Abstract
Devastating central nervous system injuries and diseases continue to occur in spite of the tremendous efforts of various prevention programs. The enormity of and annual escalation of healthcare costs due to them require that therapeutic strategies be responsibly developed. The dysfunctions that occur after injury and disease are primarily due to neurotransmission damage. The last two decades of both experimental and clinical research have demonstrated that neural and non-neural tissue and cell transplantation is a viable option for ameliorating dysfunctions to markedly improve quality of life. Moreover, significant progress has been made with tissue and cell transplantation in studies of pathophysiology, plasticity, sprouting, regeneration, and functional recovery. This chapter will review information about the ability and potential, particularly for traumatic spinal cord injury, that neural and non-neural tissue and cell transplantation has to replace lost neurons and glia, reconstruct damaged neural circuitry, and to restore neurotransmitters, hormones, neurotrophic factors, and neurotransmission. Donor tissues and cells to be discussed include peripheral nerve, fetal spinal cord and brain, central and peripheral nervous systems’ glia, stem cells, those that have been genetically engineered, and non-neural ones. Combinatorial approaches and clinical research are also reviewed.
Keywords: spinal cord injury, transplantation, regeneration, plasticity, translation
1. Introduction
Over the last two decades, neural and non-neural tissue and cell transplantation have been used extensively to study mechanisms of central nervous system (CNS) injury, plasticity, sprouting, regeneration, and recovery of function. The availability of potential donor tissues and cells (Table 1) for transplantation and the methods developed to obtain them now provide opportunity and flexibility for strategies to treat human CNS injuries and diseases. A transplant of neural tissue and cells may replace particular populations of neurons lost by injury and restore levels of neurotransmitters, hormones, neurotrophic factors, or neural circuitry. These transplants may also provide a population of neurons at the injury site which may serve as a relay to convey sensory and/or motor control to levels proximal and/or distal to the injury. Finally, neural and non-neural tissue and cell transplants, alone or in combination with other strategies, may serve as a bridge that supports axonal growth across the injury site to reach targets proximal and/or distal to the lesion. This chapter will briefly review the general principle and current advances of transplantation-mediated axonal regeneration with emphasis being placed on the advantages and disadvantages of using different neural and non-neural tissues and cell types, combinatorial approaches, as well as gene therapy in the repair of CNS injuries, particularly that of the spinal cord. For further information about some of the combinatorial approaches, the reader is referred to the chapters about neurotrophins, biomaterials, electrical stimulation, and physical activity. The reader is also referred to the chapter about clinical trials of therapies for spinal cord injury (SCI).
Table 1.
Donor tissues or cells that have been used for CNS regeneration
| Tissues | Peripheral nerve tissue |
| Fetal spinal cord or brain tissue | |
| Primary Neurons | Fetal brain or spinal cord neurons |
| PNS neurons (e.g. sympathetic dopaminergic neurons) | |
| Glial Cells | CNS: astrocytes, oligodendrocytes, microglia |
| PNS: Schwann cells, olfactory ensheathing glia (OEG) | |
| Genetically Engineered | Derived from neurons and glial cells |
| Cells | Derived from non-neuronal cells (e.g. fibroblasts) |
| Stem Cells | Embryonic stem cells (ES) |
| Neural stem cells (NSC) | |
| Glial-restricted precursors (GRP)/ Oligodendrocyte progenitor cells (OPC) | |
| Non-Neural Cells | Marrow stromal cells (MSC) |
| Reactive macrophages | |
| Meningeal fibroblasts |
2. Tissue transplantation
2.1. Peripheral nerve
In contrast to the CNS, regeneration of axons in the adult mammalian peripheral nervous system (PNS) has long been known to occur and to result in varying extents of functional recovery. This information led Spanish investigators in the early 1900s to transplant pieces of peripheral nerve into areas of the CNS to assess the capacity of CNS axons to regenerate. When axonal growth into the transplants occurred, Ramón y Cajal and colleagues concluded that CNS axons had the capacity to regenerate if the environment is suitable, i.e. if appropriate “neutritive” and “orienting” substances are provided (Ramon y Cajal 1928). This view did not flourish especially due to a number of equivocal results in experimental SCI reported in the 1940s and 1950s. In 1980, it was clearly shown with neuroanatomical tracing techniques that numerous axons of the spinal cord and dorsal root ganglion (DRG) neurons regrew into freshly dissected peripheral nerves acutely transplanted into a gap created in the adult rat spinal cord (Richardson et al. 1980). Other papers documenting growth of axons from neurons in other parts of the CNS into peripheral nerve transplants followed. In fact, these nerve grafts enabled axons in some cases to regenerate to greater lengths than they had reached initially.
Advancements in using peripheral nerve transplantation for increasing injured CNS axon regeneration have been made during subsequent investigations. More DRG axons regenerated into acute transplants of freshly dissected and, even more so, pre-degenerated peripheral nerve when a conditioning peripheral nerve lesion occurred, that is, when the DRG peripheral axons were injured prior to or at the time of laceration SCI (Richardson et al. 1984). Neurotrophins delivered to the spinal cord and brain, FK506, and knockdown of xylosyltransferase-1 mRNA acutely and chronically were seen to promote more growth of regenerated supraspinal (Ye et al. 1997), spinal cord (Novikova et al. 2002), and dorsal column (Oudega et al. 1996) axons into the spinal cord through peripheral nerve transplants. Chondroitinase ABC administration to the spinal cord to degrade inhibitory chondroitin sulfate proteoglycans also increased regenerated axon outgrowth from a peripheral nerve bypass transplant at a hemisection leading to robust functional recovery (Houle et al. 2006).
Another advancement was the use of multiple peripheral nerve grafts across a transection and gap that redirect specific axon pathways from non-permissive white to permissive gray matter (Cheng et al. 1996). Combined with the use of acidic fibroblast growth factor (aFGF, FGF1) and compressive wiring of posterior spinal processes, this study demonstrated certain degree of structural and functional recovery. This combination repair strategy was further confirmed by others (Lee et al. 2002), was applied to chronic SCI (Fraidakis et al. 2004), and was facilitated by overexpression of neurotrophin in the peripheral nerves (Blits et al. 2000). The repair strategy was not effective in spinal cord injured non-human primates (Levi et al. 2002). However, it was reported to improve motor and sensory function of a person with chronic SCI (Cheng et al. 2004). Peripheral nerves also promoted axon regeneration and improved function when transplanted alone (Rasouli et al. 2006) or combined with neurotrophic factors (Sandrow et al. 2008) at the chronic contusive injury, a model of the frequent human SCI.
Martin and colleagues (Campos et al. 2004) developed an innovative way to promote regenerated axons bypassing a spinal lesion. In this study, they disconnected the T13 spinal nerve at its distal end and inserted it caudal to a L2/3 hemisection. Four to 28 weeks later, anterograde tracing indicated that axons from the inserted T13 spinal nerve regenerated into the spinal cord distal to the injury. In addition, electrical stimulation of the T13 spinal nerve evoked contraction of back and leg muscles, spasticity and muscle wasting was reduced, and mobility of multiple joints in the ipsilateral hindlimb was improved compared to lesion alone.
Importantly, central respiratory neurons regenerated axons into acute and chronic transplants of freshly dissected and pre-degenerated peripheral nerves into the medulla oblongata or cervical spinal cord respiratory pathways (Decherchi et al. 2002). This led to phrenic nerve responses when the distal ends of the nerves were transplanted into the mid-cervical spinal cord to by-pass a rostral laceration injury (Decherchi et al. 2002) (Table 2). It remains to be determined whether some of the additional approaches mentioned above, such as, administering neurotrophins and/or chondroitinase ABC into the spinal cord to enhance regenerated axon outgrowth from the transplants will be effective for central respiratory neurons. Additionally, whether peripheral nerve transplantation around or across the various types of cervical SCI is a rationale approach for repairing respiration is unknown. Lastly, the benefits and harms of performing autologous peripheral nerve transplants for repairing respiration needs to be further examined.
Table 2.
Summary of transplantation strategies to promote recovery of respiratory function after SCI
| Species | SCI | Strategy | Results | Potential Mechanism |
|---|---|---|---|---|
| Rat young adult1 | cervical (2–3) hemisection | autologous peroneal nerve inserted into ipsilateral dorsolateral (control) or ventrolateral medulla oblongata 2–4 months pre-SCI, distal end inserted into ipsilateral cervical (C4) cord at SCI | at 4 months post-SCI: electrical stimulation of nerve bridge between ventrolateral medulla and spinal cord evoked phrenic nerve responses, unitary recordings of spontaneous activity in bridge revealed regenerated axons of medullary inspiratory neurons | axon regeneration and target site reinnervation |
| rat, adult2 | upper cervical hemisection | adult rat, eGFP-labeled OEG in matrix implanted into hemisection at SCI | at 2 months post-SCI: respiratory rhythm was in ipsilateral phrenic nerve during spontaneous breathing and asphyxial stress | axon regeneration and/or sprouting of crossed axon pathway and target reinnervation |
| rat, adult3 | cervical (2–3) hemisection | adult rat OEG transplanted into ventral & ventrolateral funiculi rostral & caudal to, at hemisection 15–30 min after SCI | at 3–6 months post-SCI: ipsilateral phrenic nerve & diaphragm electromyographic activities during spontaneous breathing, hypercapnia, upper cervical cord electrical stimulation, and after contralateral upper cervical cord hemisection | axon regeneration and/or sprouting of crossed axon pathway and target reinnervation |
2.2. Fetal spinal cord or brain
Fetal tissue transplants have been used to repair the injured spinal cord by replacing lost neurons and supplying novel components of the spinal cord circuitry as well as by providing permissive conditions for axonal growth. Transplants of fetal spinal cord tissue into the motoneuron-depleted or injured adult rat spinal cord survived and their axons grew through ventral root or peripheral nerve to innervate skeletal muscle and improve its function (Horvat 1991). Fetal brainstem tissue transplants into cavities or transections also replaced lost supraspinal adrenergic and serotoninergic input of the adult rat spinal cord, enhanced the hindlimb flexion reflex, and activated locomotion (Ribotta et al. 2000).
After spinal cord hemisection and transplantation of the fetal tissues as pieces alone or in suspension into the adult animals, host axons regenerated into the transplant but terminated near the host-transplant border (Jakeman et al. 1991; Bregman et al. 1997b). Exogenous application of neurotrophic factors increases the intrinsic capacity of mature neurons for regrowth. For example, Bregman and colleagues showed that, after spinal cord hemisection and transplantation in the adult, exogenous administration of brain-derived neurotrophic factor (BDNF) or neurotrophin-3 (NT-3) increased supraspinal axonal growth within the transplant (Bregman et al. 1997b), further prevented the atrophy of axotomized supraspinal neurons (Bregman et al. 1998), and increased the expression of regeneration-associated genes within the cell bodies of the injured axons. They also reported that both the amount of axonal regrowth from both propriospinal and supraspinal neurons within the transplant and the host cord caudal to the lesion and the extent of recovery of locomotion, including weight-supported plantar stepping on both treadmill and over-ground tasks (stair climbing), as well as skilled and unskilled forelimb function were dramatically increased when transplants and neurotrophins were administered 2–4 weeks after a spinal cord transection rather than immediately after injury(Iarikov et al. 2007). These findings suggest that the opportunity for intervention after spinal cord injury may be greater than originally envisioned and that CNS neurons with long-standing injuries can reinitiate growth, leading to improvement in motor function.
Additional strategies also enhance the effects of fetal spinal cord tissue transplants. Acute transplants after transection combined with delayed hindlimb cycling exercise restored muscle mass and motoneuron properties (Houle et al. 1999). Acute transplants after hemisection combined with delayed systemic administration of the phosphodiesterase 4 inhibitor rolipram increased axonal regrowth within them and improved forelimb function (Nikulina et al. 2004).
Clinically, pieces of human fetal spinal cord have been transplanted into a small number of patients with syringomyelia, that is, expanding cystic lesions of the spinal cord (Wirth et al. 2001; Reier 2004). The surgical procedure was found to be feasible and safe which is particularly important as the subjects had chronic SCIs. Cysts were filled and there were no negative changes of neurophysiological measures (Thompson et al. 2001). Relatively modest sensory improvement and pain reduction were reported. However, there was a transient decrease of spasticity as well as gait and proprioception deterioration in one subject. Graft survival was equivocal so it is unclear whether transplant rejection and/or cyst expansion occurred. Since detethering, cyst drainage, and immunosuppression were performed, their roles in the effects cannot be discounted. To our knowledge, there are no articles reporting the results of basic experiments using fetal tissue transplants to improve respiration. Therefore, these need to be performed before clinical trials can be considered.
3. Cell transplantation
Many kinds of cells are considered candidates for transplantation therapy (Table 1). In this section, focus is placed on those cell types with promise to be translated into clinical studies.
3.1. Schwann cells
Schwann cells were originally described by and named after the German anatomist Theodore Schwann (1810–1882). They are a major cellular component of the peripheral nerve and play a key role in its regeneration (Oudega et al. 2006). For example, following peripheral nerve injury, axons in the distal nerve stump undergo Wallerian degeneration. Schwann cells dedifferentiate into non-myelinating Schwann cells, proliferate extensively and phagocytose myelin debris actively. The proliferating Schwann cells are located within basal lamina tubes and form column-like structures known as bands of Bünger. The Schwann cells also start to increase expression of various growth factors thereby creating a growth permissive environment for axonal regeneration. It was the discovery of this key role of Schwann cells in peripheral nerve repair, the observation of CNS axonal regeneration into transplanted peripheral nerves, and the findings of Schwann cells in the damaged CNS that generated interest in them as a potential candidate for cellular approaches to repair the injured CNS.
Schwann cells have several unique features making them one of the best cell types for therapy. First, they express a variety of factors that support the growth of central axons. Among them are members of the neurotrophin family including nerve growth factor (NGF), BDNF, and NT-3, as well as ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF), and FGF. Second, Schwann cells express surface molecules such as L1/Ng-cell adhesion molecules (CAM) and N-CAM, both of which have been demonstrated to support axon growth through contact guidance. Finally, Schwann cells produce axon growth-promoting substrates such as laminin, fibronectin, and collagen [For review, see (Oudega et al. 2006)].
One principal advantage of Schwann cells over other cell types for transplantation into the injured CNS is that Schwann cells are able to myelinate both regenerating and intact central axons. This was first shown by Gilmore (1971). Many other cell types promote axonal regeneration, but do not form myelin sheaths around the new axon sprouts. Schwann cells do so and thus facilitate signal conduction in the regenerated axons. If myelination would not occur after regeneration and formation of synaptic contacts, the functioning of the new circuits would be significantly impaired similar to what is seen in multiple sclerosis.
To employ Schwann cells for transplantation into the injured CNS, obtaining large numbers is necessary to fill up large and numerous cystic cavities that develop. Fortunately, several methods have been described to harvest purified populations of numerous Schwann cells (Wood 1976; Brockes et al. 1979). To obtain these Schwann cells, several mitogens have been used, include bovine pituitary extract containing glial growth factor (GGF). Raising the intracellular level of cAMP with dibutyryl cAMP and cholera toxin (Raff et al. 1978) as well as forskolin, a potent adenylate cyclase activator (Evans et al. 1985), has been seen to be effective. In 1991, Morrissey and colleagues (Morrissey et al. 1991) developed a method to isolate and obtain large numbers of essentially pure populations of Schwann cells from the adult rat peripheral nerve. The difference between this and previously described approaches was that small nerve explants were allowed to undergo axonal and myelin breakdown rather than enzymatic dissociation of the nerve immediately after dissection. By combining this approach with the use of the mitogenic combination of GGF and forskolin for up to 10 weeks in vitro, several millions of Schwann cells at a purity of 98% could be obtained. Using this purification technique, Morrissey and co-workers were also able to obtain Schwann cell cultures from human phrenic nerve. Casella and colleagues (Casella et al. 1996) further improved the method of Morrissey by using the mitogenic combination of heregulin and forskolin for 2 weeks prior to dissociation of the human nerve explants. Importantly, the cultured Schwann cells were shown to retain their ability to myelinate and promote axonal regeneration. This opened a new avenue for preparing large quantities of autologous Schwann cell transplants for human application. One could envision that for a spinal cord injured patient, a piece of peripheral nerve can be taken from them and prepared to isolate and propagate a large number of Schwann cells for transplantation.
The efficacy of Schwann cells in promoting axonal regeneration and myelination in the injured adult mammalian PNS and CNS has been extensively studied in a variety of experimental models (Oudega et al. 2006; Bunge 2008). Schwann cells transplanted into injured peripheral nerve, optic nerve, septo-hippocampal system, diencephalon, and spinal cord survived, promoted axonal regeneration, and ensheathed or myelinated the regenerated axons (see review by (Oudega et al. 2006; Bunge 2008)). In cases where axons in the spinal cord were demyelinated but not severed, remyelination by grafted Schwann cells re-established normal conduction velocity across the lesion and thus achieved functional repair. Moreover, Schwann cells mixed with Matrigel, a commercial preparation of basement membrane components, when seeded into semipermeable polyacrylonitrile/polyvinylchoride (PAN/PVC) polymer tubes then grafted between stumps of the completely transected (Xu et al. 1997) or hemisected (Xu et al. 1999) adult rat thoracic spinal cord have been shown to promote propriospinal and supraspinal axonal growth across the gap and myelination. The cord stumps were bridged by a tissue cable that contained Schwann cells and axons were at different stages of myelination or ensheathment by Schwann cells (Xu et al. 1995a; Xu et al. 1995b; Xu et al. 1997) (Fig. 1). The absence of Schwann cells in control grafts resulted in scarce axonal growth.
Fig. 1.
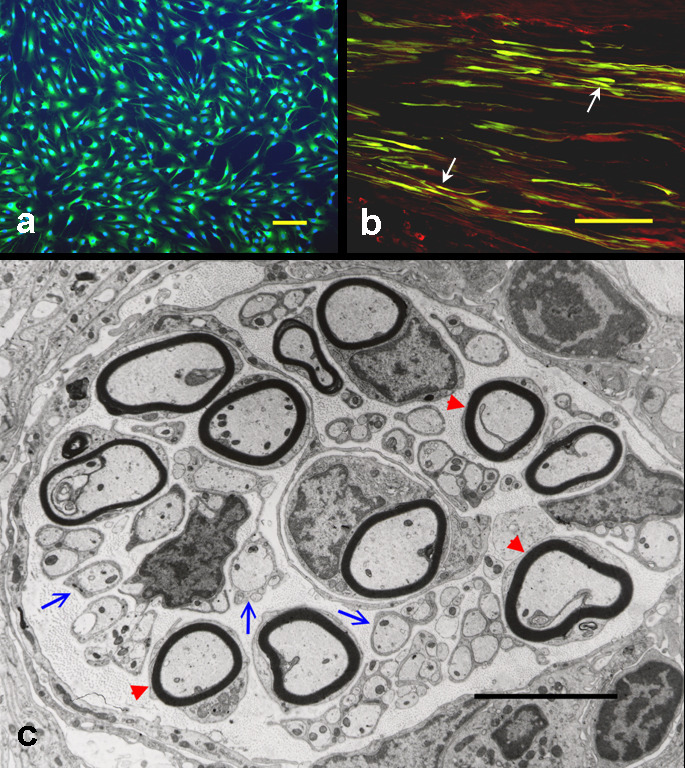
Schwann cells, isolated from the peripheral nerve, survive and promote axonal regeneration after being transplanted into the injured spinal cord. (a) Schwann cells, purified from adult rat sciatic nerves, are immunopositive for S100, a marker of Schwann cells (green). The cells are counterstained with Hoechst 33342, a fluorescent nuclear dye (blue). (b) Grafted Schwann cells (arrows), transduced with an adenoviral vector encoding green fluorescent protein (green), are associated with regenerating axons (neurofilament-positive; red) within the guidance channel grafted into the injured spinal cord. (c) At the ultrastructural level, grafted Schwann cells either ensheath (blue arrows) or myelinate (red arrowheads) regenerated axons. a: bar = 5 µm; b: bar = 10 µm; c: bar = 5µm. (Adapted from Xu, Encyclopedic Ref. Neurosci., 2005, Springer)
Although grafting Schwann cells into the lesion site has been shown to promote regeneration and myelination, the axons do not regenerate beyond the transplantation site. To achieve further axonal advancement beyond the inhibitory glial scar surrounding the injury, additional approaches such as increasing the intrinsic capacity of axons to regenerate and/or removal of the inhibitory molecules associated with reactive astrocytes and/or CNS myelin should be incorporated. Clearly, combinatorial interventions involving Schwann cells and other effective repair strategies, discussed below, are capable of achieving greater axonal regenerative responses and biologically significant functional recoveries. Moreover, a report one year after autologous Schwann cell transplantation into chronically spinal cord injured persons indicates that it is safe and feasible (Saberi et al. 2008). Continued preclinical development of these approaches to neural repair may ultimately generate strategies that could be tested in human CNS injuries such as those being proposed at The Miami Project to Cure Paralysis of the University of Miami School of Medicine.
3.2. Olfactory ensheathing glia
Over the last decade, experiments from different laboratories have demonstrated compelling evidence that olfactory ensheathing glia (OEG) too are a promising cell source for CNS repair. The OEG are also named olfactory glial cells or olfactory ensheathing cells. They ensheath axons of olfactory receptor neurons which leave the olfactory mucosa, enter the olfactory bulb where they comprise the olfactory nerve fiber layer, and finally synapse with second order neurons (mitral cells) in the glomerular layer (Franklin et al. 1997b).
The OEG have traditionally been regarded as straddling the boundary between PNS and CNS glia since they bear similarities to both Schwann cells and astrocytes. Their similarities to Schwann cells, particularly non-myelinating Schwann cells, include the expression of many shared antigenic markers (Barnett et al. 1993), ensheathment of axons, and supporting axonal growth in the adult (Doucette 1993). OEG resemble astrocytes in that they express the astrocyte-specific isoform of glial fibrillary acidid protein (GFAP) and form the cellular boundary of the olfactory bulb, similar to the astrocytic glia limitans (Franklin et al. 1997b).
OEG have been implicated in the regeneration beyond an injury and/or scar into host nervous tissue of injured axons in discrete and complete SCI models (Li et al. 1998; Ramon-Cueto et al. 1998; Ramon-Cueto et al. 2000). For example, long distance axonal regeneration of multiple supraspinal pathways, along with recovery of sensory and motor functions, and attenuation of muscle phenotypic deterioration was achieved following complete thoracic spinal cord transection and implantation of OEG on either side and into the transection site (Ramon-Cueto et al. 2000; Negredo et al. 2008). Improved function also has been observed after discrete, incomplete, and complete SCI when chronic transplants (Lopez-Vales et al. 2007), OEG overxpressing BDNF (Ruitenberg et al. 2003), and GDNF (Cao et al. 2004), xenografts of primate OEG (Guest et al. 2008), and step training (Kubasak et al. 2008) were used. However, forelimb function was impaired when OEG transplantation was combined with BDNF delivery to the red nucleus (Bretzner et al. 2008). Importantly, phrenic and diaphragmatic activities recovered (Polentes et al. 2004) and breathing was restored (Li et al. 2003) when OEG were transplanted after cervical SCI (Table 2).
The mechanisms underlying this OEG-induced growth response are still in debate. When transplanted into the injured spinal cord, they migrated considerably in the white matter tracts, gray matter, and glial scar (Ramon-Cueto et al. 1998). It may be that the OEG migrate with the growing axons, thereby preventing the axon from recognizing the non-permissive milieu. Another option is that OEG transplants do not result in adverse reactions of the nervous tissue to axonal extension. The latter option is supported by the finding that transplanting OEG into adult white matter resulted in a decreased astrocytic response and decreased expression of the growth-inhibitory molecules, chondroitin sulfate proteoglycans (CSPGs) (Takami et al. 2002).
One limitation of using of OEG is that these cells normally ensheathe but do not myelinate axons. Although previous experiments showed myelin-forming capabilities of transplanted OEGs on demyelinated or regenerated axons, the possibility that the myelin formed by endogenously- (or exogenously-) derived Schwann cells rather than OEG remains to be excluded. It was demonstrated that transplants of purified populations of OEG resulted in less extensive remyelination than those of unpurified preparations (Lakatos et al. 2003). Since OEG and Schwann cells share many growth-promoting properties but that they also exhibit different characteristics, these two cell types may be grafted together to achieve a complementary goal. For example, a combined strategy involving Schwann cell transplantation into the lesion cavity and OEG transplantation near the graft-host interfaces may utilize both growth-promoting and myelin-forming properties of Schwann cells and interface-modification properties of OEG, therefore, promoting continuous axonal regeneration through and beyond the lesion site. This strategy has been successfully used in a study when a spinal cord gap was bridged by a Schwann cell-seeded guidance channel and the adjacent cord stumps received injections of OEG (Ramon-Cueto et al. 1998). In this study, the combined transplantation resulted in regeneration of both proprio- and supraspinal axons across the lesion gap which was not achieved when a Schwann cell-seeded guidance channel alone was used. Combining this approach with Schwann cell transplantation and chondroitinase ABC spinal cord delivery was reported to improve locomotion and to prevent bladder dysfunction (Fouad et al. 2009). Locomotion also was improved when transplants of OEG and Schwann cells were combined with methylprednisolone and interleukin-10 (Pearse et al. 2004), and enriched housing (Moon et al. 2006).
Human adult olfactory neural progenitors too have been observed to promote recovery of adult rat forelimb function after dorsolateral funiculotomy SCI (Xiao et al. 2005). Clinical studies have reported that OEG transplantation is feasible and safe (Mackay-Sim et al. 2008) and that improved motor and sensory function occurred (Guest et al. 2006)(Lima et al. 2006). A number of biomedical, and ethical issues were raised about some of the fetal cell transplants performed that need to be critically considered when evaluating the results and for all future cell and tissue transplant clinical studies (Dobkin et al. 2006). These include preclinical results, randomized trials with placebo control interventions, SCI recipient selection requirements, blood typing and tissue matching between donors and recipients, type of cell transplanted, site of transplantation, use of methylprednisolone, medical complications, comprehensive plan for objective pre- and post-surgery assessments, and functional effectiveness.
3.3. Stimulated macrophages
Post-injury recovery in most tissues requires an effective dialog with macrophages. However, in the adult mammalian CNS this may be restricted possibly due to its immune-privileged status. Initial laboratory comparisons of inflammatory responses associated with PNS injury versus CNS trauma suggested that the lack of regeneration in the spinal cord and brain could be attributed to a compromised recruitment of macrophages (Hirschberg et al. 1995). Subsequent experiments, first in an optic nerve injury model, showed that regeneration could be elicited by augmenting the endogenous inflammatory response with grafts of activated macrophages that had been pre-incubated in the presence of PN tissue (Lazarov-Spiegler et al. 1996). Implantation of stimulated macrophages into transected rat spinal cord promoted tissue repair and partial recovery of motor function, manifested behaviorally and electrophysiologically (Rapalino et al. 1998). Anatomically, labeled axons were found to cross the transected site and descend distally. Re-transection of the recovered spinal cord above the primary injury site led to loss of recovery.
Although some positive results have been reported and the strategy was studied clinically by Proneuron in a Phase I safety study (Knoller et al. 2005) that led to a suspended Phase II study, controversy exists regarding the use of activated macrophages to boost post-injury inflammatory responses for axonal regeneration in the injured spinal cord (Reier 2004). Activation of intrinsic macrophages with microinjections of zymosan into a spinal contusion site resulted in deleterious effects on hindlimb functional recovery and tissue survival (Popovich et al. 2002). Furthermore, depletion of peripheral macrophages, during a time when inflammation has been shown to be maximal after spinal contusion injury in rats, led to significantly better hindlimb usage during overground locomotion, more extensive white matter sparing, and decreased tissue cavitation (Popovich et al. 1999). Thus, more work needs to be done to determine the beneficial or detrimental roles that the reactive macrophages may play in response to CNS injuries as well as the underlying mechanisms to better appreciate this therapy.
3.4. Neural stem cells and embryonic stem cells
Multipotent neural stem cells (NSCs) are found in mammals throughout their lifetime and recruiting them may be therapeutic. Neural stem cells are defined by their ability to self-renew and retain the potential to differentiate into both neurons and glia (Gage 2000). Cells with these properties were first isolated from the forebrains of adult and embryonic mice. These NSCs proliferate as neurospheres in culture in response to either epidermal growth factor (EGF) or fibroblast growth factor-2 (FGF-2) combined with heparan sulphate (HS) (Fig. 2). In addition to the forebrain, the spinal cord is another enriched source of NSCs in the CNS (Hu et al. 2004). Neural stem cells can be repetitively passaged and maintained for a long time in vitro. They express the intermediate filament nestin (abbreviated for neuroepithelial stem cell protein) but not markers for differentiated neuronal or glial cell types. In addition, NSCs retain their multipotentiality and can be induced to produce enriched populations of glial or neuronal progenitors using defined extrinsic factors in vitro. Furthermore, these cells may differentiate into neural cells with appropriate phenotype(s) after transplantation and possibly reestablish connectivity within a specific CNS region. These unique properties of NSCs relative to other neural cell types potentially allow for the design of cellular transplants that can fulfill specific needs in the repair of the damaged CNS, making NSCs a promising candidate for neural transplantation in traumatic spinal cord and brain injuries and several other neurological diseases.
Fig. 2.
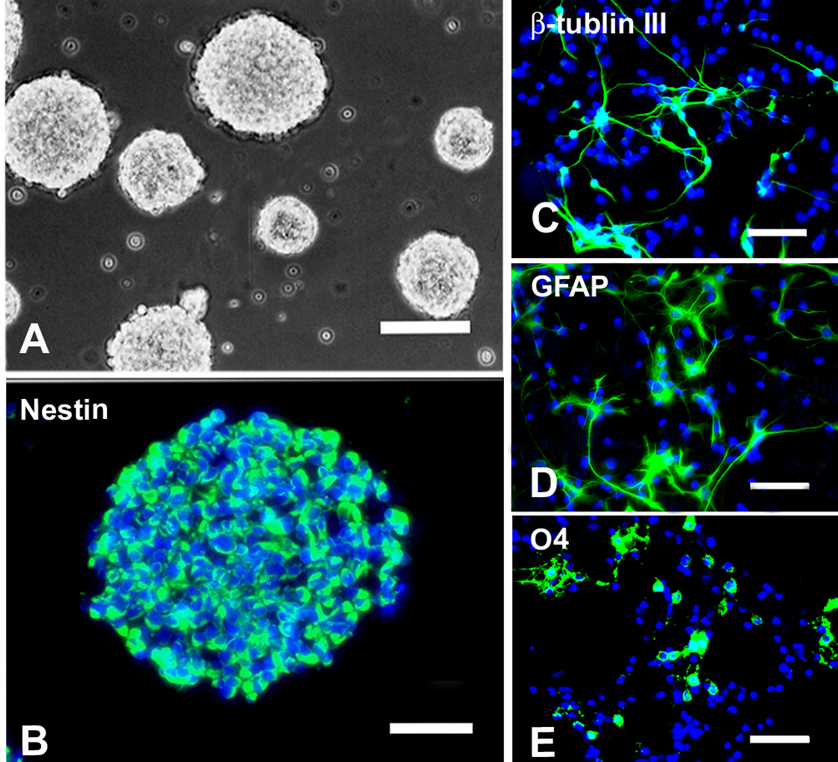
Morphological and immunocytochemical characteristics of NSCs. (A) Phase contrast photomicrograph of neurospheres cultured in growth medium supplemented with 20ng/ml EGF and 20ng/ml bFGF. (B) Cells in a neurosphere were immunopositive for nestin (green). (C~E) When cultured in medium containing no growth factors but 1% FBS for 5 days, NSCs differentiated into neurons (βIII-Tubulin+, C), astrocytes (GFAP+, D) and oligodendrocytes (O4+, E). Cells in B~E were counterstained with Hoechst 33342 (blue), a nuclear dye. A: bar = 200µm; B: bar = 50µm; C~E: bar = 25µm. (Adapted from Hu et al., J Neurosci Res 78:637–646, 2004)
Several transplantation strategies utilizing neural stem or progenitor cells have been evaluated. Using an ex vivo gene transfer approach, Liu and colleagues (Liu et al. 1999) grafted neural stem cells expressing NT-3 into the normal spinal cord at the T8 level. These cells survived, integrated into the host spinal cord, and differentiated into neurons, astrocytes, and oligodendrocytes. The cells also migrated as far as one centimeter from the site of grafting, indicating that spinal cord repair may extend rostrally and caudally. Transplants of embryonic day (E)14 spinal cord NSCs into a partial hemisection cavity of the spinal cord, combined with exogenous BDNF administration, resulted in the differentiation of these cells into neurons, astrocytes, and oligodendrocytes, however, most of the grafted cells appeared undifferentiated (Chow et al. 2000). Additionally, NSCs isolated from the E14 rat cerebral cortex or adult rat subventricular zone when transplanted into normal and contused adult rat spinal cords either remained nestin-positive or differentiated along the glial lineage (Cao et al. 2001).
Remaining undifferentiated or differentiating into predominantly glia is a potential problem for the direct transplantation of NSCs into intact or injured adult CNS regions. The differentiation fate of neural stem cells may be strongly influenced by specific cues from the local environment of engraftment (Onifer et al. 1993). It is only when NSCs are transplanted into the developing brain or areas in the adult brain undergoing active neurogenesis that significant numbers differentiate into region-appropriate neurons (Flax et al. 1998). For example, adult spinal cord-derived NSCs when transplanted into the adult rat spinal cord (non-neurogenic region) differentiated into glial cells; however, when they were transplanted into the granular layer of hippocampus (neurogenic region) they differentiated into cells characteristic of this region (Shihabuddin et al. 2000). Engraftment of these cells into other hippocampal regions resulted in the differentiation of cells with astroglial and oligodendroglial phenotypes. These data indicate that NSCs obtained from a non-neurogenic region (such as the spinal cord) are not lineage-restricted to their developmental origin but can generate region-specific neurons in vivo when exposed to the appropriate environmental cues (Shihabuddin et al. 2000). Prior growth factor induction of stem cell differentiation along neuronal or glial cell pathways may be necessary for successful repair to occur. When neuronal-restricted precursors (NRPs), a NSC-derived neuronal lineage, were grafted into the normal adult rat dentate gyrus, they showed mature neuronal morphology with robust processes. Some grafted cells showed morphological characteristics of pyramidal neurons in the CA1 region. These cells expressed the mature neuronal marker NeuN and calbindin, a protein that was not expressed by NRPs in vitro. When NRPs were transplanted into the normal rat spinal cord, they differentiated into neurons; some of them expressed γ-amino butyric acid, glutamate, and choline acetyltransferase.
An additional source of NSCs is from blastocyst-derived cells, which are expanded as totipotent embryonic stem (ES) cells (Svendsen et al. 1999). Induction of ES cells into committed precursors can yield purified populations of NSCs, precursors, or differentiated neural cell types (Svendsen et al. 1999). The resulting stem cells can be expanded in culture as neurospheres which may then be used for transplantation. Grafting of neural differentiated mouse ES cells into a rat thoracic spinal cord contusive injury resulted in the survival, migration, differentiation into astrocytes, oligodendrocytes and neurons, and improved locomotor function (McDonald et al. 1999).
An advancement of the NSC research is the development of methods in isolating human NSCs for clinical applications. Human NSCs have been isolated from embryonic forebrain by growth factor expansion in response to a defined medium containing both FGF-2 and EGF. In contrast to rodent neurospheres, a synergistic combination of these growth factors is required for survival and proliferation of human NSCs (Vescovi et al. 1999). In addition, leukemia inhibitory factor and CNTF, both members of the IL-6 cytokine family that are not required for rodent stem cell survival and proliferation, are capable of enhancing the proliferation and expansion of human stem cells as compared to neurospheres cultured only in EGF and FGF-2 (Carpenter et al. 1999). Human NSCs isolated from first-trimester embryonic forebrain have been shown to remain multipotent for at least one year in vitro (Carpenter et al. 1999).
3.5. Oligodendrocyte progenitor cells
A unique cell type that shows great promise in CNS repair is the oligodendrocyte progenitor cell (OPC). These cells likely represent the progenitor cells of mature oligodendrocytes that produce myelin in the CNS. Oligodendrocytes isolated from the rat optic nerve express A2B5 and platelet-derived growth factor (PDGF) receptor alpha (PDGFR-α) in vitro and can differentiate into oligodendroglia in serum-free medium or type-2 astroglia in serum-containing medium (Raff et al. 1983). Using this culture system, a number of growth factors or cytokines have been found to regulate the survival, proliferation, migration, differentiation, and myelination of the oligodendrocyte lineage (Barres et al. 1992). For example, a combination of PDGF and basic fibroblast growth factor (bFGF, FGF-2) induced a sustained self-renewal of the OPCs and prohibited them from differentiating into oligodendroglia (McKinnon et al. 1990). OPCs can also be differentially induced from neuroepithelial cells (NPCs) (Hu et al. 2004). The generation of OPCs from multipotent NPCs in vivo is thought to be induced by factors produced by the notochord and/or floor plate of the neural tube. When adult mouse brain-derived NPCs were transplanted with delay into the compression-injured adult rat spinal cord, OPCs, oligodendrocytes, and myelin were formed and function improved (Karimi-Abdolrezaee et al. 2006). PDGF, FGF-2, epidermal growth factor were delivered at the transplantation site and both cyclosporine A and minocycline were administered, however, these did not alter spontaneous functional recovery.
The promise of using OPCs for cell therapy primarily lies in their ability to form central myelin on demyelinated axons. Demyelination of intact axons contributes to the physiological and functional deficits after many CNS disorders and trauma. For example, in both human (Bunge et al. 1993) and contusive animal models of SCI (Blight 1985), demyelination due to oligodendrocyte cell death occurs. Apoptosis plays a major role in oligodendrocyte cell death especially in white matter tracts remote to the SCI site (Crowe et al. 1997; Liu et al. 1997). Remyelination of demyelinated intact axons, and regenerated axons as mentioned above, thus represents an important repair strategy to facilitate functional recovery. Moreover, the survival of oligodendrocytes and axons may be interwoven.
It was demonstrated that OPCs (A2B5+/O4−) produced more myelin over a wider area than did the more mature stages of the lineage (O4+/GalC− and GalC+ cells) when transplanted in neonatal shiverer mice (Warrington et al. 1993). As a consequence of this and other studies (Groves et al. 1993), the A2B5+ OPCs became the focus for a number of subsequent transplantation studies. When they were transplanted into x-irradiation + ethidium bromide (X-EB) lesions, as many as 90% of the available axons had been reinvested with central myelin sheaths at 21 d post-transplantation, indicating that transplanted OPCs were able to differentiate into myelinating oligodendrocytes within the lesion environment (Groves et al. 1993). The transplant origin of the myelinating cells was confirmed by labeling them with the marker gene LacZ (Franklin et al. 1997a). A similar degree of remyelination of X-EB lesions can be achieved using an oligodendrocyte progenitor cell line - the CG4 cell line (Franklin et al. 1995).
Demyelination in the ventral and lateral spinal white matter results in locomotor and electrophysiological deficits that also can be seen following a contusive SCI involving this region (Cao et al. 2005b). When OPCs (also called glial restricted precursors or GRPs), infected with retroviruses expressing multi-neurotrophin D15A (with both BDNF and NT-3 activities), were transplanted into the contused adult thoracic spinal cord at 9 days post-injury, they differentiated into mature oligodendrocytes at 6 weeks after transplantation (Cao et al. 2005a). These grafted D15A-GRPs formed typical central myelin around axons in the ventrolateral funiculus (VLF) of the spinal cord. Importantly, they resulted in recovery of transcranial magnetic motor-evoked potential responses, indicating that conduction through the demyelinated VLF axons was restored. Recovery of hindlimb locomotor function was also significantly enhanced in the combined D15A-GRP group indicating this approach may be a useful therapeutic strategy to repair the injured spinal cord.
In a recent study, OPCs were induced to differentiate from human ES cells (Keirstead et al. 2005). When these human-derived OPCs were transplanted into adult rat contusive spinal cord injuries, they survived, redistributed over short distances, and differentiated into oligodendrocytes. Furthermore, animals that received OPCs at 7 days post-injury exhibited enhanced remyelination and substantially improved locomotor ability. These studies demonstrated the ability of human OPCs, pre-differentiated from the human ES cells, to myelinate injured CNS axons and their therapeutic potential in the repair of CNS injuries. The United States Food and Drug Administration recently approved a safety clinical trial by Geron Corporation of OPCs derived from human ES cells for transplantation into spinal cord injured persons.
Whether OPCs could promote regeneration of injured axons remains to be elucidated. The observation that OPCs expressing neither extracellular domain of Nogo-A nor MAG were significantly more permissive than mature oligodendrocytes expressing both indicates that transplantation of OPCs may represent a feasible strategy to foster CNS axonal regeneration (Ma et al. 2009).
3.6. Bone marrow stromal cells
Bone marrow stromal cells (MSCs) are particularly attractive for CNS repair because they are easy to isolate and expand from patients without serious ethical and technical problems. MSCs initially attracted attention because of their stem cell-like attributes and pluripotency (Pittenger et al. 1999). Several laboratories have demonstrated that these cells have the ability to give rise to neurons and glia. These findings, however, have not been corroborated. Moreover, they have been subject to challenges based on issues pertaining to cell fusion and trans-differentiation (Terada et al. 2002; Camargo et al. 2004) as well as to the absence of distinct neuronal morphological features and properties despite their expression of some neuronal markers. Recent observations (Cogle et al. 2004) showing that human hemopoietic cells can trans-differentiate into neurons, astrocytes, and microglia in a long-term setting without fusing have revived the trans-differentiation potential. Whether this applies to MSCs is unclear.
Autologous MSCs can be delivered via intramedullary or intravenous routes. After being transplanted into CNS lesions, they can migrate into the surrounding tissue and differentiate into neurons or astrocytes, enhance tissue repair of the lesion, stimulate regrowth of axons across the lesion site, and promote functional recovery (Zurita et al. 2004). When combined with pegylated BDNF delivery (Ankeny et al. 2001) or BDNF gene-transfer (Lu et al. 2002), MSCs improved axonal regeneration and/or recovery of function after SCI. In related studies, Kocsis and his associates saw increased myelination after the infusion of MSCs either intravenously (Akiyama et al. 2002) or into the spinal cords (Sasaki et al. 2001) of rats damaged by x-ray irradiation. Although MSCs are a promising cell type for cell therapy, the potential mechanisms by which they may be acting remain unclear at the existing level of analysis, although neurotrophic and axonal elongation facilitating actions have been proposed. Also, the functional outcomes reported must be cautiously interpreted because many are primarily based on one evaluation protocol without other correlative behavioral/electrophysiological approaches (Reier 2004). The development of methods for efficient and specific induction of functional post-mitotic neuronal cells from both rat and human MSCs and transplantation of these cells into animal models of CNS trauma and disorders should provide a solid background for the possibility of their clinical application. Autologous MSC transplantation acutely and sub-chronically after complete SCI when combined with granulocyte macrophage-colony stimulating factor delivery was found to be safe and led to modest functional improvements in a Phase I/II open-label and non-randomized study with a control group (Yoon et al. 2007).
4. Combining transplantation-mediated therapy with other repair strategies
Traumatic injury to the CNS initially as well as inevitably disrupts axonal continuity which in turn leads to functional deficits. The extent of the deficits is determined by the severity of the primary lesion, its location, and many complex secondary injury processes. Unfortunately, most axons in the adult mammalian CNS have little intrinsic ability to regenerate. To date, it is clear from experimental and clinical evidence that no single factor accounts for the lack of axonal regeneration after CNS injuries. Failure of successful axon regeneration is particularly attributed to the diminished intrinsic capacity of neurons to regenerate, the presence of physical and chemical barriers associated with the glial scar, and the existence of myelin-associated growth inhibitors in the injured CNS. Therefore, successful functional recovery in patients suffering from CNS injuries will not simply rely on a single therapeutic strategy. There unlikely will be a ‘silver bullet’. The repair strategies discussed above may one day be developed to individually bring about certain degrees of anatomical regeneration and functional improvement. However, the extent of the repair response will not be enough to guarantee optimal biologically significant recovery of neurological function. These individual strategies, however, can be combined to achieve a greater or maximal regeneration and functional recovery. Table 3 summarizes, to our knowledge, the most promising repair strategies that may ultimately be combined. Each of these strategies addresses a specific problem associated with axonal injury, regeneration, and target reinnervation. The effects of combinations that have been evaluated experimentally have been discussed throughout this chapter.
Table 3.
Promising repair strategies that may be combined to enhance CNS regeneration
| 1. | Protecting neurons and axons from secondary injury |
| 2. | Elevating intrinsic capacity of injured CNS axons to regenerate |
| 3. | Bridging the lesion gap with permissive cellular transplants and adhesion molecules |
| 4. | Reducing glial scar formation and deposition of chondroitin sulfate proteoglycans |
| 5. | Overcoming CNS myelin-associated inhibitors |
| 6. | Enhancing directional growth with neurotrophic factors |
| 7. | Enabling reinnervation of denervated targets |
| 8. | Retraining the nervous system to use the therapeutic interventions |
5. Summary and perspectives
In spite of the tremendous efforts of various prevention programs throughout the world, neurotrauma and neurodegenerative diseases occur. To promote CNS axons to regenerate, one critical repair strategy is to stimulate the neurons’ intrinsic capacity to do so. Another effective approach is to overcome the inhibition associated with CNS myelin and/or glial scar. Since damaged axons need a physically growth-permissive terrain to regenerate across the injury site, usually characterized by large cavities, transplantation of tissues or cells, combined with permissive trophic factors and cell adhesion molecules, is essential for creating such a terrain. It now is the consensus that successful functional recovery in patients suffering from CNS injuries will not simply rely on a single therapeutic strategy. Future repair strategies will likely include combination of multiple approaches such as the ones shown in Figure 3. In addition, they may also be combined with neural protection and rehabilitation/physical therapy to maximize neural regeneration, plasticity and functional recovery after CNS injury.
Fig. 3.
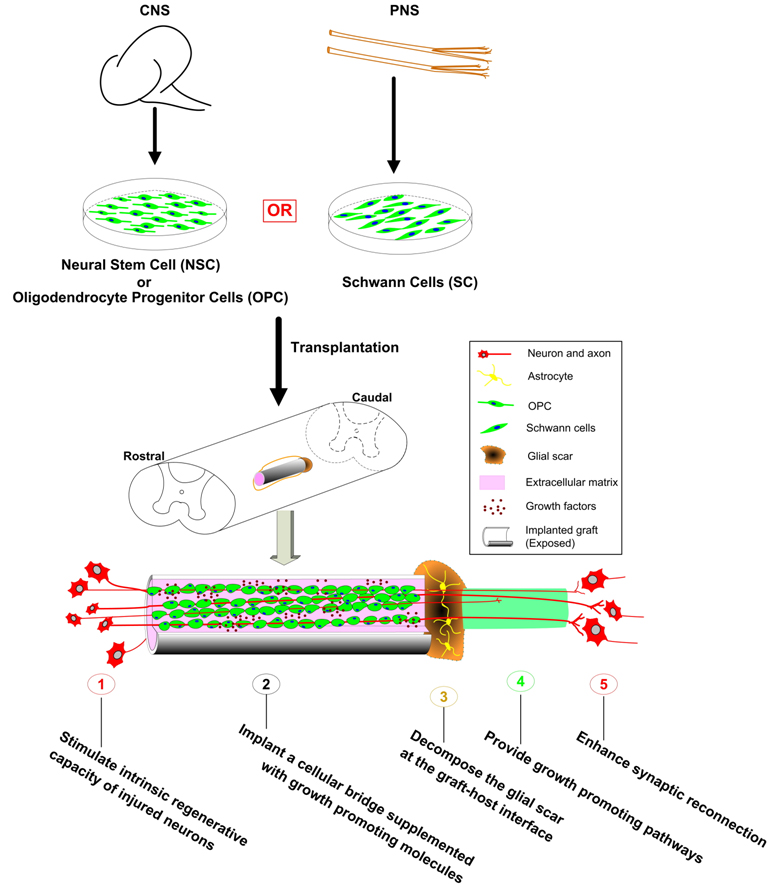
Combinatorial strategies for regeneration of the injured CNS. Both centrally- (such as NSCs or OPCs) or peripherally- (such as Schwann cells) derived cells can be used for transplantation into the CNS. These cells can be combined with other strategies such as boosting the intrinsic regenerative capacity of injured neurons, decomposing the glial scar at the graft-host interfaces, generating growth promoting pathways in the distal host spinal cord, and enhancing synaptic reconnection between regenerating axons and their targets to achieve better anatomical regeneration and functional recovery after various CNS injuries. (Adapted from He and Xu, Chapter 29, Regeneration and Transplantation, In Neuroscience (Han JS and Poo M-M, Eds) (In Press).
Significant progress has been made over the last two decades about injured CNS axon regeneration. It is no longer a matter of whether regeneration can occur. The experimental findings have already led to previous clinical trials and new ones that are underway and being planned. Very importantly, future trials will need to adhere recommendations for their scientific and ethical conduct (Hawryluk et al. 2008). The present and future challenges are to determine in the laboratory how to optimize injured CNS axon regeneration and target site reinnervation so as to maximize respiration and other functional recovery for both chronically and those to be injured persons.
Acknowledgments
This work was supported by NIH grants NS36350, NS52290, NS50243, the Daniel Heumann Fund for Spinal Cord Research, The Indiana Spinal Cord and Brain Injury Research Trust, and Mari Hulman George Endowments (XMX) and NIH 1P30 NS051220 (ED Hall for SMO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny DP, McTigue DM, Guan Z, Yan Q, Kinstler O, Stokes BT, Jakeman LB. Pegylated brain-derived neurotrophic factor shows improved distribution into the spinal cord and stimulates locomotor activity and morphological changes after injury. Exp Neurol. 2001;170:85–100. doi: 10.1006/exnr.2001.7699. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Hutchins AM, Noble M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev Biol. 1993;155:337–350. doi: 10.1006/dbio.1993.1033. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- Blits B, Dijkhuizen PA, Boer GJ, Verhaagen J. Intercostal nerve implants transduced with an adenoviral vector encoding neurotrophin-3 promote regrowth of injured rat corticospinal tract fibers and improve hindlimb function. Exp Neurol. 2000;164:25–37. doi: 10.1006/exnr.2000.7413. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Broude E, McAtee M, Kelley MS. Transplants and neurotrophic factors prevent atrophy of mature CNS neurons after spinal cord injury. Exp Neurol. 1998;149:13–27. doi: 10.1006/exnr.1997.6669. [DOI] [PubMed] [Google Scholar]
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997b;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Bretzner F, Liu J, Currie E, Roskams AJ, Tetzlaff W. Undesired effects of a combinatorial treatment for spinal cord injury--transplantation of olfactory ensheathing cells and BDNF infusion to the red nucleus. Eur J Neurosci. 2008;28:1795–1807. doi: 10.1111/j.1460-9568.2008.06462.x. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31:262–269. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. New York: Raven Press, Ltd; 1993. [PubMed] [Google Scholar]
- Camargo FD, Chambers SM, Goodell MA. Stem cell plasticity: from transdifferentiation to macrophage fusion. Cell Prolif. 2004;37:55–65. doi: 10.1111/j.1365-2184.2004.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos L, Meng Z, Hu G, Chiu DTW, Ambron RT, Martin JH. Enginnering novel spinal circuits to promote recovery after spinal injury. J Neurosci. 2004;24:2090–2101. doi: 10.1523/JNEUROSCI.5526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Liu L, Chen ZY, Wang LM, Ye JL, Qiu HY, Lu CL, He C. Olfactory ensheathing cells genetically modified to secrete GDNF to promote spinal cord repair. Brain. 2004;127:535–549. doi: 10.1093/brain/awh072. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005a;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, Devries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005b;191 Suppl 1:S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Cui X, Hu Z-Y, Jackson J, Sherman S, Seiger A, Wahlberg LU. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- Casella GT, Bunge RP, Wood PM. Improved method for harvesting human Schwann cells from mature peripheral nerve and expansion in vitro. Glia. 1996;17:327–338. doi: 10.1002/(SICI)1098-1136(199608)17:4<327::AID-GLIA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- Cheng H, Liao KK, Liao SF, Chuang TY, Shih YH. Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine. 2004;29:E284–E288. doi: 10.1097/01.brs.0000131217.61390.2c. [DOI] [PubMed] [Google Scholar]
- Chow SY, Moul J, Tobias CA, Himes BT, Liu Y, Obrocka M, Hodge L, Tessler A, Fischer I. Characterization and intraspinal grafting of EGF/bFGF-dependent neurospheres derived from embryoic rat spinal cord. Brain Res. 2000;874:87–106. doi: 10.1016/s0006-8993(00)02443-4. [DOI] [PubMed] [Google Scholar]
- Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scott EW. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet. 2004;363:1432–1437. doi: 10.1016/S0140-6736(04)16102-3. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Gauthier P. Regeneration of acutely and chronically injured descending respiratory pathways within post-traumatic nerve grafts. Neuroscience. 2002;112:141–152. doi: 10.1016/s0306-4522(02)00052-0. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette R. Glial cells in the nerve fiber layer of the main olfactory bulb of embryonic and adult mammals. Microsc Res Tech. 1993;24:113–130. doi: 10.1002/jemt.1070240204. [DOI] [PubMed] [Google Scholar]
- Evans PD, Reale V, Villegas J. The role of cyclic nucleotides in modulation of the membrane potential of the Schwann cell of squid giant nerve fibre. J Physiol. 1985;363:151–167. doi: 10.1113/jphysiol.1985.sp015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: Combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord. 2009 doi: 10.1038/sc.2009.10. [DOI] [PubMed] [Google Scholar]
- Fraidakis MJ, Spenger C, Olson L. Partial recovery after treatment of chronic paraplegia in rat. Exp Neurol. 2004;188:33–42. doi: 10.1016/j.expneurol.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Bayley SA, Milner R, Ffrench-Constant C, Blakemore WF. Differentiation of the O-2A progenitor cell line CG-4 into oligodendrocytes and astrocytes following transplantation into glia-deficient areas of CNS white matter. Glia. 1995;13:39–44. doi: 10.1002/glia.440130105. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Blakemore WF. Transplanting oligodendrocyte progenitors into the adult CNS. J Anat. 1997a;190(Pt 1):23–33. doi: 10.1046/j.1469-7580.1997.19010023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM, Barnett SC. Do olfactory glia have advantages over Schwann cells for CNS repair? J Neurosci Res. 1997b;50:665–672. doi: 10.1002/(SICI)1097-4547(19971201)50:5<665::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Groves AK, Barnett SC, Franklin RJM, Crang AJ, Mayer M, Blakemore WF, Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Guest J, Herrera LP, Qian T. Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord. 2006;44:135–142. doi: 10.1038/sj.sc.3101820. [DOI] [PubMed] [Google Scholar]
- Guest JD, Herrera L, Margitich I, Oliveria M, Marcillo A, Casas CE. Xenografts of expanded primate olfactory ensheathing glia support transient behavioral recovery that is independent of serotonergic or corticospinal axonal regeneration in nude rats following spinal cord transection. Exp Neurol. 2008;212:261–274. doi: 10.1016/j.expneurol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- Hirschberg DL, Schwartz M. Macrophage recruitment to acutely injured central nervous system is inhibited by a resident factor: a basis for an immune-brain barrier. J Neuroimmunol. 1995;61:89–96. doi: 10.1016/0165-5728(95)00087-i. [DOI] [PubMed] [Google Scholar]
- Horvat JC. Transplants of fetal neural tissue and autologous peripheral nerves in an attempt to repair spinal cord injuries in the adult rat. An overall view. Paraplegia. 1991;29:299–308. doi: 10.1038/sc.1991.44. [DOI] [PubMed] [Google Scholar]
- Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JG, Fu SL, Zhang KH, Li Y, Yin L, Lu PH, Xu XM. Differential gene expression in neural stem cells and oligodendrocyte precursor cells: a cDNA microarray analysis. J Neurosci Res. 2004;78:637–646. doi: 10.1002/jnr.20317. [DOI] [PubMed] [Google Scholar]
- Iarikov DE, Kim BG, Dai HN, McAtee M, Kuhn PL, Bregman BS. Delayed transplantation with exogenous neurotrophin administration enhances plasticity of corticofugal projections after spinal cord injury. J Neurotrauma. 2007;24:690–702. doi: 10.1089/neu.2006.0172. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: A neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead H, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3:173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, Roy RR, Edgerton VR, Ramon-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Smith PM, Barnett SC, Franklin RJ. Meningeal cells enhance limited CNS remyelination by transplanted olfactory ensheathing cells. Brain. 2003;126:598–609. doi: 10.1093/brain/awg055. [DOI] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Zeev-Brann AB, Hirschberg DL, Schwartz M. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- Lee Y-S, Hsiao I, Lin VW. Peripheral nerve grafts and aFGF restore partial hindlimb function in adult paraplegic rats. J Neurotrauma. 2002;19:1203–1216. doi: 10.1089/08977150260338001. [DOI] [PubMed] [Google Scholar]
- Levi AD, Dancausse H, Li X, Duncan S, Horkey L, Oliviera M. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg. 2002;96:197–205. doi: 10.3171/spi.2002.96.2.0197. [DOI] [PubMed] [Google Scholar]
- Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–731. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci. 1998;18:10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med. 2006;29:191–203. doi: 10.1080/10790268.2006.11753874. discussion 204–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Himes BT, Solowska J, Moul J, Chow SY, Park KI, Tessler A, Murray M, Snyder EY, Fischer I. Intraspinal delilvery of neurotrophin-3 using neural stem cells genetically modified by recombinant retrovirus. Exp Neurol. 1999;158:9–26. doi: 10.1006/exnr.1999.7079. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Navarro X, Verdu E. Chronic transplantation of olfactory ensheathing cells promotes partial recovery after complete spinal cord transection in the rat. Glia. 2007;55:303–311. doi: 10.1002/glia.20457. [DOI] [PubMed] [Google Scholar]
- Lu P, Tuszynski MH. Transplantation of bone marrow stromal cells (MSCS) and BDGF-transduced MSCS promotes robust axonal growth after spinal cord injury. Soc Neurosci Abs. 2002 [Google Scholar]
- Ma Z, Cao Q, Zhang L, Hu J, Howard RM, Lu P, Whittemore SR, Xu XM. Oligodendrocyte precursor cells differentially expressing Nogo-A but not MAG are more permissive to neurite outgrowth than mature oligodendrocytes. Exp Neurol. 2009;217:184–196. doi: 10.1016/j.expneurol.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PA, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Liu X-Z, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- Moon LD, Leasure JL, Gage FH, Bunge MB. Motor enrichment sustains hindlimb movement recovered after spinal cord injury and glial transplantation. Restor Neurol Neurosci. 2006;24:147–161. [PubMed] [Google Scholar]
- Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11:2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negredo P, Rivero JL, Gonzalez B, Ramon-Cueto A, Manso R. Slow- and fast-twitch rat hind limb skeletal muscle phenotypes 8 months after spinal cord transection and olfactory ensheathing glia transplantation. J Physiol. 2008;586:2593–2610. doi: 10.1113/jphysiol.2007.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth J-O. Differential effects of neurotrophins on neuronal survival and axonal regeneration after spinal cord injury in adult rats. J Comp Neurol. 2002;452:255–263. doi: 10.1002/cne.10381. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Whittemore SR, Holets VR. Variable morphological differentiation of a raphe-derived neuronal cell line following transplantation into the adult rat CNS. Exp Neurol. 1993;122:130–142. doi: 10.1006/exnr.1993.1114. [DOI] [PubMed] [Google Scholar]
- Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Marcillo AE, Oudega M, Lynch MP, Wood PM, Bunge MB. Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J Neurotrauma. 2004;21:1223–1239. doi: 10.1089/neu.2004.21.1223. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Polentes J, Stamegna JC, Nieto-Sampedro M, Gauthier P. Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol Dis. 2004;16:638–653. doi: 10.1016/j.nbd.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Raff MC, Hornby-Smith A, Brockes JP. Cyclic AMP as a mitogenic signal for cultured rat Schwann cells. Nature. 1978;273:672–673. doi: 10.1038/273672a0. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and regeneration of the nervous system. New York: Hafner; 1928. [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Rasouli A, Bhatia N, Suryadevara S, Cahill K, Gupta R. Transplantation of preconditioned schwann cells in peripheral nerve grafts after contusion in the adult spinal cord. Improvement of recovery in a rat model. J Bone Joint Surg Am. 2006;88:2400–2410. doi: 10.2106/JBJS.E.01424. [DOI] [PubMed] [Google Scholar]
- Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Plant GW, Hamers FP, Wortel J, Blits B, Dijkhuizen PA, Gispen WH, Boer GJ, Verhaagen J. Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci. 2003;23:7045–7058. doi: 10.1523/JNEUROSCI.23-18-07045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Smith AG. New prospects for human stem-cell therapy in the nervous system. Trends Neurosci. 1999;22:357–364. doi: 10.1016/s0166-2236(99)01428-9. [DOI] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Uthman B, Mott S, Fessler RG, Behrman A, Trimble M, Anderson DK, Wirth ED., 3rd Neurophysiological assessment of the feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18:931–945. doi: 10.1089/089771501750451848. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A, Galli R. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- Warrington AE, Barbarese E, Pfeiffer SE. Differentiation myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J Neurosci Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- Wirth ED, 3rd, Reier PJ, Fessler RG, Thompson FJ, Uthman B, Behrman A, Beard J, Vierck CJ, Anderson DK. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18:911–929. doi: 10.1089/089771501750451839. [DOI] [PubMed] [Google Scholar]
- Wood PM. Separation of functional Schwann cells and neurons from normal peripheral nerve tissue. Brain Res. 1976;196:247–252. doi: 10.1016/0006-8993(76)90355-3. [DOI] [PubMed] [Google Scholar]
- Xiao M, Klueber KM, Lu C, Guo Z, Marshall CT, Wang H, Roisen FJ. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp Neurol. 2005;194:12–30. doi: 10.1016/j.expneurol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995a;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995b;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhang SX, Li H, Aebischer P, Bunge MB. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Ye J-H, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Experimental Neurology. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, Park H, Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25:2066–2073. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004;15:1105–1108. doi: 10.1097/00001756-200405190-00004. [DOI] [PubMed] [Google Scholar]


