: Haymaker W, Anderson E, Nauta WJH, editors. The Hypothalamus. Charles C Thomas, Illinois; 1969. pp. 136–209. [Google Scholar]
- 28.Swanson LW. The hypothalamus. In: Bjorklund A, Hokfelt T, Swanson LW, editors. Handbook of chemical neuroanatomy. Vol. 5. Elsevier: 1987. pp. 1–124. [Google Scholar]
- 29.Cvetkovic V, Brischoux F, Griffond B, Bernard G, Jacquemard C, Fellmann D, Risold PY. Evidence of melanin-concentrating hormone-containing neurons supplying both cortical and neuroendocrine projections. Neuroscience. 2003;116:31–35. doi: 10.1016/s0306-4522(02)00557-2. [DOI] [PubMed] [Google Scholar]
- 30.Elias CF, Sita LV, Zambon BK, Oliveira ER, Vasconcelos LA, Bittencourt JC. Melaninconcentrating hormone projections to areas involved in somatomotor responses. J Chem. Neuroanat. 2008;35:188–201. doi: 10.1016/j.jchemneu.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Elias CF. Bittencourt, Study of the origins of melanin-concentrating hormone and neuropeptide EI immunoreactive projections to the periaqueductal gray matter. Brain Res. 1997;755:255–271. doi: 10.1016/s0006-8993(97)00104-2. [DOI] [PubMed] [Google Scholar]
- 32.Bittencourt JC, Elia CF. Melanin-concentrating hormone and neuropeptide EI projections from the lateral hypothalamic area and zona incerta to the medial septal nucleus and spinal cord: a study using multiple neuronal tracers. Brain Res. 1998;805:1–19. doi: 10.1016/s0006-8993(98)00598-8. [DOI] [PubMed] [Google Scholar]
- 33.Torterolo P, Sampogna S, Chase MH. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009;1268:76–87. doi: 10.1016/j.brainres.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 34.Kerman IA, Bernard R, Rosenthal D, Beals J, Akil H, Watson SJ. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J Comp Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- 35.Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience. 2003;122:541–550. doi: 10.1016/j.neuroscience.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 37.Presse F, Nahon JL. Differential regulation of melanin-concentrating hormone gene expression in distinct hypothalamic areas under osmotic stimulation in rat. Neuroscience. 1993;55:709–720. doi: 10.1016/0306-4522(93)90436-j. [DOI] [PubMed] [Google Scholar]
- 38.Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur. J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- 40.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 42.Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- 43.Hundahl CA, Kelsen J, Dewilde S, Hay-Schmidt A. Neuroglobin in the rat brain (II): colocalisation with neurotransmitters. Neuroendocrinology. 2008;88:183–198. doi: 10.1159/000135617. [DOI] [PubMed] [Google Scholar]
- 44.Rondini TA, Rodrigues BC, de Oliveira AP, Bittencourt JC, Elias CF. Melanin-concentrating hormone is expressed in the laterodorsal tegmental nucleus only in female rats. Brain Res. Bull. 2007;74:21–28. doi: 10.1016/j.brainresbull.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Knollema S, Brown ER, Vale W, Sawchenko PE. Novel hypothalamic and preoptic sites of prepro-melanin-concentrating hormone messenger ribonucleic acid and peptide expression in lactating rats. J. Neuroendocrinol. 1992;4:709–717. doi: 10.1111/j.1365-2826.1992.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 46.Konigsmark BW. Methods for the Counting of Neurons. In: Nauta WJH, Ebbesson SOE, editors. Contemporary Research Methods in Neutoanatomy. New York: Springer-Verlag; 1970. pp. 315–339. [Google Scholar]
- 47.Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]