Abstract
Hypoxia–ischemia (H/I) in the premature infant leads to white matter injury termed periventricular leukomalacia (PVL), the leading cause of subsequent neurological deficits. Glutamatergic excitotoxicity in white matter oligodendrocytes (OLs) mediated by cell surface glutamate receptors (GluRs) of the AMPA subtype has been demonstrated as one factor in this injury. Recently, it has been shown that rodent OLs also express functional NMDA GluRs (NMDARs), and overactivation of these receptors can mediate excitotoxic OL injury. Here we show that preterm human developing OLs express NMDARs during the PVL period of susceptibility, presenting a potential therapeutic target. The expression pattern mirrors that seen in the immature rat. Furthermore, the uncompetitive NMDAR antagonist memantine attenuates NMDA-evoked currents in developing OLs in situ in cerebral white matter of immature rats. Using an H/I rat model of white matter injury, we show in vivo that post-H/I treatment with memantine attenuates acute loss of the developing OL cell surface marker O1 and the mature OL marker MBP (myelin basic protein), and also prevents the long-term reduction in cerebral mantle thickness seen at postnatal day 21 in this model. These protective doses of memantine do not affect normal myelination or cortical growth. Together, these data suggest that NMDAR blockade with memantine may provide an effective pharmacological prevention of PVL in the premature infant.
Keywords: hypoxia–ischemia, human forebrain development, oligodendrocyte, glutamate receptor, stroke, myelin
Introduction
Despite increased survival of extremely premature infants as a result of improved intensive care, cerebral palsy and cognitive/behavioral deficits occur in up to 35% of survivors (Wilson-Costello et al., 2007). Periventricular leukomalacia (PVL) is the major neuropathological lesion in premature infants, involving focal white matter necrosis, diffuse gliosis, and subsequent hypomyelination (Kinney and Back, 1998). Magnetic resonance imaging (MRI) findings in survivors of extreme prematurity with PVL show a reduction in cortical gray matter volume associated with decreased total brain myelinated white matter in later infancy and beyond (Inder et al., 1999, 2005), consistent with pathological observations (Volpe, 2005). PVL pathophysiology is multifactorial, including hypoxia–ischemia (H/I)-induced glutamate excitotoxicity, free-radical injury, and inflammation (Volpe, 2005; Jensen, 2006). Oligodendrocytes (OLs) are the principal cellular component in white matter, and developing OLs have been shown to be uniquely susceptible to these forms of injury (Follett et al., 2000; Back et al., 2001). Notably, these developing OLs predominate in preterm human white matter at the most susceptible period for PVL (Back et al., 2001; Talos et al., 2006b). H/I results in glutamate accumulation in white matter under pathophysiological conditions from multiple sources, including vesicular release from axons (Kukley et al., 2007) and reversal of glutamate transporters (Fern and Möller, 2000; Rossi et al., 2000). Developing OL susceptibility to H/I correlates with expression of glutamate receptors (GluRs) of the AMPA subtype (AMPARs) on developing OLs in white matter of immature rodents and premature infants (Talos et al., 2006a,b). AMPARs on developing OLs are calcium permeable and mediate excitotoxic injury (Fern and Möller, 2000; Yoshioka et al., 2000; Tekkök and Goldberg, 2001; Deng et al., 2003; Follett et al., 2004), and systemic administration of AMPAR antagonists attenuates injury in a rat model of PVL (Follett et al., 2000, 2004).
Recent studies show that OLs also express the NMDA subtype of glutamate receptors (NMDARs), which are located specifically on their processes (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006). In neurons, activation of NMDARs at synapses is critical for synaptic plasticity and synaptogenesis (Cull-Candy et al., 2001), and their overactivation is implicated in H/I-induced excitotoxic injury in stroke models (MacDonald et al., 2006). In vitro studies show that simulated ischemia (oxygen glucose deprivation) activates NMDARs on OL processes in immature and adult rodent cerebellum and corpus callosum, and adult rat optic nerve (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006). Thus, we hypothesized that NMDARs may be expressed on developing OLs in the immature rat and human brain during the PVL-susceptible window and in part mediate H/I-induced white matter injury. We previously showed that the uncompetitive NMDAR antagonist memantine (adamantine) is neuroprotective against H/I-induced gray matter injury in the developing rodent without significant adverse effects (Chen et al., 1998). Unlike the open-channel NMDAR antagonist dizocilpine (MK-801), memantine is well tolerated and approved for the treatment of dementia (Chen and Lipton, 2006). Here we tested the protective efficacy of memantine against H/I-induced white matter injury in the rat PVL model.
Materials and Methods
Animal procedures.
Long–Evans male rat pups (Charles River Laboratories) were maintained in a temperature-controlled animal care facility with a 12 h light/dark cycle. All procedures were approved and in accordance with guidelines of the Animal Care and Use Committee at Children's Hospital (Boston, MA). Using a modification of the Rice-Vannucci model (Levine, 1960; Rice et al., 1981), H/I-induced injury was generated at postnatal day 6 (P6) by unilateral carotid artery ligation under ether anesthesia followed by 1 h of 6% hypoxia after a 1 h recovery period as described previously (Follett et al., 2000). Pup temperature was maintained away from the dam with a 34°C thermal blanket, and monitored before and after surgery with a rectal temperature probe. Drugs were diluted in PBS to concentrations enabling consistent intraperitoneal volume administration of 0.1 ml/10 g of pup weight. Vehicle injections of PBS of equivalent volume were given. The first dose was administered 30 min to 1 h after hypoxia to allow initial recovery. Pups were then returned to the dam. Three additional intraperitoneal drug doses were then given on a 12 h schedule until the rats were killed as per our previous study (Chen et al., 1998). Memantine (Tocris Bioscience) dosing was 20 mg/kg loading dose, then 1 mg/kg maintenance dose. We based our dosing regimen on published data of rat pharmacokinetics and doses required for neuroprotection, yielding a steady-state brain concentration of 1–10 μm (Chen et al., 1998; Hesselink et al., 1999; Parsons et al., 1999). Pup weights were monitored at the start, at each drug administration, and when the pups were killed. Pups were killed by terminal pentobarbital anesthesia followed by intracardiac PBS and then 4% paraformaldehyde perfusion-fixation. Brains were extracted and postfixed overnight in 4% paraformaldehyde, followed by cryoprotection in 30% sucrose in PBS in preparation for sectioning.
Human tissue.
Parietal lobe autopsy specimens, obtained from Children's Hospital and Brigham and Women's Hospital (Boston, MA), were collected under guidelines approved by the hospitals' Institutional Review Boards. Cases ranging from 23 to 38 postconceptional weeks (PCW; n = 9) were selected based on combined clinical and neuropathological diagnosis, as previously described (Talos et al., 2006b). Except for stillbirths (n = 2), all patients died from non-neurological causes, which included extreme prematurity (n = 4), necrotizing enterocolitis, massive pulmonary hemorrhage, and multiple congenital anomalies (n = 1 each). No brain abnormalities or lesions were detected by macroscopic and microscopic standard neuropathological diagnostic examination. The postmortem interval range in this tissue set was 4–46 h (mean 25 h), and we have previously reported no significant effect of postmortem interval on AMPAR subunit expression in similar tissue at these time points (Talos et al., 2006b). Blocks of parietal lobe cortex and underlying white matter were fixed in fresh 4% paraformaldehyde for at least 48–72 h and then cryoprotected in 30% sucrose in PBS before sectioning.
Histological and immunocytochemical methods.
For H/I experiments, 16 μm coronal cryostat sections (Leica CM3050 S) were collected on Superfrost Plus slides. For NMDAR expression in normal rat brain and human parietal lobe, 50 μm freezing microtome (HM 440E; Microm International) free-floating sections were collected. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. Immunocytochemical studies were performed as previously described (Follett et al., 2004; Talos et al., 2006a,b). The following primary antibodies were used: mouse monoclonal antibodies to myelin basic protein (MBP)/SMI-99 and glial fibrillary acidic protein (GFAP)/SMI-22 (Covance); mouse monoclonal IgM antibodies to O4 and O1 (gifts from Dr. S. Pfeiffer, University of Connecticut Medical School, Farmington, CT); mouse monoclonal antibodies to CD68/MCA341GA and CD11b/MCA275G (Serotec); and mouse monoclonal antibody to NeuN/MAB 377 and rabbit polyclonal antibody to NR1/AB1516 (Millipore Bioscience Research Reagents). Sections were blocked and incubated overnight at 4°C with the primary antibody. Fluorescent goat Alexa Fluor 488 or 568 secondary antibodies (Invitrogen) appropriate to the primary antibody species were applied for 1 h at room temperature. For NR1, a biotinylated anti-rabbit IgG followed by an avidin-FITC conjugate (Vector Laboratories) was applied. Double labeling was performed sequentially with O4 or O1 (detergent free) first. Slides were coverslipped with an antifade medium (Fluoromount-G; Southern Biotechnology) or a mounting medium containing nuclear stain 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories). Fluorescence images were obtained on a Zeiss Axioscope, using a Spot digital camera and Advanced 4.5 software (Diagnostic Instruments). In H/I experiments, coronal sections in the region of the mid-dorsal hippocampus were examined. Stereotaxic coordinates used were as follows: for P9, 2.8–3.1 mm from bregma, 2.6–3.0 mm lateral to midline; for P21, 3.0–3.4 mm from bregma, 2.4–3.4 mm lateral to midline (Sherwood and Timiras, 1970). At P9 (72 h study), sections were immunostained for O1 and MBP to evaluate white matter loss. At P21 (15 d study), sections were H&E stained to evaluate combined parietal cortex and white matter (cerebral mantle) thickness. All scoring was performed by an observer blinded to treatment group or side of carotid artery ligation. ImageJ software was used to quantitate O1 immunostaining as a proportion of the white matter capsule area. ImageJ analysis of immunocytochemical staining was represented as a ratio of ipsilateral (to carotid ligation) to contralateral staining to take account of interanimal developmental variations. MBP staining was scored using a five-point semiquantitative ranked injury score modified from our previous three-point scale (Follett et al., 2000): 0 = no MBP loss; 1 = some loss of processes perpendicular to capsule; 2 = moderate loss of processes; 3 = complete loss of processes with intact capsule; 4 = loss of processes with thinning or breaks in capsule; 5 = loss of processes with complete loss of capsule. This methodology was validated with ImageJ software quantitation of total area of MBP immunostaining within each 2.4 mm2 field. MBP immunostaining was represented as a ratio of ipsilateral (to carotid ligation) to contralateral staining to take account of interanimal developmental variations. Parietal cerebral mantle thickness was measured on 25× photomicrographs with ImageJ software, and the ipsilateral-to-contralateral ratio was calculated for each brain. Confocal images were taken on a Zeiss LSM 510 scanning laser microscope with a 63× objective. Z-stacks were composed with confocal serial images (10 images/specimen) collected at 1 μm (rat tissue) or 2 μm (human tissue) intervals.
Brain slice electrophysiology.
The coronal slice preparation method used in this study has been described in previous reports (Jensen et al., 1998; Sanchez et al., 2001). Normal P6–P7 rat pups were decapitated, and the brains were rapidly dissected from the skull and placed in ice-cooled sucrose-based dissection solution bubbled with 95% O2/5% CO2. Coronal slices (250–300 μm) were prepared with a vibratome (World Precision Instruments) in cold dissection solution containing 210 mm sucrose, 2.5 mm KCl, 1.02 mm NaH2PO4, 0.5 mm CaCl2, 10 mm MgSO4, 26.19 mm NaHCO3, and 10 mm d-glucose, pH 7.4. Slices were incubated in a chamber at 32°C for 30 min with continuously oxygenated artificial CSF (ACSF) and then maintained at room temperature for at least 1 h before starting electrophysiological recordings. We used coronal mid-dorsal hippocampal white matter slices consistent with the regions analyzed in H/I and normal rat immunohistochemistry studies. As in our previously published protocols (Sanchez et al., 2001, 2005), whole-cell patch-clamp recordings were made from white matter developing OLs by using Nikon infrared/differential interference contrast optics microscopy, and the slices were continuously superfused with ACSF bubbled with 95% O2/5% CO2 at 1–1.5 ml/min containing 124 mm NaCl, 5 mm KCl, 1.25 mm NaH2PO4, 2 mm CaCl2, 1.2 mm MgSO4, 26 mm NaHCO3, and 10 mm d-glucose, pH 7.4. All recordings were performed at room temperature (22–24°C) using filled electrodes (6–10 MΩ). The internal solution of the patch pipette contained 110 mm Cs-sulfonate, 10 mm TEA-Cl, 4 mm NaCl, 2 mm MgCl2, 10 mm EGTA, 10 mm HEPES, 4 mm ATP-Mg, and 0.3 mm GTP, pH 7.25. No correction was made for the junction potential of 8.9 mV during recordings. To isolate NMDAR-mediated currents, 50 μm picrotoxin and 10 μm 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxaline (NBQX) were used to block GABA and AMPA/kainate receptors, respectively. NMDAR-mediated excitatory currents were recorded in the presence of 10 μm glycine and 2 μm strychnine. Developing OLs were clamped with a ramp from −90 to +50 mV while 50 μm NMDA was bath perfused. K+-based internal solution was used when characterizing inward and outward currents. With this K+-based solution, 10 mm TEA, 5 mm 4-AP, and 1 μm TTX were used to block the potassium channels to examine NMDAR-mediated currents. 10 μm NBQX (Sigma), 30 μm picrotoxin (Tocris Bioscience), 10 or 50 μm memantine (Sigma), 100 μm dl-AP-5 (Tocris Bioscience), and 1 μm TTX (Tocris Bioscience) were applied by gravity superfusion. All drug-induced alterations in synaptic currents were completely or partially reversed by 20 min washout in ACSF. Data were collected using an Axopatch 200A amplifier and Clampex 9.2 software (Molecular Devices) with compensation for series resistance (70%) and cell capacitance, filtered at 2 kHz, and digitized at 20 kHz using a Digidata 1320A. The data analysis was performed with Clampfit 9.2 (Molecular Devices) to construct I–V curves. Alexa Fluor 594 was included in the electrode solution to mark the recorded cells. After recordings, slices were fixed for 1–2 h in 4% paraformaldehyde and stained (overnight at 4°C) with rabbit polyclonal antibody to Olig2/AB9610 (Millipore Bioscience Research Reagents), a pan-OL nuclear marker, and fluorescent goat Alexa Fluor 488 secondary antibody (1 h at room temperature) and visualized using confocal microscopy (63× objective; Z-stack = 10 serial images at 2 μm intervals) to confirm that the recorded cells were developing OLs by colocalization and morphology.
Statistical analysis.
Data were analyzed with SigmaStat 3.11 software (Systat Software 2004). Data is expressed as mean ± SEM. Normally distributed data differences between two groups were compared using Student's t test. Multiple groups were compared using one-way ANOVA with the Bonferroni multiple-comparison post hoc test. Nonparametric datasets were compared using the Mann–Whitney rank sum test. A p value of ≤0.05 was considered statistically significant (denoted by an asterisk in the figures).
Results
NMDARs are expressed on multiple cell types, including developing OLs, in immature rat white matter
Using our model of PVL in Long–Evans rats, we have shown that white matter is highly vulnerable to H/I injury at P6 (Follett et al., 2000, 2004). To evaluate whether the NMDAR represents a potential therapeutic target in our rat PVL model, we examined the cell-specific expression of NMDARs in parietal subcortical white matter in the P6 Long–Evans rat. We qualitatively assessed the cellular expression of the obligate NR1 subunit of NMDARs in multiple white matter cell types. The NR1 subunit containing the glycine binding site is thought to be required for functional NMDAR expression (Stys and Lipton, 2007). Hence, we double labeled for the NR1 subunit, together with the developing OL markers O4 and O1, microglial markers CD11 and CD68, astrocytic marker GFAP, or neuronal marker NeuN. The NR1 subunit was highly expressed at P6 in OLs stained with O4 (Fig. 1 A–C) and O1 (supplemental Fig. 1A–C, available at www.jneurosci.org as supplemental material), with staining predominantly on the OL processes (Fig. 1M). NR1 expression was present at low levels on most GFAP-positive cell bodies, although it was highly expressed in some astrocytic processes (Fig. 1 D–F). CD11- and CD68-positive microglia revealed strong NR1 staining that was present primarily on the somata and proximal processes (Fig. 1 G–I; supplemental Fig. 1D–F, available at www.jneurosci.org as supplemental material). Subplate neurons are also present in developing white matter and were identified by their characteristic morphology, NeuN immunoreactivity, and location at the cortical/white matter border (Talos et al., 2006a). These subplate neurons demonstrated high NR1 expression throughout their somata and processes (Fig. 1 J–L). Together, these results demonstrate the presence of NMDARs on developing OLs as well as other white matter cells at P6, coincident with the window of increased white matter susceptibility to H/I injury.
Figure 1.

NR1, the NMDAR obligate subunit, is present on multiple white matter cell types in the P6 Long–Evans rat pup during the window of susceptibility for PVL. Double-fluorescent staining of normal P6 rat pup 50 μm frozen sections is shown. A, D, G, J, Cell-specific antibody labeling of developing OLs (O4), astrocytes (GFAP), microglia (CD11), and subplate neurons (NeuN). B, E, H, K, NR1 obligate NMDAR antibody labeling and nuclear DAPI stain. C, F, I, L, Overlay showing representative NR1 expression pattern within these four white matter cell types. M, Confocal Z-stack of O4 (red), NR1 (green), and DAPI (blue), showing colocalization of NR1 with O4-positive developing OL processes. Scale bars: C, F, I, 10 μm; L, M, 50 μm.
NMDARs are expressed on developing OLs in preterm human white matter during the window of susceptibility to PVL
Having demonstrated NMDAR expression on OLs in the rodent during a developmental window of white matter susceptibility, we next evaluated whether NMDARs are present in developing human white matter in the preterm period when PVL occurs. Serial sections of parietal lobe cortex and associated white matter from cases between 23 and 38 PCW were double labeled for the NMDAR obligate subunit NR1 and the O4 and O1 markers of developing OLs. O4-positive developing OLs (comprising late progenitor and immature premyelinating OLs) predominate in preterm human white matter at 17–32 weeks gestation, although the proportion of O1-positive immature premyelinating OLs increases prominently at that time (Kinney and Back, 1998; Back et al., 2001). In all nine samples from preterm human brain, we found NR1 highly expressed on O4-positive developing OLs in subcortical parietal white matter, localized preferentially on the processes through their entire extent (Fig. 2 A–C, arrowheads). In addition, NR1 was variably expressed on O4-negative white matter cell types (Fig. 2E,F, arrows). A similar finding of NR1 localization on processes was also seen when OLs were labeled with the O1 marker for premyelinating OLs (Fig. 2G). Thus, these results demonstrate that NMDARs are present in developing OLs in premature white matter during the window of susceptibility to PVL, mirroring NMDAR expression in the rat at a similar developmental stage.
Figure 2.

Preterm human periventricular white matter developing OLs express NR1, the NMDAR obligate subunit, during the window of susceptibility for PVL. A–F, Human O4-positive (late progenitor and immature premyelinating) developing OLs express NR1, predominantly in their processes, at both 26 (A–C, arrowheads) and 31 (D–F) PCW. Other O4-negative white matter cell types also express NR1 (E, F, arrows). Scale bars, 20 μm. G, Confocal Z-stack of the immature premyelinating OL marker, O1 (red), and NR1 (green), showing colocalization of NR1 with O1 throughout premyelinating OL processes. Scale bars, 20 μm.
Post-H/I treatment with memantine prevents acute white matter injury in the rat PVL model
We tested the in vivo protective efficacy of memantine administered systemically for 48 h after unilateral cerebral H/I-induced injury in P6 Long–Evans rats. White matter injury in the immature rodent acutely results in loss of the immature OL marker O1 and the mature OL marker MBP (Follett et al., 2000; Liu et al., 2002; McCarran and Goldberg, 2007). Thus, we assessed for acute white matter injury by immunostaining for both O1 and MBP in parietal white matter of P9 rats (72 h after H/I). O1 expression was assessed using ImageJ (NIH analysis software) and revealed unilateral decreases in white matter ipsilateral to the carotid ligation compared with contralateral in vehicle-treated rats at 72 h after H/I (n = 11; Mann–Whitney rank sum test, p < 0.004) (Fig. 3A,B,E). In contrast, memantine post-H/I treatment significantly attenuated this loss of O1 immunostaining ipsilateral to the ligation (memantine, 72.1 ± 7.3%, n = 12; vehicle, 41.3 ± 8.6%, n = 11; t test, p = 0.012) (Fig. 3 C–E).
Figure 3.
Memantine significantly attenuates loss of the developing OL cell surface marker O1 ipsilateral to the carotid ligation at 72 h after injury. A–D, Representative O1 immunohistochemistry showing loss of O1 staining, more severe ipsilateral (A, C) to the carotid artery ligation, in vehicle-treated pups (A, B), with attenuation of O1 loss in memantine-treated pups (C, D). Scale bar, 100 μm. E, Ipsilateral O1 loss represented as a ratio of contralateral stain (1 = no loss; 0 = complete loss). Memantine significantly attenuates loss of the O1 marker of developing OLs (n = 11 vehicle; n = 12 memantine; t test, p = 0.012). Error bars denote SEM.
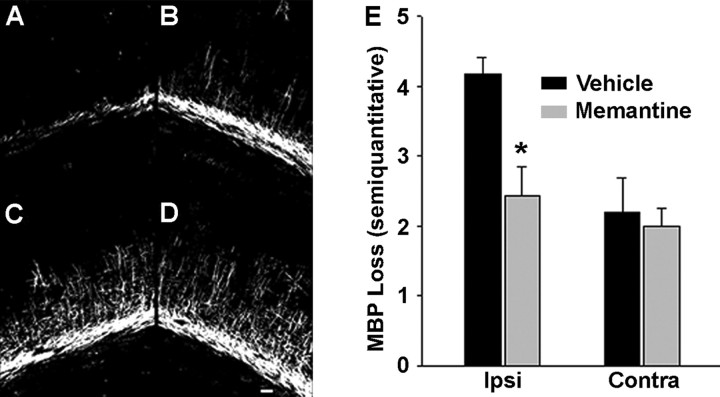
We next scored MBP immunostaining in the ipsilateral and contralateral hemispheres using a semiquantitative method modified from our previously published work (Follett et al., 2000, 2004), where 0 = no myelin loss and 5 = complete myelin loss (see Materials and Methods). At 72 h, H/I resulted in a significant loss of MBP immunostaining ipsilateral to the carotid ligation in vehicle-treated pups, with ablation of radiating OL processes and disruption of the pericallosal fiber bands (Fig. 4A). Analysis of the hemisphere contralateral to the ligation did show a mild loss of radiating processes compared with littermate non-H/I controls (Fig. 4B), consistent with our previous observations (Follett et al., 2000, 2004). In contrast, post-H/I memantine treatment resulted in significant attenuation of MBP loss ipsilateral to the ligation, with most pups showing preservation of processes radiating into cortex as well as pericallosal process bundles compared with vehicle-treated controls (t test, p = 0.008) (Fig. 4 C–E). To confirm these results, we used ImageJ to quantitate MBP immunostaining area. Vehicle-treated animals showed significant injury ipsilateral to the carotid ligation (n = 11; t test, p < 0.001) with loss of MBP at 72 h after H/I (23.9 ± 7.4%; n = 11). In contrast, memantine-treated animals had significant attenuation of MBP loss ipsilateral to the ligation, almost approaching the amount of contralateral staining (82.1 ± 13.5%; n = 14; t test, p = 0.018). These results demonstrate therapeutic efficacy of memantine in preventing acute white matter injury in a rat model of PVL. Most importantly, memantine was effective when administered systemically and after the H/I insult, suggesting that post-H/I NMDAR activation plays a role in white matter injury in this model.
Figure 4.
Memantine significantly attenuates loss of MBP at 72 h after injury. A–D, Representative MBP immunohistochemistry: vehicle-treated (A, B) and memantine-treated (C, D), ipsilateral (A, C) and contralateral (B, D) to carotid artery ligation. Scale bar, 100 μm. E, MBP loss based on a five-point semiquantitative scale (0 = no loss; 5 = maximum loss). Memantine significantly attenuates MBP loss ipsilateral to carotid artery ligation (n = 11 vehicle; n = 14 memantine; t test, p = 0.008). Error bars denote SEM.
Post-H/I treatment with memantine prevents long-term injury in the rat PVL model
Long-term MRI follow-up in later infancy and childhood, with a prior diagnosis of PVL as premature infants, reveals a reduction in the cerebral mantle, constituted by cortical and white matter volume (Inder et al., 1999, 2005), consistent with neuropathological studies (Volpe, 2005). Thus, we evaluated rat pups at P21, 15 d after P6 H/I, for cerebral mantle thickness of coronal sections in the region of the mid-dorsal hippocampus. We represented injury as a ratio of ipsilateral (to carotid ligation) to contralateral thickness. Pups that underwent H/I had a significant reduction in cerebral mantle thickness (74 ± 11%; n = 9) compared with control (no H/I) littermates (n = 8; t test, p = 0.041). Post-H/I memantine treatment (48 h dosing as in the short-term study) resulted in significantly greater preservation of cerebral mantle thickness ipsilateral to the carotid ligation (98 ± 2%; Mann–Whitney rank sum test, p = 0.037) (Fig. 5). These results demonstrate that in addition to its acute protection, memantine also has a protective effect on later outcome after H/I at P6.
Figure 5.
Memantine reduces long-term sequelae of white matter injury at 15 d after injury. ImageJ quantification of cerebral mantle (CM) thickness represented as ratio of ipsilateral (to carotid ligation) to contralateral values (1 = no loss; 0 = complete loss) (n = 9 vehicle; n = 10 memantine; Mann–Whitney rank sum test, p = 0.037). Error bars denote SEM.
Memantine reduces NMDAR-mediated currents in P6 rat developing OLs in situ
Given that developing OLs express NMDARs at P6 and that white matter injury is attenuated by memantine, we next evaluated whether memantine could modulate NMDA-evoked currents in developing OLs in P6 rat subcortical white matter in situ. Whole-cell patch-clamp recordings were performed in 250–300 μm P6 rat brain coronal slices, and NMDA was bath applied. As previously reported, only a subset of OLs exhibited NMDA currents (Lin and Bergles, 2002). In those OLs exhibiting NMDA currents, memantine (10 and 50 μm) significantly decreased peak current amplitudes at both negative (45.48 ± 22.09% of control for 10 μm; 6.02 ± 6.95% for 50 μm; one-way ANOVA, p = 0.036) and positive (34.21 ± 19.12% of control for 10 μm; 10.21 ± 9.07% for 50 μm; one-way ANOVA, p = 0.024) voltages in a dose-dependent manner (Fig. 6 A–C). Developing OLs were identified morphologically and by their characteristic inward and outward currents (Lin and Bergles, 2002) (Fig. 6D). Additional cell identification was provided by injecting recorded cells with Alexa Fluor 594 and double labeling with the nuclear pan-OL marker Olig2 (Fig. 6E). These findings demonstrate the presence of functional NMDARs on developing OLs of the age used in our PVL model, and suggest that memantine may attenuate white matter injury in vivo via modulation of H/I-induced excitotoxic injury to developing OLs.
Figure 6.
NMDA-evoked currents in P6–P7 rat white matter OLs in situ are significantly attenuated by memantine in a dose-dependent manner. A, B, Raw data showing NMDA-evoked current in developing OL after application of 50 μm NMDA and attenuation with application of 10 μm (A) and 50 μm (B) memantine. C, Bar graph showing percentage reduction in NMDA peak current amplitudes at negative and positive voltages after application of 10 μm memantine (n = 5 recordings) and 50 μm memantine (n = 4 recordings) normalized to control peak currents (n = 6 recordings). Memantine significantly reduced peak current amplitudes at −15 mV (1-way ANOVA, p = 0.036) and at +50 mV (1-way ANOVA, p = 0.024). Error bars denote SEM. D, E, We identified cells as developing OLs by current signature, morphology, and immunohistochemistry. D, The outward and inward currents in morphologically identified developing OLs in P6 white matter slice are typical of developing OLs. Currents were recorded by stepping cells from −90 mV to +50 mV in 10 mV intervals. The calibration is indicated. E, Example of confocal projection of a recorded cell injected with the fluorescent dye Alexa Fluor 594 (red) and stained for the nuclear pan-OL marker Olig2 (green), confirming that memantine attenuation of NMDA-evoked currents occurs in OLs. Scale bar, 10 μm.
White matter maturation is not affected by protective doses of memantine
In contrast to MK-801, memantine administration in the early postnatal period in rats does not induce acute changes in neuronal morphology or alter synaptic plasticity (Chen et al., 1998; Chen and Lipton, 2006). Here we evaluated the effects of memantine administration at protective doses on white matter development. P6 rats (not exposed to H/I) were given the same 48 h neuroprotective memantine treatment and evaluated for white matter development (MBP) and were compared with control littermates that received equivalent injections of PBS vehicle. Memantine treatment did not result in any change in MBP staining assessed at P9 (n = 10 per group; t test, p = 0.64), or later at P21 (n = 8 per group; t test, p = 0.45) (supplemental Fig. 2A, available at www.jneurosci.org as supplemental material). In addition, assessment of cortical and white matter (cerebral mantle) thickness at P21 was similarly unchanged by memantine treatment (n = 8 per group; t test, p = 0.54) (supplemental Fig. 2B, available at www.jneurosci.org as supplemental material). Finally, there was no difference in weight gain up to P21 between memantine-treated and control littermates (data not shown).
Discussion
This study supports a role for NMDAR-mediated OL excitotoxicity in the pathophysiology of H/I-induced white matter injury in developing rat brain. NMDARs are present on multiple cell types in rat pup white matter, including developing OLs during the first postnatal week, when they are most susceptible to H/I-induced white matter injury. Notably, NMDARs are also expressed on developing OL processes in human preterm subcortical white matter at a time when they are most susceptible to PVL. Here we show that post-H/I treatment with the NMDAR antagonist memantine significantly attenuates acute white matter injury in a P6 rat model of PVL. Furthermore, this early beneficial effect appears to result in a long-term reduction in cerebral mantle thinning in this rat model, a consequence of PVL seen in preterm infants. In situ whole-cell patch-clamp recordings in P6 rat brain slices reveal that developing OLs express functional NMDARs, and memantine significantly reduces NMDAR-mediated currents. Control rats treated with memantine at P6 had no significant alteration in white matter (MBP) or cortical (cerebral mantle) development.
NMDAR expression in P6 rat and preterm human white matter
Here we show that NMDARs are expressed on rat white matter developing OLs at P6, an age of developmental susceptibility to H/I-induced white matter injury. Similar to other reports, NR1, the obligate NMDAR subunit, staining patterns suggest that NMDARs are predominantly localized on the processes of developing OLs (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006). We show that the NR1 subunit also is located on other cellular components of white matter in our P6 rat model, including rat astrocytes, microglia, and subplate neurons, consistent with previous reports (Gottlieb and Matute, 1997; Schipke et al., 2001; Hanganu et al., 2002). A novel finding in this study is the presence of NMDARs on developing OLs in human preterm parietal subcortical white matter, expressed through the extent of the processes, similar to that seen in the rat. Overactivation of NMDARs resulting from accumulation of extracellular glutamate during H/I leads to excessive calcium entry, and/or cell death mediated by free radical formation, and lipid membrane peroxidation (Choi, 1992; Michaelis, 1998; Vannucci and Hagberg, 2004). Thus, the presence of NR1 on cellular components of white matter in the P6 rat and preterm human suggests that NMDARs may contribute to H/I-induced injury. Indeed, elevated glutamate levels have been reported in the CSF of infants after perinatal H/I injury (Gücüyener et al., 1999), suggesting that excitotoxic mechanisms may be involved in human PVL.
Memantine administration after H/I protects against white matter injury in the rat PVL model
Given the presence of NMDARs on developing OLs and other cells intrinsic to white matter, we investigated whether the NMDAR antagonist memantine would attenuate H/I-induced white matter injury in the developing rat brain. Using both O1 and MBP as markers of injury to OLs in white matter, this is the first study to show an in vivo effect of memantine on developing white matter in the rat PVL model. In addition, memantine treatment is effective when initiated after injury. This novel protective effect of memantine against white matter injury in vivo is consistent with many reports of neuronal protection by memantine in vivo in rodent ischemia models (Block and Schwarz, 1996; Chen et al., 1998; Stys and Lipton, 2007), and in vitro against glutamate- and NMDA-induced death (Erdö and Schäfer, 1991). Memantine, like MK-801, is an uncompetitive antagonist but exerts a use-dependent NMDAR blockade because of a faster off-rate than MK-801, and consequently exhibits less toxicity (Chen et al., 1992; Chen and Lipton, 2006). While this manuscript was under revision, a recent report emerged reporting protection of compound axon potentials from simulated ischemia in P28 rat myelinated optic nerve in vitro by memantine in combination with NBQX (Bakiri et al., 2008).
In addition to acute protection, we also examined whether there was long-term protection. Recent clinical observations demonstrate that in later infancy and childhood, thinning of cortical volume and cerebral mantle thickness is the major anatomical sequela of PVL (Inder et al., 1999, 2005). Strikingly, we show here that thinning of the cerebral mantle is recapitulated in the animal model, and that early post-H/I memantine treatment protects against this outcome. The mechanism of how acute white matter injury mediates this more diffuse injury is not known, and suggests that this model may be appropriate to study the contribution of OLs to developing cortical architecture.
Potential mechanisms of protection by memantine in the rat PVL model
Developing OLs are exquisitely sensitive to excitotoxic injury (Itoh et al., 2002; Deng et al., 2003; Rosenberg et al., 2003). In white matter, glutamate can accumulate because of reversal of transport under pathophysiological conditions (Fern and Möller, 2000; Rossi et al., 2000). We and others have previously shown that activation of OL-expressed AMPA/kainate receptors contribute to OL injury in models of PVL. Developing OLs exposed to simulated ischemia in vitro are protected from death by the AMPA/kainate receptor antagonists CNQX and NBQX in culture and in organotypic slices (Tekkök and Goldberg, 2001; Deng et al., 2003), and AMPA/kainate-receptor antagonists NBQX and topiramate prevent white matter injury in vivo in the rat PVL model (Follett et al., 2000, 2004). Here we now show evidence for a protective effect in vivo by an NMDAR antagonist, suggesting that protection may occur through direct blockade of NMDARs in white matter. OLs in subcortical white matter brain slices in situ at P6, an age characterized by increased susceptibility to H/I injury (Follett et al., 2000), also express functional NMDARs, and these can be blocked by memantine. Supporting this observation, Bakiri et al. (2008) recently reported that in P12 callosal white matter slices memantine reduced NMDA-evoked currents in MBP-positive mature OLs, and that the NMDAR antagonist d-AP5 reduced an ischemia-induced slowly developing inward current in both mature and developing OLs in vitro. Together, these results suggest that the direct blockade of NMDAR-mediated excitotoxicity intrinsic to OLs may contribute to the in vivo effect seen here. Importantly, NMDARs on OLs and developing OLs appear distinct from their neuronal counterparts, exhibiting relative magnesium insensitivity and rundown, likely related to novel subunit composition (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006). Further characterization of OL stage-specific NMDAR subunit composition will have functional implications and inform the future use of subunit specific blockers (Chenard and Menniti, 1999). NMDARs are preferentially expressed on OL processes (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006). Consistent with this observation, blocking AMPA/kainate receptors protects developing OL somata, whereas blocking NMDARs protects developing OL processes (Salter and Fern, 2005). The distinct spatial expression of these GluRs suggests that combined therapy with both AMPA/kainate receptor and NMDAR antagonists may result in enhanced protection.
The presence of the NR1 subunit on other white matter cell types at P6 suggests that memantine may, in part, attenuate white matter injury through NMDAR blockade of non-OL cellular components. Selective death of subplate neurons has been reported in a younger rat pup H/I model of white matter injury (McQuillen et al., 2003), perhaps explaining some of the more extensive neurocognitive sequelae of H/I in premature infants than cannot be accounted for by OL injury alone (Volpe, 1996). Microglia and astrocytes express NMDARs and may aggravate developing OL injury (Tahraoui et al., 2001). Adult models of H/I show NMDAR subunit upregulation on activated microglia and reactive astrocytes in hippocampus (Gottlieb and Matute, 1997; Krebs et al., 2003). Indeed, NMDAR blockade by memantine attenuates microglial activation in models of neuroinflammation (Rosi et al., 2006). Because microglia are also activated in developing white matter after H/I (Cai et al., 2006; Lechpammer et al., 2008), the NMDARs on microglia may also be a target for memantine treatment.
Therapeutic implications and safety
Memantine is FDA-approved for use in Alzheimer-type dementia (Robinson and Keating, 2006), and appears to be clinically well tolerated. In contrast to the poorly tolerated uncompetitive antagonist MK-801, memantine has no significant effect on neuronal morphology or synaptic plasticity in immature rat brain at the same concentrations used in this study (Chen et al., 1998), likely because of its rapid off-rate binding kinetics (Chen et al., 1992; Chen and Lipton, 2006). In addition, memantine has not demonstrated any teratogenic potential in pregnant rats or rabbits (Forest Pharmaceuticals, 2003). However, NMDARs could play a role in normal myelination given their expression on myelin sheaths and responsiveness to axonally released glutamate (Yuan et al., 1998; Micu et al., 2006, 2007). We show in vivo in the immature rat that myelination is not altered by the 48 h memantine doses effective in attenuating white matter injury. Furthermore, this treatment does not appear to have long-term effects on myelination and cortical growth (cerebral mantle thickness) in control rats evaluated 15 d after initial doses at P6–P8.
No specific treatment currently exists for premature infants at risk for PVL, the leading cause of cerebral palsy. Here, we show a novel mechanism of NMDAR-mediated excitotoxicy to developing OLs that is attenuated by memantine posttreatment. The observation that memantine is protective when administered after the H/I insult suggests clinical feasibility, because the majority of infants are identified after an insult, given the lack of clinical predictors of risk. In addition, we show a lack of adverse effects on myelination memantine treatment, suggesting that this agent may have potential as an age-specific therapy in human premature infants at risk for PVL. The demonstration of similar patterns of NMDAR expression on developing OLs in human white matter supports future studies evaluating their role as a therapeutic target.
Footnotes
This work was supported by National Institutes of Health Grants NS038475 (J.J.V., F.E.J.) and NS031718 (F.E.J.) and by the William Randolph Hearst Foundation (S.M.M., D.M.T.), in addition to core support from the Mental Retardation Developmental Disorders Research Center (National Institutes of Health–National Institute of Child Health and Human Development Grant P30 HD18655). Dr. Rebecca Folkerth provided invaluable assistance with evaluation of human tissue neuropathological diagnosis and immunostaining. We are most grateful to Dr. Meayoung Chang, Dr. An Li, Kathia Cordero, and Griffin Boll for help with tissue preparation and to Dr. Lihong Bu for help with confocal microscopy.
References
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Hamilton NB, Káradóttir R, Attwell D. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia. 2008;56:233–240. doi: 10.1002/glia.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block F, Schwarz M. Memantine reduces functional and morphological consequences induced by global ischemia in rats. Neurosci Lett. 1996;208:41–44. doi: 10.1016/0304-3940(96)12545-3. [DOI] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-d-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE. Neuroprotective concentrations of the N-methyl-d-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des. 1999;5:381–404. [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdö SL, Schäfer M. Memantine is highly potent in protecting cortical cultures against excitotoxic cell death evoked by glutamate and N-methyl-d-aspartate. Eur J Pharmacol. 1991;198:215–217. doi: 10.1016/0014-2999(91)90625-z. [DOI] [PubMed] [Google Scholar]
- Fern R, Möller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci. 2000;20:34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest Pharmaceuticals. Namenda™ package insert. St. Louis: Forest Pharmaceuticals; 2003. [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Gücüyener K, Atalay Y, Aral YZ, Hasanoğlu A, Türkyilmaz C, Biberoglu G. Excitatory amino acids and taurine levels in cerebrospinal fluid of hypoxic ischemic encephalopathy in newborn. Clin Neurol Neurosurg. 1999;101:171–174. doi: 10.1016/s0303-8467(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 2002;22:7165–7176. doi: 10.1523/JNEUROSCI.22-16-07165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselink MB, De Boer BG, Breimer DD, Danysz W. Brain penetration and in vivo recovery of NMDA receptor antagonists amantadine and memantine: a quantitative microdialysis study. Pharm Res. 1999;16:637–642. doi: 10.1023/a:1018856020583. [DOI] [PubMed] [Google Scholar]
- Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Itoh T, Beesley J, Itoh A, Cohen AS, Kavanaugh B, Coulter DA, Grinspan JB, Pleasure D. AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem. 2002;81:390–402. doi: 10.1046/j.1471-4159.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–633. doi: 10.1097/MOP.0b013e328010c536. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Wang C, Stafstrom CE, Liu Z, Geary C, Stevens MC. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia in vivo. J Neurophysiol. 1998;79:73–81. doi: 10.1152/jn.1998.79.1.73. [DOI] [PubMed] [Google Scholar]
- Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Back SA. Human oligodendrocyte development: relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180–189. doi: 10.1016/s1071-9091(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Krebs C, Fernandes HB, Sheldon C, Raymond LA, Baimbridge KG. Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro. J Neurosci. 2003;23:3364–3372. doi: 10.1523/JNEUROSCI.23-08-03364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Manning SM, Samonte F, Nelligan J, Sabo E, Talos DM, Volpe JJ, Jensen FE. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathol Appl Neurobiol. 2008 doi: 10.1111/j.1365-2990.2007.00925.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- Liu Y, Silverstein FS, Skoff R, Barks JD. Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr Res. 2002;51:25–33. doi: 10.1203/00006450-200201000-00007. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Xiong ZG, Jackson MF. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 2006;29:75–81. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- McCarran WJ, Goldberg MP. White matter axon vulnerability to AMPA/kainate receptor-mediated ischemic injury is developmentally regulated. J Neurosci. 2007;27:4220–4229. doi: 10.1523/JNEUROSCI.5542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Micu I, Ridsdale A, Zhang L, Woulfe J, McClintock J, Brantner CA, Andrews SB, Stys PK. Real-time measurement of free Ca2+ changes in CNS myelin by two-photon microscopy. Nat Med. 2007;13:874–879. doi: 10.1038/nm1568. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Robinson DM, Keating GM. Memantine: a review of its use in Alzheimer's disease. Drugs. 2006;66:1515–1534. doi: 10.2165/00003495-200666110-00015. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE, Volpe JJ. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–245. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142:1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Koh S, Rio C, Wang C, Lamperti ED, Sharma D, Corfas G, Jensen FE. Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures. J Neurosci. 2001;21:8154–8163. doi: 10.1523/JNEUROSCI.21-20-08154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J Neurosci. 2005;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-d-aspartate receptors. FASEB J. 2001;15:1270–1272. doi: 10.1096/fj.00-0439fje. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. Berkeley, CA: University of California; 1970. [Google Scholar]
- Stys PK, Lipton SA. White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol Sci. 2007;28:561–566. doi: 10.1016/j.tips.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Tahraoui SL, Marret S, Bodénant C, Leroux P, Dommergues MA, Evrard P, Gressens P. Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 2001;11:56–71. doi: 10.1111/j.1750-3639.2001.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J Comp Neurol. 2006a;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Follett PL, Folkerth RD, Fishman RE, Trachtenberg FL, Volpe JJ, Jensen FE. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006b;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkök SB, Goldberg MP. AMPA/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21:4237–4248. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Subplate neurons—missing link in brain injury of the premature infant. Pediatrics. 1996;97:112–113. [PubMed] [Google Scholar]
- Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–225. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D, Friedman H, Minich N, Siner B, Taylor G, Schluchter M, Hack M. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000–2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Yamaya Y, Saiki S, Kanemoto M, Hirose G, Beesley J, Pleasure D. Non-N-methyl-d-aspartate glutamate receptors mediate oxygen–glucose deprivation-induced oligodendroglial injury. Brain Res. 2000;854:207–215. doi: 10.1016/s0006-8993(99)02359-8. [DOI] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]