Abstract
Objective
To examine the effects of ACL transection (ACLT) in a rat model on lubricin metabolism and its relationship to markers of inflammation and cartilage damage, and to determine if blocking the metabolic effects of tumor necrosis factor-alpha (TNF-α), by etanercept increases chondroprotection provided by lubricin.
Methods
Unilateral ACLT was performed in Lewis rats. Synovial fluid (SF) lavage levels of lubricin, TNF-α, IL-1, synovial tissue lubricin gene expression, and sulfated glycoaminoglycans (sGAG) were evaluated at 1 and 4 weeks following ACLT. Histological evaluation of articular cartilage included staining with lubricin-specific monoclonal antibody 9G3, and Safranin O. The percentage lubricin staining on the surface of articular cartilage in weight-bearing areas was estimated by digital imaging. Blocking of TNF-α was performed by etanercept, administered subcutaneously at 0.5 mg/kg around the ACLT joints using different dosing strategies. Knee joints were harvested 4 weeks after ACLT.
Results
Four weeks following ACLT, SF lubricin concentrations and the percentage surface lubricin staining were significantly lower in the injured joints compared to the contralateral joints. A significant decrease in the synovial tissue lubricin gene expression was associated with elevated TNF-α and IL-1β concentrations in SF lavages. Blocking of TNF-α significantly increased the lubricin bound to cartilage for all etanercept treatment strategies, coupled with a significant decrease in sGAG release. However, there were variable changes in SF lubricin concentrations.
Discussion and Conclusions
Blocking TNF-α resulted in a chondroprotective effect, exemplified by increased lubricin deposition on articular cartilage and a decrease in sGAG release from articular cartilage in a post-traumatic arthritis animal model.
Keywords: ACL Injury, Lubricin, TNF-α, cartilage damage
Introduction
Acute anterior cruciate ligament (ACL) injury is a significant risk factor for the development of secondary osteoarthritis (OA)1,2. Many contributing factors such as joint instability, changes in kinematics, and tissue degradative pathways are postulated to play a significant role in the pathogenesis of OA following injury. Recently, we reported that synovial fluid (SF) lubricin3–5 concentrations in injured joints from patients with ACL injury were significantly lower than SF lubricin concentrations in uninjured contralateral joints6. Animal models of meniscectomy7, medial collateral ligament and ACL disruption8, and adjuvant induced arthritis9 have all showed an association of cartilage degeneration with a decrease in either SF or cartilage bound lubricin. Lubricin gene expression is differentially regulated by a variety of cytokines including tumor necrosis factor-alpha (TNF-α)10, interleukin-1 (IL-1)11, transforming growth factor-beta (TGF-β)12, and by bone morphogenetic protein-7 (BMP-7)13, which can impact chondroprotection following recovery from an ACL injury6.
There were two objectives of this study. First was to establish the early effects of acute ACL injury on lubricin metabolism and the resultant effects on cartilage integrity and lubrication in the rat model. Second was to determine if treatment with etanercept, a soluble TNF-α inhibitor, could preserve lubricin and cartilage integrity. We hypothesized that early inhibition of TNF-α would increase lubricin concentrations in the SF and on the surface of articular cartilage providing better chondroprotection. Synovial fluid lubricin lavage concentrations, whole joint coefficient of friction, sulfated glycosaminoglycans (sGAG) and lubricin deposition on the surface of articular cartilage, as measured by histological staining, were measured at 1 and 4 weeks following ACL transection (ACLT) and following etanercept administration. Etanercept was administered: A) every other day immediately following ACLT, B) on post-operative days 7 & 14 and C) on post-operative days 14 & 21. Synovial tissue lubricin gene expression in ACL transected and contralateral (CL) joints, SF lavage TNF-α, and IL-1 concentrations were determined in ACL transected and CL joints at 1 and 4 weeks following ACLT. These measurements were correlated with lubricin SF lavage concentrations, synovial tissue lubricin gene expression, and intensity of lubricin staining on the surface of articular cartilage.
Methods
ACL transection (ACLT) animal model
Twelve male Lewis rats 7–8 weeks of age were assigned to an ACLT group, and another 12 were assigned to a sham group that underwent capsulotomy without disturbing the ACL. In the ACLT group, 6 animals were harvested at 1 week and 6 animals were harvested at 4 weeks. Similarly, 6 animals in the sham group were harvested at 1 week and 6 animals were harvested at 4 weeks. After the animals were anesthetized with intraperitioneal ketamine and xylozine, the skin was prepped with a topical antiseptic, and an incision was made in the skin laterally to the right knee joint. After the joint capsules were opened, the ACL was transected using a surgical scalpel. In all animals, the right knee joint underwent surgery and the left knee was the CL joint. Sham surgery was performed in the same manner as the ACLT surgery without transecting the ACL. All experimental procedures were approved by the Rhode Island Hospital Animal Welfare Committee.
Etanercept dosing
In a separate set of experiments, 24 animals underwent ACLT as described above. Eighteen animals received etanercept according to three dosing strategies (6 in each group) and 6 did not receive etanercept. The ACLT and CL joints were harvested 4 weeks following surgery. Etanercept was given at a dose of 0.5 mg/kg by subcutaneous injection around the ACLT joints. Dosing groups included three different treatment regimens: A, B, and C. Treatment A animals received early and high doses of etanercept administered 1,3,5,7,9,11, and 13 days following ACLT. Treatment B dosing was performed only on days 7 and 14 following ACLT, while treatment C dosing was performed only on days 14 and 21. The differences between ACLT and CL limbs of etanercept treated animals was compared to that of the 4 week ACLT animal group as a measure of the effect of etanercept.
Histological processing
Paraffin-embedded coronal sections were taken from weight-bearing areas of the articular cartilage of ACLT and CL joints of animals described above. Histological staining was performed using lubricin-specific monoclonal antibody 9G3 (provided by M. Warman) at 1:1,000 dilutions and by Safranin O/Fast Green stain for qualitative assessment of cartilage sGAG content.
Analysis of lubricin bound to the surface of articular cartilage
Representative sections of the ACLT and CL joints, stained with monoclonal antibody 9G3, were imaged by an Olympus BX51 system (Olympus, Center Valley, PA, USA) using 200× magnification. Images were captured using Image Pro-Plus software (Media Cybernetics, MD, USA) with pre-determined threshold parameters for lubricin staining. Regions of interest included surfaces of the tibial plateau and the femoral condyle cartilage between the tip of the meniscus and the origin of the cruciate ligaments in both joint compartments. The length of a contour drawn to follow the surface of the articular cartilage in the region of interest was calculated. The segmental length of lubricin immunopositivity on that same contour was calculated using Image Pro Plus and expressed as a percentage of its total length of normal histologic immunostaining.
Determination of whole joint coefficient of friction
The whole joint coefficient of friction was determined by the modified Stanton pendulum as previously described9. The whole joint coefficient of friction was determined for ACLT and CL joints of 1 and 4 weeks animals as well as etanercept-treated animals.
SF lavage lubricin concentrations
Joints were lavaged by injecting 100 µl of normal saline in the joint capsule, then aspirating 30 µl of fluid. A sandwich ELISA using peanut agglutinin (PNA) (EY Labs, San Mateo, CA, USA) and monoclonal antibody 9G3 was used to quantitiate SF lavage lubricin concentrations. High binding 96 well plates (Costar, Cole-Parmer, Vernon Hills, IL, USA) were coated overnight with PNA in 50 mM sodium bicarbonate buffer, pH 9.5 at a final concentration of 10 µg/ml. 24 hours following coating, serial dilutions of purified human lubricin6 and diluted lavaged SF samples were incubated on the PNA-coated plates for 60 minutes at room temperature. The plate was then washed with phosphate buffered saline (PBS) + 0.1% Tween 20. Subsequently, 9G3 was added at 1:10,000 dilutions and incubated for 60 minutes at room temperature, followed by washing with PBS+0.1% Tween 20. Goat anti-mouse IgG-peroxidase (Invitrogen, Carlsbad, Ca, USA) was added at 1;1,000 dilution to the plate for 60 minutes, followed by washing with PBS + 0.1% Tween 20 and lastly PBS alone. Finally, TMB reagent was added (Pierce, Rockford, IL, USA), and the absorbance was measured at 450 nm.
Quantification of sulfated glycosaminoglycan (sGAG), IL-1β and TNF-α in SF lavages
Quantification of the sGAG concentrations in SF lavages of the ACLT and CL joints were performed using Alcian Blue dye binding assay (Alpco Diagnostics, Windham, NH, USA). The assay is based on the formation of an insoluble blue colored complex between sGAG and Alcian blue, which can be quantified by absorbance at 590 nm. The sGAG concentrations were determined using serially diluted chondroitin sulfate-6 as a standard. Quantification of TNF-α and IL-1β in rat SF lavages was performed using commercially available ELISA kits (Invitrogen, Carlsbad, CA, USA). The reported minimum detection limits were 0.3, and 0.1 pg/ml, respectively.
Isolation of total RNA from synovial tissues from ACLT and CL joints and real-time quantitative PCR assay
Synovial tissue was removed from ACLT and CL joints at 1 and 4 weeks following ACLT (n=6 at each time point of ACLT and CL joints) and was quickly snap-frozen in liquid nitrogen and stored at −80°C. Isolation of total RNA, conversion to cDNA and quantitative PCR of lubricin gene expression using primers complimentary for exon 6 of the mucin domain in accession #NW 047397 in the National Center for Biotechnology Information (Bethesda, MD) was performed as previously described9.
Data analysis
All statistical analyses were conducted using SAS version 9.1.3 (service pack 4, SAS Institute, Cary, NC, USA). The distributions of several variables were positively skewed and so a logarithmic (base 2) transformation of the coefficient of friction, sGAG, IL-1, and TNF-α was applied prior to analysis. Values, means, and confidence intervals were back translated for presentation.
Effects were tested by comparing the group differences in the ACLT to CL limb differential to adjust for overall differences between animals. Using the CL limb as a reference within animal removed any innate differences between animals or day-to-day variation in instrumentation, decreasing the potential for bias and increasing statistical power. These comparisons were conducted using mixed linear models with correlated errors, similar to repeated measures ANOVA, containing fixed effects for group, limb (correlated errors within rat), and their interaction. An unstructured variance-covariance matrix was used for the correlated errors and the model was fit using residual estimation of maximum likelihood. The Kenwood-Rogers method was used to calculate denominator degrees of freedom. Least-squares means within groups and their 95% confidence intervals are reported. Where data from CL limbs were not available, these models were reduced to standard ANOVA or regression. Planned comparisons were conducted using orthogonal contrasts to test the limb differentials within each group against zero, as well as comparing the differentials between all possible pairings of groups. Family wise alpha was maintained at 0.05 across all planned comparisons using the Holm test.
The relationship between observed measures of lubricin and coefficient of friction were also tested in correlated error (within animal across limbs) mixed linear models. Initially, a fixed effect for limb and its interaction with the lubricin covariate was run. If the interaction term was statistically significant, the relationships were tested within ACLT and CL limbs individually. If the interaction was not statistically significant, the fixed effects of limb and the interaction of limb with the covariate were removed leaving only the fixed effect of lubricin and the correlated error within animal.
Results
ACLT model in rats and effects on lubricin metabolism
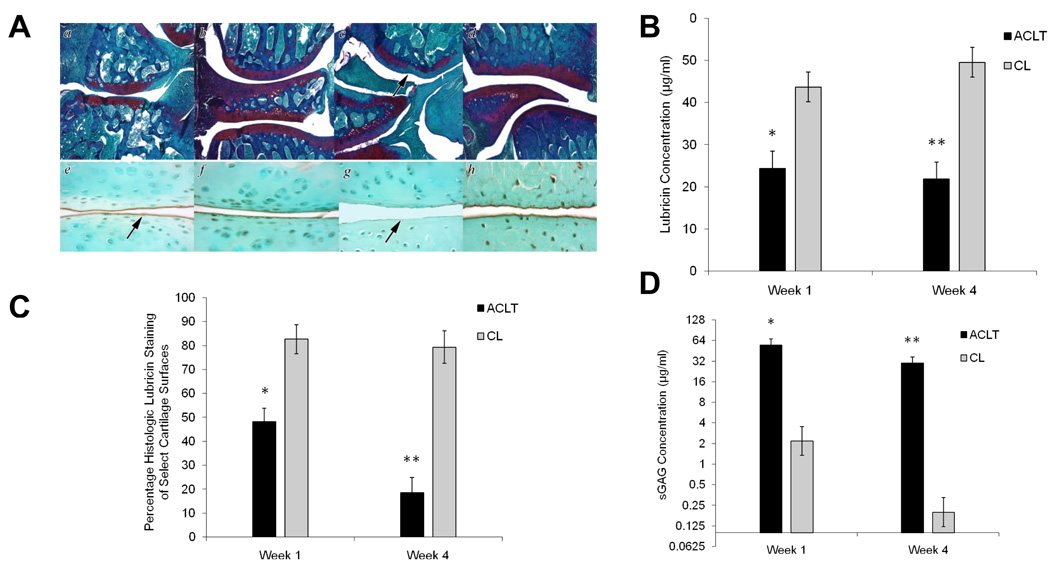
Safranin O and lubricin immunostaining of a representative ACLT and CL joint at 1 and 4 weeks following ACLT is depicted in Figure 1A. The ACLT and CL joints exhibited staining for proteoglycans (Fig 1, panels a and b respectively) at 1 week following ACLT. Lubricin immunostaining in the ACLT joint at 1 week following ACLT exhibited immunopositivity on the surface of articular cartilage, with reduced staining in the superficial zone of articular cartilage (Panel e). The CL joint at 1 week following ACLT exhibited immunopositivity on the surface of articular cartilage and in the superficial zone of articular cartilage (Panel f). The ACLT joints at 4 weeks showed decreased proteoglycan staining in the ACLT joint (Panel c) compared to the CL joint (Panel d). There was no lubricin immunostaining, either on the surface of articular cartilage or in the superficial zone of articular cartilage in the ACLT joint at 4 weeks following ACLT (Panel g). By contrast, the CL joint exhibited positive lubricin immunostaining on the articular cartilage surface and superficial zone (Panel h).
Figure 1.
Anterior cruciate ligament transection (ACLT) rat model at 1 and 4 weeks following transection: A Safranin O (a – d) and lubricin immunostaining using lubricin-specific monoclonal 9G3 (e – h) of ACLT and CL joints from representative rats at 1 and 4 weeks following ACLT. a;e: ACLT joints at 1 week, b;f: CL joints at 1 week, c;g: ACLT joint at 4 weeks and d;h: CL joint at 4 weeks. Arrow points to decreased proteoglycan staining in ACLT joint at 4 weeks (c), lubricin staining (arrow) on the surface of articular cartilage in ACLT joint at 1 week (e), and lack of lubricin staining (arrow) on the surface of articular cartilage in ACLT joint at 4 weeks (g). B Synovial fluid (SF) lavage lubricin concentrations in ACLT and CL joints at 1 and 4 weeks following transection. C Percentage lubricin staining of select cartilage surfaces in ACLT and CL joints at 1 and 4 weeks following transection. D Synovial fluid (SF) lavage sulfated glycosaminoglycan (sGAG) concentrations in ACLT and CL joints at 1 and 4 weeks following transection. A single asterisk (*) denotes significance (p<0.05) at 1 week between ACLT and CL, two asterisks (**) denotes significance (p<0.001) at 4 weeks between ACLT and CL.
SF lavage lubricin concentration of the ACLT joint of week 1 was significantly lower (adj. p<0.001) compared with SF lavage lubricin concentration in the CL joint of week 1 (Fig 1B). The SF lavage lubricin concentration of the ACLT joint of week 4 was significantly lower (adj. p<0.001) than the SF lavage lubricin concentration of the CL joint of week 4. There was no significant difference between weeks after adjustment for multiple comparisons (adj. p = 0.0605). Animals at each time of sacrifice exhibited larger effects than their sham controls (adj. p < 0.001 for both), neither of which exhibited significant differences between limbs. The percentage of lubricin staining on the surface of select cartilage areas of ACLT and CL joints at 1 and 4 weeks is represented in Figure 1C. At 1 week following ACLT, lubricin staining was significantly lower (adj. p<0.001) in the ACLT joint than in the CL joint. At 4 weeks following ACLT, lubricin staining remained significantly lower (adj. p<0.001) in the ACLT joint compared with the CL joint. Additionally, lubricin staining in the ACLT joints at 1 week was significantly higher (adj. p = 0.0002) than in the ACLT joints at 4 weeks, adjusting for CL limbs. Animals at each time of sacrifice exhibited larger effects than their sham controls (adj. p < 0.001 for both), neither of which exhibited significant differences between limbs.
The SF lavage sGAG concentrations of ACLT and CL joints at 1 and 4 weeks following ACLT is depicted in Figure 1D. SF lavage sGAG concentrations in ACLT joints were significantly higher than those of CL joints of both week 1 and week 4 groups (adj. p =0.0003, adj. p<0.0001, respectively), with a statistically significantly greater fold increase for the week 4 group (adj. p=0.0099).
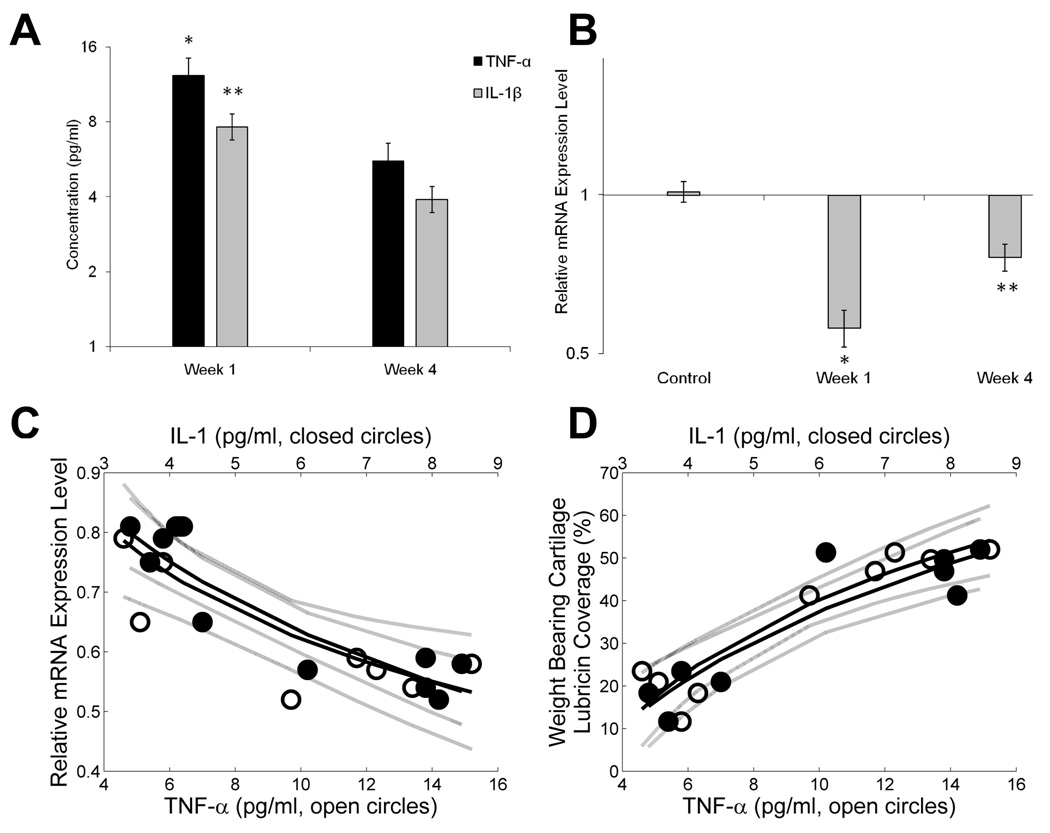
There were no detectable TNF-α and IL-1β in the CL joints at either time point. In the ACLT joints, TNF-α and IL-1β concentrations were significantly higher (p<0.0001 for both) in week 1 compared with week 4 (Fig 2A). The ratios of synovial tissue lubricin mRNA expression in the ACLT joints to the CL joints at 1 and 4 weeks following ACLT are presented in Figure 2B. In control animals, there was no difference in lubricin gene expression between sham operated and CL joints represented by a ratio of 1.0. At 1 week following ACLT, the ratio of lubricin mRNA expression in the ACLT joints to the CL joints was significantly lower (adj. p<0.001) compared with that of the control joints. Similarly, at 4 weeks following ACLT, the ratio of lubricin mRNA expression in the ACLT joints to the CL joints was significantly lower (adj. p < 0.001) than that same ratio in the control joints.
Figure 2.
Association of cytokine levels and lubricin expression in ACL transected joints. A Synovial fluid (SF) lavage TNF-α and IL-1β concentrations in ACLT joints at 1 and 4 weeks following transection. There were no detectable levels in the CL joints. B Lubricin mRNA expression of ACLT joints and sham operated joints at weeks 1 and 4 compared to CL joints. C Correlation of lubricin relative mRNA expression level and SF lavage IL-1β and TNF-α levels in ACLT joints at 1 and 4 weeks following ACLT. Significant correlations between lubricin expression and IL-1β (p<0.001; adjusted R2=0.833), and between lubricin expression and TNF-α (p=0.006; adjusted R2=0.594) existed. D Correlation of surface lubricin staining and SF lavage IL-1β and TNF-α levels in ACLT joints at 1 and 4 weeks following ACLT. Significant correlations between surface lubricin staining and IL-1β (p<0.001; adjusted R2=0.817), and between percentage lubricin staining and TNF-α (p<0.001; adjusted R2=0.852) existed.
The variability in whole joint coefficient of friction measures was high. The whole joint coefficient of friction in the ACLT limbs at 4 weeks was approximately 36% higher than that of CL limbs (adj. p=0.0247). By comparisons, the whole joint coefficient of friction values of ACLT joints at 1 week following ACLT was approximately 11% higher than CL, but did not reach statistical significance (adj. p=1.0), nor differ from that of 4 weeks (adj. p=0.9590). The limbs of 1 and 4 week sham controls did not differ (adj. p=1.0 and 0.8852, respectively), nor did ACLT animals differ from their sham controls (adj. p=1.0 and adj. p=0.4255).
The SF lavage TNF-α concentrations at weeks 1 and 4 following ACLT were significantly negatively related to quantitative lubricin PCR from synovial tissues in these joints (p=0.006; adjusted R2=0.594) (Fig 2C). Similarly, the IL-1 at weeks 1 and 4 following ACLT were significantly negatively related to quantitative lubricin PCR from tissues in these joints (p<0.01; adjusted R2=0.833) (Fig 2C). Conversely, there were no significant relationships between either SF lavage TNF-α concentrations and SF lavage lubricin concentrations (p=0.35), or IL-1 and SF lavage lubricin concentrations (p=0.632). There was a significant positive relationship between percentage lubricin coverage and SF lavage IL-1β concentrations (p<0.0005; adjusted R2=0.817), as well as with SF lavage TNF-α concentrations (p<0.0002; adjusted R2=0.852) (Fig 2D).
The effect of etanercept on lubricin metabolism
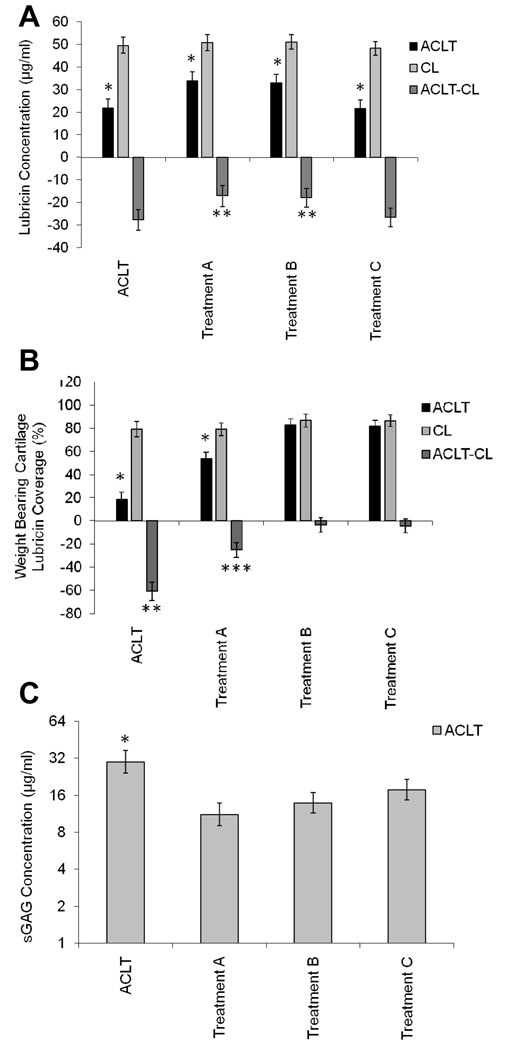
The SF lavage lubricin concentrations in ACLT joints of both treated (Treatments A, B, and C) and untreated animals were significantly lower (adj. p<0.001) than SF lavage lubricin concentrations in CL joints (Fig 3A). When adjusted for CL lubricin SF concentrations, Treatment A SF lubricin concentrations were significantly higher (adj. p=0.021) than untreated ACLT lubricin SF concentrations. Similarly, Treatment B SF lubricin concentrations were significantly higher (adj. p=0.025) than untreated ACLT lubricin SF concentrations. There were no differences in Treatment C adjusted SF lubricin concentrations when compared with untreated ACLT lubricin SF concentrations (adj. p=1.0). There was no significant difference (adj. p=0.762) between adjusted SF lubricin concentrations in Treatments A and B.
Figure 3.
ACLT and CL joints from etanervept treated and untreated animals (n=5 in each group). A SF lavage lubricin concentrations in ACLT joints receiving treatments A, B and C and untreated animals were significantly lower* (p<0.001) than in CL joints. ACLT joints receiving treatments A and B were significantly different** (p<0.001) than joints in treatment C and untreated joints. B Percentage lubricin staining on the surface of cartilage in ACLT and CL joints at 4 weeks following ACLT, treated with etanercept (Treatments A, B, C), and untreated animals. *ACLT joints of untreated animals and treatment A were significantly lower (p<0.001) than CL joints. **ACLT joints of untreated animals showed less lubricin staining (p<0.001) than ACLT joints in treatments A, B, and C. ***ACLT joints in treatment A were significantly different (p<0.001) in percentage surface lubricin staining in ACLT joints of untreated animals and treatments B, and C. There were no differences between treatments B and C. C SF lavage sGAG concentrations in ACLT joints, treated with etanercept (Treatments A, B, C), were lower* (p<0.001) than untreated animals.
The percentage lubricin staining on the surface of articular cartilage in ACLT joints of Treatment A and untreated animals was significantly lower (adj. p<0.001) than in CL joints, while there was no significant difference for Treatments B & C (Fig 3B). When adjusted for CL percentage lubricin staining, Treatments A, B, and C percentage staining values were significantly higher (adj. p<0.001) than that of untreated ACLT joints. Treatments B and C percentage lubricin staining were significantly higher (p<0.001) than that of Treatment A, with no significant difference between Treatments B and C.
The SF lavage sGAG concentrations in ACLT joints of untreated and etanercept-treated animals are presented in Fig 3C. SF lavage sGAG concentrations were lower in Treatments A (adj. p<.0001), B (adj. p=0.0001), and C (adj. p=0.0055), relative to untreated ACLT limbs. Treatment A was significantly lower than Treatment C (adj. p=0.0117), and Treatment B did not differ significantly from either treatment A (adj. p=0.1358), or treatment C (adj. p=0.1448).
The relationships between whole joint coefficient of friction, percentage cartilage surface lubricin staining, and SF lavage lubricin concentrations in ACLT and CL joints
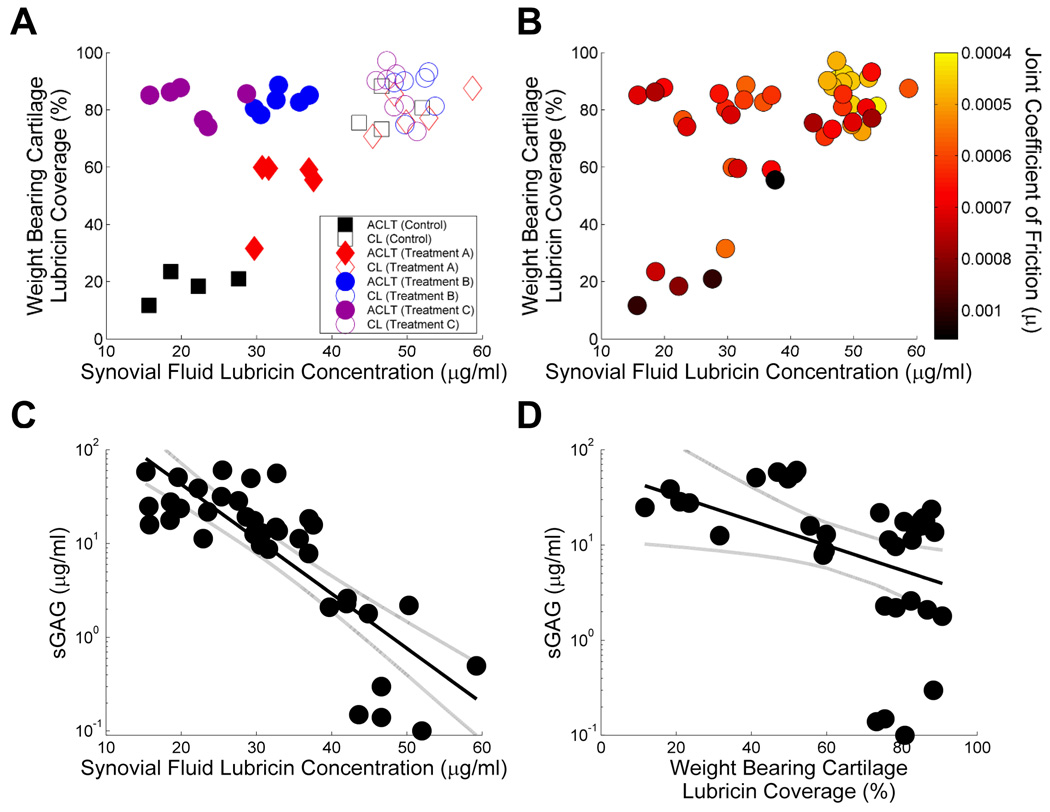
The relationship between surface lubricin staining and SF lavage lubricin concentration of ACLT and etanercept-treated ACLT joints is depicted in Figure 4A. The CL joints of ACLT and etanercept-treated ACLT joints exhibited a combination of high percentage lubricin staining and SF lavage lubricin concentrations. In contrast, non-treated ACLT joints exhibited a combination of low lubricin staining and SF lavage lubricin concentrations. None of the etanercept treated joints fully recapitulated lubricin in both locations to the levels of the CL joints. However, Treatments B and C demonstrated more lubricin staining compared with Treatment A, which had a higher SF lavage lubricin concentration compared with Treatment C.
Figure 4.
Summary of ACLT rat joints treated with etanercept and untreated, and the corresponding CL knee joints. A Association of percentage lubricin staining of cartilage surfaces and SF lavage lubricin concentration. B Relationship between percentage lubricin staining of cartilage surfaces and synovial lavage lubricin concentrations of ACLT and contralateral rat joints as a function of whole joint coefficient of friction. The grouped joints were harvested from rats treated with three different etanercept dosing strategies as well as untreated animals. C Correlation of SF lavage lubricin and sGAG concentrations. There was no significant correlation between SF lavage lubricin and sGAG concentrations. D Correlation of percentage lubricin staining on the surface of articular cartilage and SF lavage sGAG concentration. There was a significant correlation between surface lubricin staining and SF lavage sGAG concentration (p=0.044).
The relationships between percentage of surface lubricin staining and SF lavage lubricin concentrations as a function of coefficient of friction values of ACLT and CL joints of etanercept-treated and non-treated animals are depicted in Figure 4B. The lowest coefficient of friction values clustered around a combination of high lubricin cartilage staining and SF lavage lubricin concentrations. The significant negative relationship between SF lavage lubricin and SF lavage sGAG is presented in Figure 4C (p=0.0095). The relationship between lubricin surface coverage and SF lavage sGAG is presented in Figure 4D. There was evidence of different relationships between limbs (interaction p=0.044). Follow-up analyses revealed a statistically significant negative relationship between SF lavage sGAG and lubricin surface coverage in the ACLT limbs (regression line and 95% CI plotted adj. p=0.015), but not within the contralateral limbs (adj. p=0.074), which was also lower in general (p=0.0052).
Discussion
ACL injury is an acute traumatic injury leading to increased risk of long-term development of degenerative joint diseases. Following ACL injury, SF concentrations of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, have been shown to be highest within 24 hours14, and associated with an increase in SF proteoglycans15. These findings corroborated our study of SF from patients with an acute unilateral ACL injury6.
In this present study, we examined the time course of the impact of an ACL injury on cartilage chondroprotective abilities. Following the ACL injury in the rat model, an early increase in pro-inflammatory cytokines in the SF was detected at 1 week, and was also detected at week 4 (Fig 1). The elevated levels were significantly related to the levels of decreased synovial tissue lubricin gene expression, and with lubricin deposition on the articular cartilage surface. This association is consistent with previous reports that the pro-inflammatory cytokines TNF-α and IL-1 to significantly decrease lubricin gene expression in synoviocytes and superficial zone chondrocytes10,11. However, the larger quantities of lubricin detected on the cartilage surface as IL-1 and TNF-α is increased (Fig 2D) suggests that lubricin already present on the surface has a long half-life. This has been observed in other work where cartilage surface interaction of labeled lubricin increased its half-life to 6.3 days 16.
At 1 week following ACLT, SF lavage lubricin concentrations were significantly lower in the ACLT joints than in the uninjured joints (Fig 1C). This decrease was also paralleled with a significant decrease in lubricin deposition on weight-bearing areas of cartilage surfaces. These results, coupled with the observation that the coefficient of friction was significantly elevated in the ACLT limb relative to CL in the 4-week untreated ACLT group (worst damage), indicate compromised joint lubrication at a relatively early stage following an ACL injury. The compromised lubrication may be due to a significant reduction in lubricin gene expression leading to decreased SF lubricin concentrations and decreased lubricin deposition on the articular cartilage surface. These results corroborate earlier findings that demonstrated an early decrease of SF lubricin concentrations in ACL injured joints compared with uninjured joints in humans6 and another animal model8.
Etanercept is a soluble TNF-α receptor that competes with endogenous TNF receptors for binding to TNF-α, resulting in attenuation of the effects of TNF-α. Along with other TNF-α targeted therapies, etanercept has significantly advanced the treatment of rheumatoid arthritis17–20. Clinically, etanercept has been used in other clinical conditions where TNF-α plays a significant role in pathogenesis21,22. Dosing schedules of etanercept were aimed to examine the effects of early vs. late blocking of the effects of TNF-α as well as the effects of high vs. low dose etanercept. Given the effect of TNF-α on lubricin expression10, we anticipated that etanercept administration would increase SF and cartilage lubricin. Across all treatment strategies, cartilage lubricin was higher compared to untreated animals in the injured joints. However, SF lavage lubricin elevation was variable. While Treatments A and B resulted in higher SF lavage lubricin than in the untreated joints, Treatment C did not result in any significant SF lavage lubricin difference (Fig 3A). Furthermore, the percentage of the cartilage surface staining for lubricin was higher in Treatments B and C compared to Treatment A (Fig 3B). Taken together, these results indicate that blocking the effects of TNF-α leads to an increase in total lubricin in the joint suggesting improved chondroprotective ability. Treatment B may have provided the best combination of increased SF lavage and percentage surface lubricin staining, though whole joint lubrication was still compromised.
The relationship between SF and cartilage lubricin is complex and is not entirely understood23. Nugent et al examined the effect of bathing fluid conditions on lubricin concentration at the articular surfaces and concluded that articular surface lubricin concentration was independent of lubricin concentration in the fluid phase24. Following removal of lubricin from the surface of articular cartilage, lubricin in the fluid phase saturated the articular surface24. These results may be in agreement with findings from our model where at 1 week following injury, the decrease in lubricin was greater in the SF than at the surface of articular cartilage (Fig 1). As the post-traumatic period progressed, surface lubricin decreased and was associated with cartilage damage. Conversely, in etanercept-treated joints, lubricin on the surface of articular cartilage was replenished independent of or preferentially to SF lubricin.
ACL injuries, meniscus tears and menisectomies are examples of acute joint injuries that lead to the development of OA and involve down regulation of lubricin expression as a part of the disease process7,25. Relevant to these observations is the fact that meniscus cells also secrete lubricin26. Intra-articular lubricin supplementation has been advocated as a potential new therapeutic modality for these pathologies27,28. In our study, inhibiting the effects of TNF-α was investigated as an alternative approach to intra-articular lubricin supplementation. To the extent of the measured effects, TNF-α inhibition increased both SF and cartilage surface lubricin, and it also decreased proteoglycan release from articular cartilage.
Conclusions and Limitations
At an early stage following an acute ACL injury, lubricin gene expression is down regulated by the effects of pro-inflammatory cytokines IL-1β and TNF-α. This down regulation results in decreased lubricin concentrations in SF and on the surface of articular cartilage, compromising joint lubrication, and increasing the risk of wear induced damage that may exceed the limited regenerative ability of cartilage. Early inhibition of the effects of TNF-α restores lubricin in the SF and on the surface of articular cartilage, lowers whole joint coefficient of friction, and limits cartilage damage. The authors acknowledge that in this model it is difficult to differentiate this effect of TNF-α inhibition from more general degradative enzyme activity upon cartilage itself. In assessing lubricin staining, areas were selected that were likely weight-bearing regions and unsupported by menisci. However, the entire articular cartilage surface was not examined for lubricin coverage, and so the results should not be generalized to the entire surface. The lack of surface lubricin in some joints served to validate the lack of recoverable lubricin in SF lavages. Due to the method of quantification, the authors did not factor in the intensity of lubricin staining at areas of measurement, but relied solely on the segmental length of the immunostained surface.
Acknowledgements
The authors would like to thank Dr. Mathew Warman from Boston Children’s Hospital for providing lubricin-specific Mab 9G3 and Ms. Margaret Case for manuscript review. This research was funded by NIH/NIAMS R21AR055937, RO1AR050180 and NCRR COBRE P20 RR024484-01.
References
- 1.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 2.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction, and osteoarthritis. Curr Opin in Orthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jay GD. Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 5.Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225(1):195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 9.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 10.Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40–45. doi: 10.22203/ecm.v013a04. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL. Differential regulation of proteoglycan 4 metabolism in cartilage by IL1- α, IGF-I, and TGF-β1. Osteoarthritis Cartilage. 2008;16:90–97. doi: 10.1016/j.joca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Nikura T, Reddi AH. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312–2321. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 13.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 14.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25:751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 16.Glasson SS, Rivera-Bermudez TJ, Mark LS, Whiteside G, Resmini C, et al. Intra-articular lubricin supplementation modifies disease progression and ameliorates pain in a rat model of osteoarthritis. 55th Orthop Res Soc. 2009:1116. presentation. [Google Scholar]
- 17.Moreland LW, Weinblatt ME, Keystone EC, Kremer JM, Martin RW, Schiff MH, Whitemore JB, White BW. Etanercept treatment in adults with established rheumatoid arthritis: 7 years of clinical experience. J Rheumatol. 2006;33:854–861. [PubMed] [Google Scholar]
- 18.Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls AA. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:3–13. doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- 19.Navarro-Sarabia F, Ariza-Ariza R, Hernandez-Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. J Rheumatol. 2006;33:1075–1081. [PubMed] [Google Scholar]
- 20.Jones RE, Moreland LW. Tumor necrosis factor inhibitors for rheumatoid arthritis. Bull Rheum Dis. 1999;48:1–4. [PubMed] [Google Scholar]
- 21.Kircik L, Bagel J, Korman N, Mentor A, Elmets CA, et al. Utilization of narrow-band ultraviolet light B therapy and etanercept for the treatment of psoriasis (UNITE): efficacy, safety, and patient-reported outcomes. J Drugs Dermatol. 2008;7:245–253. [PubMed] [Google Scholar]
- 22.Hoy SM, Scott LJ. Etanercept: a review of its use in the management of ankylosing spondylitis and psoriatic arthritis. Drugs. 2007;67:2609–2633. doi: 10.2165/00003495-200767170-00009. [DOI] [PubMed] [Google Scholar]
- 23.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, Elsaid KA, Kim KS, Cui Y, Warman ML. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nugent-Derfus GE, Chan AH, Schumacher BL, Sah RL. PRG4 exchange between articular cartilage surface and synovial fluid. J Orthop Res. 2007;25:1269–1276. doi: 10.1002/jor.20431. [DOI] [PubMed] [Google Scholar]
- 25.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, Mechrefe AP. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orhtop Res. 2008;26:231–237. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SY, Niikura T, Reddi AH. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A. 2008;14(11):1799–1808. doi: 10.1089/ten.tea.2007.0367. [DOI] [PubMed] [Google Scholar]
- 27.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 28.Wimmer MA, Schmid TM, Jacobs JJ. Tribology; a portal to understand joint failure? Arthritis Rheum. 2007;56:3511–3513. doi: 10.1002/art.22973. [DOI] [PubMed] [Google Scholar]