Abstract
Background
Environmental factors including seasonal changes are important to guide physical activity (PA) programs to achieve or sustain weight loss. The goal was to determine seasonal variability in the amount and patterns of free-living PA in women.
Methods
PA was measured in 57 healthy women from metropolitan Nashville, TN, and surrounding counties (age: 20 to 54 years, body mass index: 17 to 48 kg/m2) using an accelerometer for 7 consecutive days during 3 seasons within 1 year. PA counts and energy expenditure (EE) were measured in a whole-room indirect calorimeter and used to model accelerometer output and to calculate daily EE and intensity of PA expressed as metabolic equivalents (METs).
Results
PA was lower in winter than in summer (131 ± 45 vs. 144 ± 54 × 103 counts/d; P = .025) and in spring/fall (143 ± 48 × 103 counts/d; P = .027). On weekends, PA was lower in winter than in summer by 22,652 counts/d (P = .008). In winter, women spent more time in sedentary activities than in summer (difference 35 min/d; P = .007) and less time in light activities (difference −29 min/d, P = .018) and moderate or vigorous activities (difference −6 min/d, P = .051).
Conclusions
Women living in the southeastern United States had lower PA levels in winter compared with summer and spring/fall, and the magnitude of this effect was greater on weekends than weekdays.
Keywords: accelerometry, prediction of energy expenditure, free-living
In the last decade, the prevalence of overweight and obesity among women in the United States has increased from 42.4% to 52.3%.1 Low levels of physical activity (PA) may have contributed to this increase. Participation in regular PA decreases the risk of cardiovascular disease,2 type 2 diabetes,3 osteoporosis,4 depression,5 obesity,6 breast cancer,7 and colon cancer.8 Despite the numerous benefits of PA9 and the recent attention to specific PA guidelines,10,11 47.9% of women in the United States and only 32.8% of women in the southeastern United States reported regular participation in moderate and vigorous PA outside the workplace.12
PA is the largest variable component of daily total energy expenditure (TEE) and in free-living humans, may vary from day to day,13 seasonally,14,15 and in response to environmental factors.16 It has been suggested that seasonal variation in PA coincides with changes in blood lipid levels, blood pressure, and bone density.17–19 Seasonal variation in leisure-time PA has been described in cross-sectional surveys20 and in small longitudinal studies in homogenous groups.21,22 Most of these studies assessed PA by means of self-report,15,22 pedometry,23 a combination of self-report and accelerometry,15 and a combination of doubly labeled water (DLW) method and accelerometry.24 If PA behavior is related to seasonal climate changes, this should be considered when developing intervention and health promotion efforts designed to increase PA in the general population. Unfortunately, information about the impact of seasons on the amount of PA, PA associated EE, times spent in various PA levels, and interactions between these factors in diverse populations is limited.
The goal of this study was to examine the amount and patterns of free-living PA across seasons in a heterogeneous group of women living in the southeastern United States. Our primary hypothesis was that there would be a significant seasonal influence on the amount, intensity, and EE of free-living PA. Our secondary goal was to explore the relationship between PA and body weight, body fat, and fitness level in our study population.
Research Methods and Procedures
Participants
Sixty-three women, 18 to 55 years of age, were recruited from the Nashville metropolitan area located in Tennessee using advertisements, posters, e-mail, or through informal contacts. They received written information about the nature, purpose, and eligibility criteria of the study and signed informed consent approved by the Institutional Review Board at Vanderbilt University before enrolling into the study. Women were eligible for participation if they were apparently healthy with no evidence of past or present thyroid disorders, diabetes mellitus, heart disease, did not use drugs or supplements known to affect energy metabolism, were not actively participating in a weight loss program, were eating a normal habitual diet, and were nonsmokers.
Protocol
Each participant was studied for 6 to 8 months, and the measurements of PA were recorded during summer (June to August), either fall (September to November) or spring (March to May), and winter (January and February). Fall and spring were used interchangeably based on previous studies showing no significant differences between spring and fall in the amount of PA14,25 and because of similar weather and temperature patterns in the southeastern United States. During the current study, the average daily temperature between December and February (39°F) was lower than the average temperature between June and August (77°F). The average temperature between March and May (59°F) was similar to the temperature between September and November (61°F). The study was conducted during 3 consecutive seasons. Each monitoring period extended over 7 consecutive days, which included 5 to 6 weekdays and 1 to 2 weekend days. The triaxial accelerometer Tritrac-R3D (Reining International, Madison, WI, USA) readings were recorded on a minute-by-minute basis throughout waking hours except for instances when this was not feasible (eg, during showering or swimming). All parameters used to describe the amount of PA (PA counts) were analyzed for the entire experimental period and then were separated into the weekday and weekend categories. Weekday PA was defined as any activity that took place from 7:00 AM Monday through 7:00 AM Saturday. Weekend PA included all activities that took place 7:00 AM Saturday to 7:00 AM Monday.
Health history and a physical examination that included blood pressure and EKG were completed at the baseline visit. Body composition, resting energy expenditure (REE), and maximal oxygen consumption (VO2max) were also measured at that visit. Study visits before the second- and third-season monitoring periods included changes in health history and anthropometrics.
Calculation of Free-Living Physical Activity (PA)
PA was measured in terms of body acceleration in 3 dimensions. The primary outcome was the square root of the raw activity counts, or vector magnitude, defined as the amount of PA (PA counts). PA counts are, thus, independent of individual characteristics such as weight, height, age, body composition, and REE.
Calculation of Free-Living Energy Expenditure (TEE)
A previously validated 2-component power-fitting model was used to individually calibrate accelerometer output from the Tritrac-R3D to EE measured in the room calorimeter.26 Total EE and REE were measured during each participant’s 24-hour stay in a whole-room indirect calorimeter where they followed a structured protocol. In the morning, participants performed three 10-minute walking bouts at various speeds separated by 10-minute rest periods. In the afternoon, they performed three 10-minute stepping bouts at various cadences separated by rest periods. Sedentary activities included typing, sweeping the floor, writing, and viewing TV. All activities except for REE measurements were performed in the fed state. Energy and nutrient needs were calculated based on the individual’s body weight, and food choices were made according to the individual’s preferences. REE was measured in the room calorimeter after an overnight fast and with the participant remaining awake but not arising from the bed for 35 minutes with minimal movement. EE was calculated from oxygen consumption and carbon dioxide production according to Weir’s formula.27 REE was used together with the calculations of EE of PA (total and leisure) to calculate total free-living EE. The model used to estimate EE of PA in free living was
where a, b, p1, and p2 are parameters optimized by a computer modeling program (MatLab v. 8.1, Math-Works Inc, Natick, MA) written specifically for this study. Body motion in the horizontal plane (x- and y-axes) was combined as 1 component (denoted H) and acceleration in the vertical plane (z-axis) as the other component (denoted V). The minimum least-square difference was used to optimize the model.
Patterns of Free-Living Physical Activity (PA)
Time of accelerometer wearing was derived from Tritrac measures of uniaxial acceleration over investigator-specified time intervals, or epochs (1 minute in this study). The computer program was used to determine valid wearing time. Epochs contained within strings of 20 or more minutes of consecutive zeros were eliminated because it was assumed the accelerometer was not worn. The minimum criterion for days worn was 4 days (3 weekdays and 1 weekend day). A minimum of 8 valid hours defined a valid day. The average wearing time for the 57 participants who completed the study was 11.2 hours per day and 5.7 days worn. Patterns of free-living PA were characterized by the time spent on different activities of varying intensities. To calculate the amount of time (min/d) spent in PA of different intensities, minute-by-minute PA counts were classified into 4 categories according to their predicted metabolic equivalents (METs). METs were calculated as the ratio of the minute-by-minute predicted EE for the specific activity and the measured REE. Sedentary activities were classified as activities equal to 1 METs, light activities as 1.1 to 2.9 METs, moderate activities as 3.0 to 5.9 METs, and vigorous activities as more than 6.0 METs.11 In the analyses, moderate and vigorous activities were combined because of low frequency and small amount of PA in the >6 METs category.
Anthropometrics and Body Composition
Body weight was measured to the nearest 0.01 kg with a monthly calibrated digital scale (Detecto-Medic, Detecto Scales, Inc, Northbrook, IL) with the participants wearing light clothing and no shoes. Height was measured using a wall-mounted stadiometer calibrated upon wall installation and recalibrated yearly (Perspective Enterprises, Portage, MI). Body fat and fat free mass were determined with underwater weighing previously described.28 The participants were weighed underwater a minimum of 6 times, and the mean of the 3 heaviest weights was used to calculate body density. Residual lung volume was measured before underwater weighing using the closed-circuit nitrogen dilution technique.29 Total percentage of body fat (%BF) was calculated from body mass density using Siri’s equation for Caucasians and Hispanics or Schutte’s equation for African Americans, and fat mass and fat free mass were calculated from body weight.30,31
Maximal Fitness Testing (VO2max)
Physical fitness was assessed at baseline by measuring the maximal oxygen consumption (VO2max) during the standard Bruce treadmill protocol using metabolic cart (Med Graphics, St. Paul, MN).32 The VO2peak was subjectively determined by each participant as the point of fatigue or by the maximum heart rate (220 – age). The VO2max score is expressed in milliliters per kilogram of body weight per minute (mL/kg/min). Safety monitoring rules during the test were based on the American College of Sports Medicine guidelines.32
Statistical Analysis
All data are presented as means ± SD and ranges. Before proceeding with the main analyses to examine seasonal differences, we tested seasonal differences in time of wearing the monitor for weekdays and weekends using Wilcoxon sign rank tests. Spearman rank correlations were used to test any correlation between time of wearing the monitor and percentage of body fat or age for each season.
For the main analyses, we conducted a longitudinal analysis for each outcome (PA counts and TEE) using generalized estimating equations (GEE)33 to take into account the correlation among repeated measurements obtained from individual subjects over time.
In primary analyses, we examined the differences in the PA counts and the TEE for a whole week, on weekends, and on weekdays between seasons (summer–winter, summer–spring/fall, and spring/fall–winter). To examine the importance of PA in seasonal differences, we tested also for seasonal differences in total time spent on each of the 3-MET categories. The main longitudinal analysis models included VO2max, %BF, and age as potential confounding factors to be adjusted for when the seasonal differences in each outcome were tested. We chose the mean differences as unstandardized measures of effect as recommended by Lenth and Wilkinson et al.34,35 The 95% confidence intervals (CI) were reported for these adjusted analyses. Seasonal changes in body weight and %BF were also analyzed using GEE. All tests were 2-tailed, and a P value of <.05 was considered significant. All analyses were performed using the software STATA 9.2 (StataCorp, College Station, TX) or R (www.r-project.org).
Results
Participant Characteristics
Participants’ baseline characteristics are in Table 1. Body weight, height, BMI, and %BF did not change between consecutive seasons (data not shown, P > .05).
Table 1.
Participant Characteristics
| Variable | Women (n = 57)d mean ± SD (range) |
|---|---|
| Weight (kg)a | 79.5 ± 19.5 (46.8–130.3) |
| Body mass index (kg/m2)a | 29.3 ± 6.8 (17.2–47.9) |
| Body fat (%)a | 39.0 ± 8.1 (21.5–52.4) |
| Height (cm)b | 164.7 ± 6.9 (149.8–182.8) |
| Age (y)b | 36.5 ± 9.2 (20–54) |
| VO2max (mL/kg/min)bc | 28.4 ± 7.6 (15.4–46.7) |
No significant interindividual differences between seasons (P > .05).
Measured at baseline.
Maximal oxygen (O2) consumption during the treadmill test measured at baseline.
41 Caucasian, 14 African American, 2 Hispanic.
Compliance With Measurement Protocol
Sixty-three women enrolled into the study. One became pregnant, 2 moved from the area, and 3 withdrew from the study because of non-study-related factors. The 57 participants who completed the study (41 Caucasian, 14 African American, and 2 Hispanic) were no different (age, weight, BMI) from those who withdrew from the study. The average wearing time of the Tritrac-R3D monitor was 13.1 ± 2.3 hours per day. The duration of wearing the accelerometer was not significantly different between seasons during weekdays or weekends (all seasonal differences P > .05). However, the wearing time was generally longer during weekdays than during weekends (1.6 ± 0.3 h/d in winter, 1.5 ± 0.3 h/d in spring/fall, and 1.2 ± 0.3 h/d in summer; all P < .001). There was no significant correlation between the average daily time of wearing the Tritrac-R3D and %BF or age during weekdays or weekends (all seasons P > .05).
Physical Activity Counts (PA Counts) Between Seasons
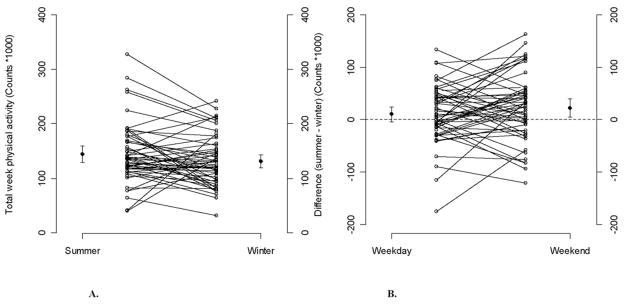
The mean PA counts (Table 2 and Figure 1 A) for the week were lower in winter compared with summer (the mean difference: −15,058 counts/d, 95% CI: −1933 to −28,182, P = .025) and spring/fall (the mean difference: −14,055 counts/d, 95% CI: −1579 to −26,531, P = .027). However, there were no differences between summer and spring/fall (the mean difference: 1003 counts/d, 95% CI: −11,888 to 13,893, P = .879). The mean PA counts on weekends in winter were lower compared with summer (the mean difference: −22,652 counts/d, 95% CI: −5852 to −39,451, P = .008) and slightly lower compared with spring/fall (the mean difference: −15,720 counts/d, 95% CI: 446 to −31,885, P = .057). There was no difference in PA counts between spring/fall and summer (the mean difference: 6932 counts/d, 95% CI: −7149 to 21,014, P = .335). The mean PA counts during weekdays in winter were not different from summer (the mean difference: −12,100, 95% CI: 1313 to −25,514, P = .077) but were lower than in spring/fall (the mean difference: −13,654 counts/d, 95% CI: −108 to −27,199, P = .048). The mean PA counts difference between summer and winter was higher on weekends than on weekdays (Figure 1 B).
Table 2.
Daily Averages of Physical Activity (Activity Counts) and Total Energy Expenditure for the Week and Separately for Weekdays and Weekends From the Raw Data (Unadjusted Analyses)
| Winter | Spring/Fall | Summer | ||||
|---|---|---|---|---|---|---|
| Variable | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range |
| Physical activity (Total counts ×103/d) | ||||||
| weekc | 131 ± 45ab | (32–241) | 143 ± 48 | (48–285) | 144 ± 54 | (39–327) |
| weekdays | 137 ± 52b | (33–252) | 149 ± 50 | (54–316) | 147 ± 56 | (29–327) |
| weekends | 111 ± 50a | (26–314) | 126 ± 60 | (37–322) | 132 ± 59 | (31–327) |
| Total energy expenditure (kcal/d) | ||||||
| weekd | 2505 ± 442a | (1629–3696) | 2529 ± 441 | (1464–3870) | 2571 ± 454 | (1706–3803) |
| weekdays | 2538 ± 461 | (1634–3841) | 2565 ± 452 | (1476–4044) | 2575 ± 470 | (1708–3753) |
| weekends | 2417 ± 440a | (1536–3497) | 2440 ± 435 | (1438–3660) | 2522 ± 454b | (1699–4073) |
Significantly different from summer in the adjusted analysis (P < .05).
Significantly different from spring/fall in the adjusted analysis (P < .05).
Mean of weighted daily physical activity averages (Total counts × 103/d) calculated by dividing total number of counts per week by a sum of monitored weekdays (3 to 5) and weekend days (1 to 2).
Mean of weighted daily total energy expenditure averages (kcal/d) calculated by dividing total weekly energy expenditure (kcal/wk) by a sum of monitored weekdays (3 to 5) and weekend days (1 to 2).
Figure 1.
Individual plots for total unadjusted physical activity(PA) (counts/d × 103) (A) during summer and winter; (B) difference in physical activity (counts/d × 103) between summer and winter during weekday and weekends along with the means and the corresponding 95% confidence intervals. Dots are the means of PA during summer or winter or the mean difference in PA between summer and winter, and bars represent the corresponding 95% confidence intervals.
Total EE Between Seasons
Mean TEE (Table 2) was lower in the winter compared with summer (mean difference: −58 kcal/d, 95% CI: −7 to −109 kcal/d, P = .025). However, there was no difference between spring/fall and summer (P = .366) and spring/fall and winter (P = .192). On weekends, TEE was lower in the winter compared with summer (the mean difference: −96 kcal/d, 95% CI: −26 to −166 kcal/d, P = .007) and was lower in spring/fall than in summer (the mean difference: −66 kcal/d, 95% CI: −1 to −132 kcal/d, P = .048). However, there was no difference between winter and spring/fall (P = .343). The mean TEE on weekdays was slightly lower during the winter compared with summer (the mean difference: −49 kcal/d, 95% CI: 4 to −102 kcal/d, P = .072). There were no differences between summer and spring/fall (P = .670) and between spring/fall and winter (P = .182). Individual plots for PA in summer and winter and the differences in PA between summer and winter during weekdays and weekends are illustrated in Figure 1.
PA Time Between Seasons
During the winter and spring/fall, participants spent more time in sedentary activities (MET <1) than in the summer (the mean difference: 35 min/d, 95% CI: 11 to 71 min/d, P = .007 and 38 min/d, 95% CI: 5 to 77 min/d, P = .025, respectively; Table 3). During winter, study participants spent less time in light (1.1 to 2.9 METs) PA category activities (the mean difference: −29 min/d, 95% CI: −62 to −6 min/d, P = .018) and moderate/vigorous (>3.0 METs) PA category activities (the mean difference: −6 min/d, 95% CI: 15 to 0, P = .051) than during summer. Our study participants also spent less time in moderate/vigorous (>3.0 METs) PA category activities in winter than in spring/fall (the mean difference: −9 min/d, 95% CI: −17 to −2 min/d, P = .009). We observed similar patterns in time spent in all PA intensity categories on weekdays and on weekends. Time spent in moderate and vigorous (>3.0 METs) activities was lower on weekends than on weekdays in all seasons (P < .05).
Table 3.
Daily Averages of Time Spent on Various Activity MET Categories During the Week and Separately on Weekdays and Weekends in Seasons From the Raw Data (Unadjusted Analyses)
| Winter (min/d) | Spring/Fall (min/d) | Summer (min/d) | ||||
|---|---|---|---|---|---|---|
| Activity | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range |
| Weekc | ||||||
| Sedentary (≤1 MET) | 675 ± 104a | (498–941) | 678 ± 115 | (503–1027) | 640 ± 100b | (400–868) |
| Light (1.1–2.9 METs) | 714 ± 104a | (462–895) | 702 ± 110 | (396–893) | 743 ± 93b | (565–1015) |
| Moderate + Vigorous (>3 METs) | 49 ± 29b | (3–162) | 58 ± 28 | (7–134) | 55 ± 29 | (4–173) |
| Weekdays | ||||||
| Sedentary (≤1 MET) | 649 ± 112a | (474–892) | 655 ± 126 | (425–1025) | 616 ± 105b | (370–825) |
| Light (1.1–2.9 METs) | 738 ± 108 | (507–929) | 721 ± 122 | (388–922) | 764 ± 99b | (595–1052) |
| Moderate + Vigorous (>3 METs) | 52 ± 32b | (1.2–143) | 63 ± 30 | (5.8–150) | 58 ± 30 | (5.4–179) |
| Weekends | ||||||
| Sedentary (≤1 MET) | 753 ± 150a | (545–1139) | 748 ± 175 | (399–1222) | 703 ± 152 | (335–1064) |
| Light (1.1–2.9 METs) | 646 ± 145 | (284–860) | 645 ± 166 | (197–964) | 686 ± 139 | (349–948) |
| Moderate + Vigorous (>3 METs) | 40 ± 39a | (0–229) | 45 ± 36 | (0–177) | 49 ± 34 | (0–158) |
Significantly different from summer in the adjusted analysis (P < .05).
Significantly different from spring/fall in the adjusted analysis (P < .05).
Mean of weighted average time (min) spent daily in sedentary, light, and moderate + vigorous physical activity (MET) calculated by dividing total weekly time (min/wk) spent in this activity category by a sum of monitored weekdays (3 to 5) and weekend days (1 to 2).
Association Between PA and VO2max and Body Fat Percentage
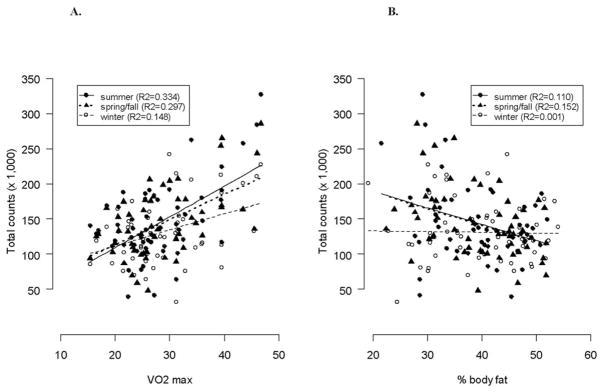
PA (counts/d) was significantly and positively associated with VO2max (P < .001; Figure 2). The slope representing the association between PA counts and VO2max was lower during winter than during spring/fall and summer (P = .042 and P = .041, respectively). There was a negative association between PA and %BF, but it was not statistically significant (P = .104). The absolute slopes representing the relationship between PA and %BF were lower in winter than summer and spring/fall (P = .062, R2 = .110 and P = .051, R2 = .152, respectively).
Figure 2.
Scatterplots of total week physical activity (raw activity counts × 103) and (A) maximal oxygen consumption (VO2max [L/kg]) and (B) body fat (%) during seasons in women.
Discussion
This study examined detailed seasonal variations in PA using objective measurements in a relatively large group of women in this region that has relatively low levels of PA and higher rates of obesity than other regions of the United States.12 The main finding from this study is that there are significant differences in the amount and patterns of PA between winter and other seasons in young and middle-age women in the southeastern United States. This effect was evident on both weekends and weekdays, but the magnitude of the effect was greater on weekends. Although the group mean followed this seasonal trend, perhaps an interesting finding is that some women decreased or did not change their amount of PA between winter and other seasons. Moreover, most participating women (75%) did not perform any vigorous activity in any season, and only 7% spent any time in vigorous activities (>6 METs) in all seasons.
Participants performed less PA during winter than summer and spring/fall, and this difference was more visible on weekends than weekdays. More specifically, PA counts were about 9% lower in winter than in summer. This difference between seasons could be related to many factors including environmental factors such as weather and number of daylight hours but cannot be established based on results of our study. There are other data to support the notion of seasonal variation in PA. In the Framingham Offspring Study, Dannenberg et al25 found that women and men expended more energy in PAs in the summer than the winter (P < .001). Data from the Behavioral Risk Factor Surveillance System also show seasonal variation; the percentage of adults reporting no participation in leisure-time PA was highest in the winter (about 34%) and lowest in the summer (about 25%).10 In the third National Health and Nutrition Examination Survey (NHANES III), approximately 60% of the respondents reported similar activity patterns for the past month as compared with the previous 12 months, whereas the remaining 40% reported different activity patterns.36 The authors suggested that differences reported in the prevalence of inactivity may have been the result of seasonal variation.36
When expressed as total EE, the difference between these 2 seasons (58 kcal/d) was more pronounced on weekends (96 kcal/d) than on weekdays (49 kcal/d). We also observed a similar trend but smaller difference between winter and spring/fall on both weekdays (36 kcal/d) and weekends (30 kcal/d). Our results are comparable to those from a study by Matthews and colleagues15 relying on self-reports of PA conducted in the northeastern United States. In their study of 580 adults who reported their PA, men (n = 300) reported a 121 kcal/d increase in PA in the summer. Women (n = 280) reported a 70 kcal/d summer PA increase. Household and leisure-time activity, particularly on weekend days, contributed substantially to the overall effect. Our study is also in agreement with a study by Levin et al,14 in which 77 healthy adults wore a Caltrac accelerometer for 48 hours every 26 days for 1 year. The results showed a significant seasonal trend for more activity in the summer compared with winter.
During the winter, women in our study spent significantly more time (41 min/d) in sedentary activities (<1 MET) than during the summer. This significant difference was compensated by less time spent in light, moderate, and vigorous PA intensity categories. In our analyses, we combined the moderate and vigorous PA categories because only 9 participants (16%) performed activity in the vigorous PA category on weekdays and 6 (11%) on weekends. Most participants (75%) did not perform any vigorous activity in any season. In contrast, only 4 participants (7%) spent time in the vigorous PA category in all seasons. Analysis that is more detailed showed that the average time spent on vigorous activity was 5.6 and 10.2 min/d for weekdays and weekends, respectively. This pattern is similar to the pattern observed in our previous study of 120 men and women in which women spent most of their active time on light-level PA (10–12 h/d) and moderate PA (1 h/d).37 Women in that study spent on average of 6.4 minutes in vigorous (>6 METs) activities. In the study by Van Staveren et al,22 114 young women were assessed monthly 14 times by a 24-hour PA recall reflecting time spent in 8 PA categories but not calculating EE. During spring and summer, they spent slightly more time in walking and sports than in winter (17 minutes) and less time in sitting (15 minutes). Furthermore, these authors also found no significant variation in vigorous leisure-time activity between seasons.
Our data showing larger seasonal differences during weekends than weekdays suggest that participants in our study tend to be more active on weekends in summer, but not in winter or spring/fall. Analysis that is more detailed shows that the variability across participants in the seasonal differences was greater during weekends compared with weekdays. This observed high interindividual variation in PA patterns change between seasons is well illustrated in Figure 1. These results confirm the results of Pivarnik et al38 who reported a relatively high interseasonal variability of calculated leisure-time EE of PA. The authors determined the effect of seasons on self-reported PA in 2843 adults and found that EE of PA was 15% to 20% lower in winter when compared with spring and summer.
In our study, we found a significant relationship between VO2max and PA in participants. This suggests that women who were more physically fit performed more PA than their less-fit counterparts. This is in agreement with our previous observations and other studies.16,37,39 We also analyzed the association between PA and %BF. Although not significant (P = .101) in all seasons, there was a slight trend for PA to be associated with %BF in summer and spring/fall but not in winter. This finding is in agreement with our previous findings and findings from other reports.37,40,41 The overall trend in the relationship between body weight and the amount of PA observed in our study is also in agreement with Hemmingsson and Ekelund,42 who in a recent study have shown that the relations between body mass index (BMI) and PA depend on BMI.
Our study has some limitations that have to be considered when interpreting the results. The first limitation is an assumption that average daily PA counts used in a consequent calculation of TEE reflected all physical activities performed by the individual. PA is a more complex behavior than can be assessed accurately using accelerometer. Accelerometers, especially triaxial, are considered a relatively objective measure of PA under free-living conditions.43–45 However, it is known that they do not adequately measure body movements of upper and lower extremities when the center of body mass (waist) remains motionless, such as in cycling or rowing. Other underestimations include activities such as walking uphill because ground slope gradients cannot be detected.
To minimize the prediction error, we individually calibrated the monitor for various forms of PA using a previously validated whole-room indirect calorimeter.26 It is possible, however, that energy predicted was different from actual EE of PA spent by our study participants due to the errors in prediction inherent to such models. In a recent study, however, Plasqui et al39 showed that triaxial accelerometer output modeled using sleeping metabolic rate and body composition explained 90% in TEE variation. An additional potential limitation is that we measured VO2max only at baseline. However, it has been shown that in adult women and men, physical fitness is not different between summer and winter.39 The VO2max test used a subjective assessment of exhaustion that was not supported by physiological data. Therefore, it is possible that some participants did not reach their peak effort. The small sample could be considered a limitation, but it could also be considered a strength because it allowed collection of detailed, individual-level data. The amount of monitor wear time in our study (13.1 h/d) was about 0.8 h/d less than the average monitor wear time reported in a recent national (NHANES IV) survey.46 Thus, our estimates of time spent in sedentary and light activities are likely to underestimate the actual time spent in these behaviors. Another potential limitation may be that our study population is not a good representation of women in general. Our participants volunteered to participate in the study and may have different attitudes of PA compared with other women. The extent that such factors may have influenced our findings is unknown and may limit generalizability of this study.
Finally, another possible confounder may be related to the study environment. The southeastern United States has usually mild winters and relatively hot summers. It is known that extreme environmental factors (eg, humidity and high or low temperatures) can modify PA behaviors. Therefore, our findings may be applicable more to regions with environmental conditions similar to those of the southeastern United States than to other regions.
In summary, we found that women living in the southeastern United States had a lower amount of PA performed in the winter compared with summer and spring/fall, and the magnitude of this effect was greater on weekends than on weekdays. The decrease in PA was associated with an increase in sedentary behaviors and a decrease in both light and moderate/vigorous PA intensity on weekends. These results suggest that seasonal changes are affected mostly by fluctuations in PA performed during leisure time. Our findings may influence guidance for clinical programs that include moderate PA as a tool to achieve or sustain weight loss. These programs may recommend an increase in PA on weekends and during winter.
Acknowledgments
We are indebted to our participants for their time and commitment. We thank Vanderbilt University CRC staff for help with conducting this study. This study was supported in part by the Vanderbilt Institute for Clinical and Translational Research (VICTR) grant 1UL1 RR024975 from NCRR/NIH and DK20593. MSB was supported by DK69465 and CA92447.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PD, Buchner D, Pina IL, et al. exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (subcommittee on exercise, rehabilitation, and prevention) and the Council on Nutrition, Physical Activity, and Metabolism (subcommittee on physical activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vuori I. Health benefits of physical activity with special reference to interaction with diet. Public Health Nutr. 2001;4(2B):517–528. doi: 10.1079/phn2001137. [DOI] [PubMed] [Google Scholar]
- 5.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 6.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21(1):323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 7.Breslow RA, Ballard-Barbash R, Munoz K, Graubard BI. Long-term recreational physical activity and breast cancer in the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Cancer Epidemiol Biomarkers Prev. 2001;10(7):805–808. [PubMed] [Google Scholar]
- 8.Slattery M, Potter J. Physical activity and colon cancer: confounding or interaction? Med Sci Sports Exerc. 2002;34(6):913–919. doi: 10.1097/00005768-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Marcus BH, Williams DM, Dubbert PM, et al. Physical activity intervention studies: what we know and what we need to know: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (subcommittee on physical activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation. 2006;114(24):2739–2752. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Dept of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 11.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2005. [Google Scholar]
- 13.Lakka TA, Salonen JT. Intra-person variability of various physical activity assessments in the Kuopio Ischaemic Heart Disease Risk Factor Study. Int J Epidemiol. 1992;21(3):467–472. doi: 10.1093/ije/21.3.467. [DOI] [PubMed] [Google Scholar]
- 14.Levin S, Jacobs DR, Ainsworth BE, Richardson MT, Leon AS. Intra-individual variation and estimates of usual physical activity. Ann Epidemiol. 1999;9(8):481–488. doi: 10.1016/s1047-2797(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 15.Matthews CE, Freedson PS, Hebert JR, et al. Seasonal variation in household, occupational, and leisure time physical activity: longitudinal analyses from the Seasonal Variation of Blood Cholesterol Study. Am J Epidemiol. 2001;153(2):172–183. doi: 10.1093/aje/153.2.172. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson G, Drust B. Seasonal rhythms and exercise. Clin Sports Med. 2005;24(2):e25–e34. doi: 10.1016/j.csm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Manttari M, Javela K, Koskinen P, et al. Seasonal-variation in high-density-lipoprotein cholesterol. Atherosclerosis. 1993;100(2):257–265. doi: 10.1016/0021-9150(93)90212-d. [DOI] [PubMed] [Google Scholar]
- 18.Robinson D, Bevan EA, Hinohara S, Takahashi T. Seasonal-variation in serum-cholesterol levels - evidence from the UK and Japan. Atherosclerosis. 1992;95(1):15–24. doi: 10.1016/0021-9150(92)90171-c. [DOI] [PubMed] [Google Scholar]
- 19.Mundal R, Kjeldsen S, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Seasonal covariation in physical fitness and blood pressure at rest and during exercise in healthy middle-aged men. Blood Press. 1997;6(5):269–273. doi: 10.3109/08037059709062081. [DOI] [PubMed] [Google Scholar]
- 20.Uitenbroek D. Seasonal variation in leisure time physical activity. Med Sci Sports Exerc. 1993;25(6):755–760. [PubMed] [Google Scholar]
- 21.Haggarty P, McNeill G, Abumanneh MK, et al. The influence of exercise on the energy-requirements of adult males in the UK. Br J Nutr. 1994;72(6):799–813. doi: 10.1079/bjn19940086. [DOI] [PubMed] [Google Scholar]
- 22.Van Staveren W, Deurenberg P, Burema J, De Groot L, Hautvast J. Seasonal variation in food intake, pattern of physical activity and change in body weight in a group of young adult Dutch women consuming self-selected diets. Int J Obes. 1986;10(2):133–145. [PubMed] [Google Scholar]
- 23.Lee CJ, Lawler GS, Panemangalore M, Street D. Nutritional status of middle-aged and elderly females in Kentucky in two seasons: Part 1. Body weight and related factors. J Am Coll Nutr. 1987;6(3):209–215. doi: 10.1080/07315724.1987.10720183. [DOI] [PubMed] [Google Scholar]
- 24.Plasqui G, Joosen AMCP, Kester AD, Goris AHC, Westerterp KR. Measuring free-living energy expenditure and physical activity with triaxial accelerometry. Obes Res. 2005;13(8):1363–1369. doi: 10.1038/oby.2005.165. [DOI] [PubMed] [Google Scholar]
- 25.Dannenberg AL, Keller JB, Wilson PWF, Castelli WP. Leisure time physical activity in the Framingham Offspring Study: description, seasonal variation, and risk factor correlates. Am J Epidemiol. 1989;129(1):76–88. doi: 10.1093/oxfordjournals.aje.a115126. [DOI] [PubMed] [Google Scholar]
- 26.Chen KY, Sun M. Improving energy expenditure estimation by using a triaxial accelerometer. J Appl Physiol. 1997;83(6):2112–2122. doi: 10.1152/jappl.1997.83.6.2112. [DOI] [PubMed] [Google Scholar]
- 27.Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behnke A, Wilmore J. Evaluation and Regulation of Body Build and Composition. Englewood Cliffs, NJ: Prentice Hall; 1974. [Google Scholar]
- 29.Wolfe W, Carlson L. Studies of pulmonary capacity and mixing with the nitrogen meter. J Clin Invest. 1950;47:1568–1575. doi: 10.1172/JCI102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schutte JE, Townsend EJ, Hugg J, Shoup RF, Malina RM, Blomqvist CG. Density of lean body mass is greater in blacks than in whites. J Appl Physiol. 1984;56(6):1647–1649. doi: 10.1152/jappl.1984.56.6.1647. [DOI] [PubMed] [Google Scholar]
- 31.Siri W. Body composition from fluid spaces and density: analysis of methods. In: Brozek J, Henschel A, editors. Techniques for Measuring Body Composition. Washington, DC: National Academy of Sciences; 1961. pp. 223–244. [Google Scholar]
- 32.American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 33.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrica. 1986;73:13–22. [Google Scholar]
- 34.Lenth R. Some practical guidelines for effective sample size determination. Am Stat. 2001;55:187–193. [Google Scholar]
- 35.Wilkinson L APA Task Force on Statistical Inference. Statistical methods in psychology journals: guidelines and explanations. Am Psychol. 1999;54:594–604. [Google Scholar]
- 36.Crespo CJ, Keteyian S, Heath G, Sempos C. Leisure time physical activity among US adults: results from the third National Health and Nutrition Examination Survey. Arch Intern Med. 1996;156:266–275. [PubMed] [Google Scholar]
- 37.Buchowski M, Acra S, Majchrzak K, Sun M, Chen K. Patterns of physical activity in free-living adults in the Southern United States. Eur J Clin Nutr. 2004;58(5):828–837. doi: 10.1038/sj.ejcn.1601928. [DOI] [PubMed] [Google Scholar]
- 38.Pivarnik JM, Reeves MJ, Seasonal APR. Variation in adult leisure-time physical activity. Med Sci Sports Exerc. 2003;35(6):1004–1008. doi: 10.1249/01.MSS.0000069747.55950.B1. [DOI] [PubMed] [Google Scholar]
- 39.Plasqui G, Westerterp KR. Seasonal variation in total energy expenditure and physical activity in Dutch young adults. Obes Res. 2004;12(4):688–694. doi: 10.1038/oby.2004.80. [DOI] [PubMed] [Google Scholar]
- 40.Tucker LA, Peterson TR. Objectively measured intensity of physical activity and adiposity in middle-aged women. Obes Res. 2003;11(12):1581–1587. doi: 10.1038/oby.2003.210. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein MS, Costanza MC, Morabia A. Association of physical activity intensity levels with overweight and obesity in a population-based sample of adults. Prev Med. 2004;38(1):94–104. doi: 10.1016/j.ypmed.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 42.Hemmingsson E, Ekelund U. Is the association between physical activity and body mass index obesity dependent? Int J Obes. 2006;31(4):663–668. doi: 10.1038/sj.ijo.0803458. [DOI] [PubMed] [Google Scholar]
- 43.Kochersberger G, McConnell E, Kuchibhatla MN, Pieper C. The reliability, validity, and stability of a measure of physical activity in the elderly. Arch Phys Med Rehabil. 1996;77(8):793–795. doi: 10.1016/s0003-9993(96)90258-0. [DOI] [PubMed] [Google Scholar]
- 44.Westerterp K, Bouten C. Physical activity assessment: comparison between movement registration and doubly labeled water method. Z Ernahrungswiss. 1997;36(4):263–267. doi: 10.1007/BF01617795. [DOI] [PubMed] [Google Scholar]
- 45.Chen KY, Acra SA, Majchrzak K, et al. Predicting energy expenditure of physical activity using hip- and wrist-worn accelerometers. Diabetes Technol Ther. 2003;5(6):1023–1033. doi: 10.1089/152091503322641088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. doi: 10.1093/aje/kwm390. [published online ahead of print February 25, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]