Abstract
Secretory phospholipases A2 (sPLA2s) are a subfamily of lipolytic enzymes which hydrolyze the acyl bond at the sn-2 position of glycerophospholipids to produce free fatty acids and lysophospholipids. These products are precursors of bioactive eicosanoids and platelet-activating factor (PAF). The hydrolysis of membrane phospholipids by PLA2 is a rate-limiting step for generation of eicosanoids and PAF. To date, more than 10 isozymes of sPLA2 have been found in the mammalian central nervous system (CNS). Under physiological conditions, sPLA2s are involved in diverse cellular responses, including host defense, phospholipid digestion and metabolism. However, under pathological situations, increased sPLA2 activity and excessive production of free fatty acids and their metabolites may lead to inflammation, loss of membrane integrity, oxidative stress, and subsequent tissue injury. Emerging evidence suggests that sPLA2 plays a role in the secondary injury process after traumatic or ischemic injuries in the brain and spinal cord. Importantly, sPLA2 may act as a convergence molecule that mediates multiple key mechanisms involved in the secondary injury since it can be induced by multiple toxic factors such as inflammatory cytokines, free radicals, and excitatory amino acids, and its activation and metabolites can exacerbate the secondary injury. Blocking sPLA2 action may represent a novel and efficient strategy to block multiple injury pathways associated with the CNS secondary injury. This review outlines the current knowledge of sPLA2 in the CNS with emphasis placed on the possible roles of sPLA2 in mediating CNS injuries, particularly the traumatic and ischemic injuries in the brain and spinal cord.
Keywords: Phospholipases A, spinal cord injury, ischemia, excitatory amino acids, reactive oxygen species, inflammation, lipid metabolism, cytokines
INTRODUCTION
Phospholipases A2 (EC 3.1.1.4) are enzymes that catalyze the hydrolysis of the sn-2 position of membrane glycerophospholipids, leading to the production of free fatty acids and lysophospholipids. These enzymes are of particular interest since free fatty acids can be converted to bioactive ecosainoids via the cycloxygenase pathway leading to increased inflammation. Additionally, the other reaction product, lysophospholipids, such as lysophosphatidic acid and lysophosphatidylcholine (LPC), are also bioactive [1] and can be converted into platelet-activating factor (PAF). Since lipids are a main constituent of the CNS and phospholipids constitute 44% of myelin [2], understanding the role of phospholipases in CNS disorders becomes a major priority.
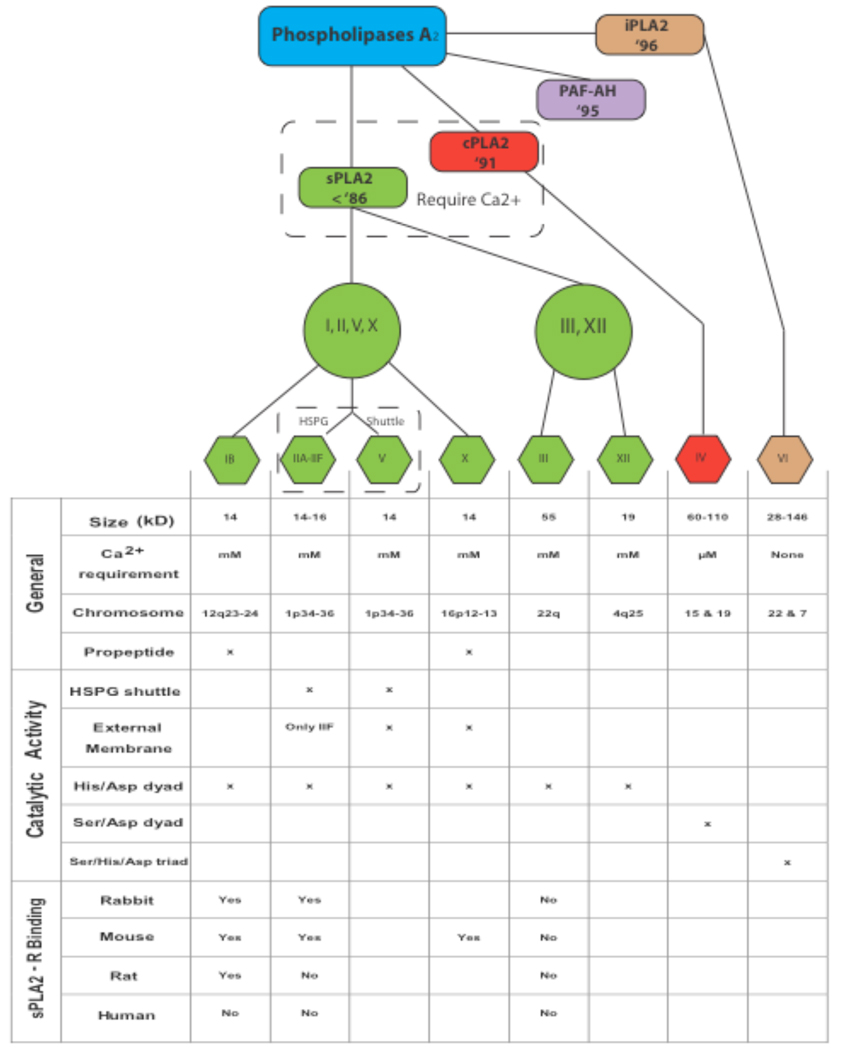
To date, more than 27 mammalian isoforms of PLA2 have been found which can be classified into four major categories: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), Ca2+-independent PLA2 (iPLA2), and PAF acetylhydrolases (PAF-AH) [3–6] (Fig. 1). The 11 mammalian isozymes in the sPLA2 subfamily have a low molecular mass of about 14–18 kD, require the presence of submillimolar to millimolar concentrations of Ca2+ for effective hydrolysis of a substrate phospholipid, and lack fatty acid selectivity [3, 6, 7].
Fig. (1). Classification of the mammalian PLA2 isoforms.
The top panel shows a branching diagram indicating the relative subdivisions of the PLA2 subfamily and their years of discovery. The mammalian PLA2 family of enzymes is grossly divided into the sPLA2, cPLA2, iPLA2, and PAF-AH. The sPLA2 subfamily is further divided into groups IB, group II and V, group X, and group III and XII based on structural and functional differences presented in the table below. HSPG: heparin sulfate proteoglycans.
The functions of sPLA2s are far reaching, including digestion, exocytosis [8], and anticoagulation [9]. However, sPLA2s most prominent role is in pathological conditions such as neurotrauma [10], antimicrobial activity [11–13], ischemia [14], atherosclerosis [15–17], and cancer [18–21]. In this review we will focus on the role of sPLA2s in CNS pathology, particularly spinal cord injury (SCI).
Role in General Inflammation
sPLA2 has had an established role in inflammation and inflammatory diseases for some time [22]. The blockade of PLA2 holds a particular interest for pharmacologists since inhibition of sPLA2 would in theory prevent the formation of inflammatory eicosanoids prior to the cyclooxygenase (COX; EC 1.14.99.1) reaction. In fact, PLA2 is the rate limiting precursor in arachidonic acid (AA) production [23]. Therefore its blockade should eliminate the need for COX-1 versus COX-2 specificity in anti-inflammatory therapeutics. This theory has spurred the development of a large number of sPLA2 inhibitors that unfortunately, have not produced the desired clinical efficacy to date [24].
sPLA2s have been linked to many inflammatory diseases. sPLA2 activity is elevated in several body fluids of patients with acute pancreatitis [25]. Synovial fluid from arthritic joints of rheumatic patients contains sPLA2-IIA [26, 27]. Total PLA2 activity and sPLA2-IIA is enhanced in bronchoalveolar lavage fluids from patients with adult respiratory distress syndrome [28]. Increased levels of sPLA2-IIA were seen in the skin of patients with psoriasis [29]. Increased group II, PLA2 expression was found in colonic mucosa of patients with Crohn’s Disease and ulcerative colitis [30] and experimental models of ischemic bowel disease in rodents [31, 32]. Additionally, serum levels of sPLA2, particularly group IIA, increase in patients with sepsis [33, 34] and injuries [22, 35], and following many types of surgeries such as cardiac surgery [36], aortobifemoral reconstruction [36], and splenectomy [12]. Levels of serum sPLA2-IIA correlate with C-reactive protein in several disease states, supporting the notion that sPLA2-IIA is an acute phase protein [37]. Some suggest that elevations in serum levels of sPLA2-IB are a specific marker of pancreatic damage whereas elevation in sPLA2-IIA are a more general marker of inflammation [22].
sPLA2 has been either found in or produced by various inflammatory cells including platelets [38], mast cells [39], fibroblasts [40], macrophages [41, 42] and neutrophils [43]. Macrophages isolated from peritoneal exudates of mice and rabbits secrete PLA2 [42]. Additionally, sPLA2-IIA is constitutively expressed in immune tissues, such as the spleen, thymus, tonsils, and bone marrow [26, 27]. Proinflammatory cytokines such as interferon gamma (IFN-γ), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) can induce its production in a variety of cell types such as human arterial smooth muscle cells, HepG2, HEK293, and BRL-3A in culture [44–46]. Interestingly the AA release from cells treated with IIA, IID, IIE and V were found to be dependent on IL-1, while treatment with group X released AA irrespective of IL-1 [6]. More relevant to the CNS, TNFα, IL-1, and lipopolysaccharide (LPS) were shown to induce sPLA2-IIA production in cultured astrocytes and direct injection of LPS into brain increased IIA mRNA [47]. Similarly LPS injections have been shown to increase sPLA2-IIE production [48]. Consistent with its inducible nature, the promoter region of sPLA2-IIA gene contains TATA and CAAT boxes as well as several elements homologous with consensus sequences for binding of transcription factors such as AP-1, C/EBPs, CREB, NF-κB, STAT, and PPARγ [37, 49]. Finally, sPLA2 induction can be blocked by anti-inflammatory cytokines, such as platelet-derived growth factor, transforming growth factor β, and IL-10 as well as glucocorticoids [50–52].
CLASSIFICATION, STRUCTURE, AND PROPERTIES OF sPLA2
General Structure
Eleven mammalian sPLA2s exist which are further divided into groups I, II, III, V, X, XII, and XIII (Fig. 1). All sPLA2s are structurally related, and generally are 14–17 kD secreted enzymes, with six absolutely conserved disulfide bonds, which contribute to the high degree of stability of these enzymes. The enzymes do not have strict fatty acid specificity, unlike cPLA2, but instead tend to act on anionic phospholipids in the presence of high concentrations of Ca2+. The central, core protein consists of a highly conserved Ca2+-binding loop (XCGXGG) and a catalytic site (DXCCXXHD) [6]. Group differentiations are then made based on the presence or absence of up to two additional unique disulfide bonds, the presence or absence of an N- or C-terminal extension, and alterations in the conserved catalytic site.
Intracellular Handling
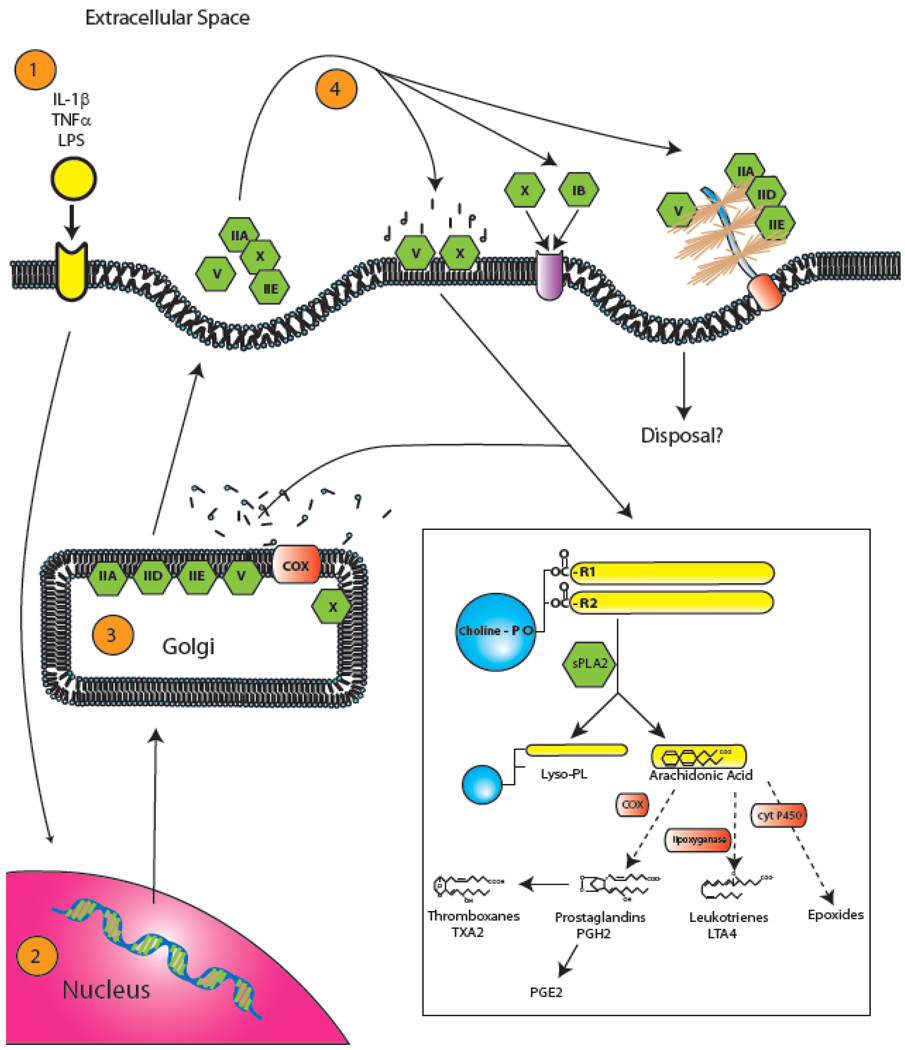
The site of sPLA2 activity has been a point of exhaustive study in recent years. While all sPLA2 isoforms are capable of secretion by definition, recent work has indicated that some heterogeneity exists among their site of activity [53]. Recent studies in CHO and HEK293 cell lines that have been stably transfected with human groups IIA and X have shed light on this subject. Following stimulation by fetal bovine serum and IL-1β, both sPLA2-IIA and X are transcribed within the cell nucleus and synthesized in the endoplasmic reticulum prior to packaging in the Golgi apparatus [54] (Fig. 2). This post synthesis packaging most likely results in the perinuclear puncta observed after stimulation, an observation which was previously ascribed to invagination of heparin sulfate proteoglycans (HSPG)-bound sPLA2, a process described later. It is within the Golgi apparatus and later microvesicles, prior to initial secretion, that sPLA2-IIA is primarily active [53]. The reason for this is that most group II sPLA2s as well as sPLA2-IB show a marked preference for anionic phospholipids, such as phosphatidylglycerol, phosphatidylethanolamine and phosphatidylserine, which are generally segregated to the inner leaflet of plasma membranes [55]. In contrast, sPLA2-V and X can hydrolyze both anionic phospholipids and charged-neutral phosphatidylcholines. This difference in phospholipid preference results in secreted groups I and II having decreased abilities to act on the outer layer of plasma membranes [5, 53]. Once secreted, sPLA2 isoforms can then 1) metabolize the external plasma membrane, 2) bind to the sPLA2 receptor (sPLA2-R), or 3) be internalized by the HSPG shuttle. Again, each of these actions is governed by isoform and species specificity (Fig. 2).
Fig. (2). Intracellular handling and sPLA2 activity.
Following stimulation by various cytokines [1], sPLA2 is synthesized in the nucleus [2] and endoplasmic reticulum prior to packaging for secretion in the Golgi apparatus [3]. It is within the Golgi apparatus and later microvesicles that certain isoforms, particularly IIA, are predominantly active. Following secretion [4], sPLA2 can metabolize the extracellular lipid membrane directly, bind to the sPLA2 receptor (sPLA2-R), and/or be endocytosed via the heparin sulfate proteoglycan shuttle (HSPG shuttle). Of course, each of these actions is governed by species and isoform specificity. The inset shows the general metabolism of phospholipids by sPLA2. sPLA2 first hydrolyzes the acyl bond at the sn-2 position of glycerophospholipids to produce free fatty acids (such as arachidonic acid) and lysophospholipid (Lyso-PL). Arachidonic acid can then be further modified by COX to form prostaglandins, lipoxygenase to form leukotrienes, or cytochrome P450 to form epoxides. Prostaglandins can be further modified to form thromboxanes. These eicosanoids have metabolic activities including proinflammatory and vasoconstrictive functions.
HSPG Shuttling
Some of the group II subfamily of sPLA2s, including IIA, IID, and V are highly cationic and bind tightly to anionic heparanoids such as heparin and heparin sulfate [56, 57] (Fig. 2). Since cell surfaces are usually rich in heparin sulfate proteoglycans (HSPG), significant portions of these sPLA2s are membrane-bound rather than being secreted into the culture media [58, 59]. Other sPLA2s (IB, IIC, and X) with neutral to acidic pHs show no, or very low, heparanoid affinity and are secreted into the medium. Some authors suggest that this binding facilitates phospholipid digestion since treatment of stably transfected cells with heparinase, exogenous heparin, and GPI-specific phospholipase C [45, 46, 60, 61], or mutation of the heparin binding domain in sPLA2-IIA [56–58] markedly attenuates sPLA2-IIA-mediated prostaglandin generation. In contrast, one recent study found that treatment of cells with heparin had little or no effect on sPLA2-IIA activity [54]. Also of note, HSPG has been shown to increase following cerebral stab injury with an implicated role in storage, nuclear trafficking, and cell-specific injury responses in CNS wounds [62]. The closely related chondroitin sulfate proteoglycans are also greatly expressed following SCI [63, 64].
Receptor Binding Domain
In addition to its enzymatic function, some sPLA2s mediate their biological function through a membrane receptor. Two sPLA2 receptors have been identified to date, the M-type [65–67] and the N-type [68], named for their discovery in muscle and neural tissue respectively. However, mammalian sPLA2s only bind to the M-type which was later discovered to be relatively widespread and not merely confined to muscle [69]. Since only the M-type has the ability to initiate an intracellular signal we will restrict our discussion to its properties and further refer to it as sPLA2-R. The receptor is a type 1 transmembrane glycoprotein with a molecular mass of 180–220 kDa and is a member of the C-type animal lectin family (subgroup VI) [70]. The intracellular region consists of approximately 40 amino acids and contains both a consensus sequence for casein-kinase II phosphorylation [71] and a consensus sequence for coated-pit-mediated endocytosis [70]. Accordingly, the receptor undergoes internalization after sPLA2 binding [72]. Interestingly the isozymes of sPLA2 show varying affinities for the sPLA2-R in different species. For example, the rabbit sPLA2-R is very promiscuous, binding to almost all sPLA2 tested to date. The mouse sPLA2-R on the other hand binds only IB, IIA, and X, while the rat sPLA2-R only binds sPLA2-IB [73] (Fig. 1). Likewise sPLA2-IIA does not seem to bind to the sPLA2-R in humans [73]. In general, sPLA2-IB and X appear to be the predominant ligand of this receptor [74] and most of the research has therefore focused on their effects [71, 75, 76]. This specificity suggests that the biological function of sPLA2-R could vary wildly among species. However, the generalized functions of the sPLA2-R are far reaching including cell growth [77], proliferation [78], and migration [79]. It has also been suggested that sPLA2-R functions in the clearance of extracellular sPLA2s to protect against enzymatic over activity, particularly sPLA2-X which has potent activity against the extracellular membrane, unlike IB and IIA as stated above [68, 80]. Additionally, sPLA2-R knockout mice have significantly reduced levels of TNFα and IL-1β after systemic LPS administration suggesting an inflammatory role [81]. For an excellent review on the role of the sPLA2-R in sPLA2 function, please see [68, 82].
Group I –
sPLA2-IB was the first sPLA2 to be discovered and is predominantly present in pancreatic juices [83]. sPLA2-IB lacks a C-terminal extension and is secreted as a catalytically inactive propeptide that is later proteolytically cleaved [84]. Group IB has a unique five amino acid extension termed the pancreatic loop in the middle of the molecule as well as a group specific disulfide bond between Cys11 and Cys77 [83, 84]. sPLA2-IB is almost exclusively secreted into the medium of transfected cells [6]. Interestingly, sPLA2-IB shows low affinity for both heparinoids and phosphatidylcholine (PC) on the external membrane leaflet. Subsequently, it was discovered that sPLA2-IB can only release AA indirectly through the M-type sPLA2 receptor-dependent pathway via cPLA2α activation [71, 75, 76].
Groups II and V –
The second member of the sPLA2 family, later named group IIA, was first cloned in 1989 [85] and is constitutively expressed in immune tissues, such as the spleen, thymus, tonsils and bone marrow [26, 27] as well as the digestive system of some mouse strains [86]. sPLA2-IIA [26, 27, 85], IID [87, 88], and IIE [89, 90] are the archetypical group II enzymes. Typically, the enzymes have a C-terminal extension and a disulfide bond linking Cys50 with a Cys in the C-terminus. IIC [91] and IIF [92] have minor variations in amino acids and disulfide structure. Similar to IIA, sPLA2-IIE is constitutively expressed in human lung and mouse uterus, brain, heart, liver and testis at low levels [48, 87, 90]. However, LPS has been shown to induce IIE expression in macrophages, suggesting an inflammatory role as well as [90]. sPLA2-IIF is highly expressed in the mouse embryo, suggesting a roll in development and it is upregulated by LPS as with other group II enzymes [87, 93]. Finally, groups IIA, IIC, IID, IIE, and V all utilize the HSPG-shuttling pathway.
Often considered in the same breath with group II enzymes is group V. Group V shows the highest homology with the group II enzymes and is similarly located on human chromosome 1 (1p34–36) [92]. However group V lacks the typical group II C-terminal region, thus justifying its isolation. sPLA2-V functions as the primary mouse sPLA2. This is since mice express group V at higher levels than any of the group II enzymes [94] and since some species of mice have a frame shift mutation resulting in a natural sPLA2-II knock out [86]. However, as with group II, group V is closely linked with inflammation as well as being found in mast cells [94], macrophages [95], and type 2 T helper cells [96]. As with group II, group V is upregulated by LPS [94].
Group X –
Group X possesses characteristics of both group I and group II enzymes. sPLA2-X contains the disulfide bonds of both group I and group II as well as the group II, C-terminal extension [97]. Additionally, like group I, it is secreted as a zymogen with cleavage of the N-terminal propeptide for activation [98]. Like sPLA2-IB, cells transfected with group X, secrete this sPLA2 almost exclusively into the culture medium rather than having it bound to the membrane like group II enzymes [6]. This is not surprising since unlike group II, group X does not readily bind HSPG and shows high activity towards PC, a dominant phospholipid enriched in the outer leaflet of the plasma membrane [19, 58, 61, 74, 98]. Typically, sPLA2-X is expressed in the digestive organs such as intestine, colon and stomach and in some immune organs [97]. However, unlike group II, group X is constitutively expressed with little or no change in most tissues [6].
Groups III and XII –
A distinct class of soluble sPLA2s, distantly related to groups I and II, were later discovered in bee and lizard venom and are classified as group III. A mammalian homolog of group III was discovered in 2000 [89]. Groups III and XII only share the Ca2+-binding loop and catalytic site with groups I, II, V, and X. At 55kDa, Group III is considerably large than all the other sPLA2 isozymes. While maintaining all the sPLA2 signature characteristics such as, 10 cystines, the Ca2+-biniding loop, and catalytic site, it additionally has a large N- and C- terminal flanking regions that add to its molecular weight [89]. Within humans, sPLA2-III was found to be in high abundance in heart, skeletal muscle, liver, and kidney, but had only weak expression in the brain [89]. Group-XII is a much smaller enzyme than group III (19 kDa), lacks an N- or C-terminal flanking regions, and has deviations in the Ca2+ bind loop, that are inconsistent with other sPLA2s [96, 99]. Group XII is expressed in human kidney, heart, and skeletal muscle [89, 99]. Relatively little is known about the function of either group III or XII in the mammalian system.
MECHANISM UNDERLYING sPLA2-INDUCED CNS INJURY
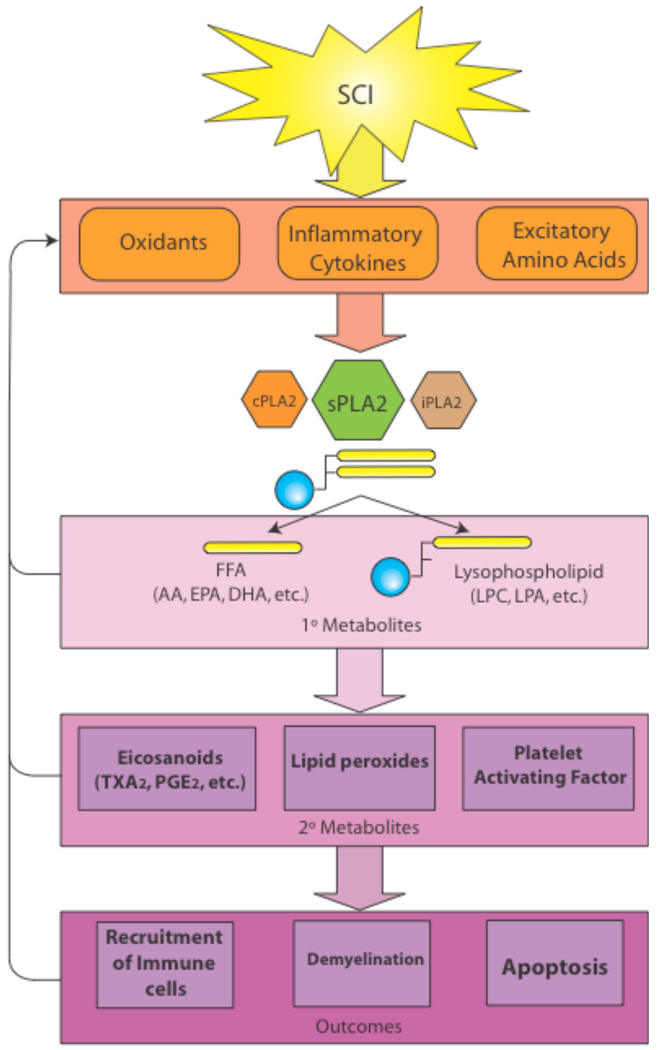
The sPLA2 family of enzymes results in CNS damages by multiple injury mechanisms such as attacking cellular membranes, releasing pro-inflammatory mediators, generating free radicals, increasing release of excitotoxic neurotransmitters and enhancing apoptosis [100–102]. These insults in turn exacerbate activation of sPLA2 by a positive feedback loop (Fig. 3). Our discussion will begin by looking at several injury mechanisms common to CNS pathology and how each induces and is exacerbated by sPLA2.
Fig. (3). Overview of sPLA2’s role in spinal cord injury.
The toxicity of sPLA2 is compounded by three factors. 1) sPLA2 is upregulated by commonly known neurotoxic mechanisms such as oxidative stress, cytokines, and EAA. 2) Both the primary metabolites of sPLA2 activity, such as free fatty acids and lysophospholipids, and the secondary metabolites, such as eicosanoids and platelet activating factor, are toxic to the CNS. 3) Finally, sPLA2 has been shown to reciprocally upregulate oxidative stress, cytokines, and EAA thus propagating a positive feedback loop resulting in cytotoxicity and secondary SCI. It must also be noted that sPLA2 does not work in isolation from cPLA2 and iPLA2, rather a reciprocal activity is often demonstrated among the PLA2 subfamilies.
Oxidative Stress
Oxidative injury is a common pathological mechanism in neurological disorders. Free radicals induce not only lipid peroxidation of neural membranes, but also the oxidation of proteins, RNAs, and DNAs. Reactive oxygen species (ROS) including hydrogen peroxide (H2O2) and superoxide radicals are produced by a number of cellular oxidative and metabolic processes including metabolism of AA. PLA2 metabolism of phospholipids is a well-established source of ROS [103, 104]. Application of pathophysiological concentrations of free fatty acids has been demonstrated to induce oxidative injury to cultured spinal cord neurons [133]. Microinjections of PLA2 into the normal spinal cord induced expression of 4-hydroxynonenal, a product of lipid peroxidation and marker for oxygen free radical-mediated membrane injury [105]. Nethery et al. [106], found that ROS production in contracting muscle required the presence of sPLA2 but not cPLA2 or iPLA2. In the presence of 15-lipoxygenase the addition of sPLA2-IIA or IB greatly enhances the accumulation of hydroperoxides of oxidized free fatty acids [107]. Finally, it can be assumed that the proinflammatory effects of sPLA2 would result in an immigration of immune cells, which will release copious amounts of ROS [108–113]. For a review of the ROS species produced by the enzymatic effects of sPLA2 please see [104].
While sPLA2 production of ROS is well established, the induction of sPLA2 by ROS is an immerging concept. One study found that mesangial cells treated with H2O2 utilized both cPLA2 and sPLA2 during AA release and that elimination of sPLA2 greatly inhibited AA release [114], thus suggesting the first of many positive feedback loops.
Inflammation and Inflammatory Cytokines
Inflammation has been implicated in many CNS neurological disorders including SCI. PLA2 may serve as a key molecule that controls the biosynthesis of several well-known bioactive mediators of inflammation such as eicosanoids (prostaglandins, thromboxanes, leukotrienes and lipoxins) and PAF in a rate-limiting manner [102, 115]. In addition, our recently findings showed that sPLA2 induced expression of cytokines including TNF-α and IL-1β in the injured spinal cord [105]. On the other hand, there is also strong evidence to suggest that cytokines upregulate sPLA2-IIA in vitro and in vivo. sPLA2-IIA is up-regulated by TNF-α and IL-1α/β after transient focal cerebral ischemia in rats [116]. sPLA2-IIA mRNA is expressed in cultured astrocytes and can be induced in response to proinflammatoy cytokines TNFα, IL-1β, and, INFγ [47, 117–119]. Astrocytes isolated from C57Bl/6 mice, which lack the sPLA2-IIA gene, were less responsive to cytokines in the production of PGE2 than were astrocytes expressing sPLA2-IIA [119]. Additionally, IL-1β and TNFα can also activate COX-2 continuing the proinflammatory pathways [19, 45, 59, 94, 120]. sPLA2 also induced AA release and COX-2 expression in cultured neurons independent of other cytokines [121].
Excitatory Neurotoxicity
Elevated levels of excitatory amino acids (EAA) have been implicated in the pathogenesis of neural injury and death in many disorders and evidence suggests that sPLA2 may stimulate the release of EAA following CNS trauma. First, injections of sPLA2 into the brain caused epileptic seizures and neurotoxicity in vivo [122]. Secondly, application of sPLA2 to the rat ischemic cerebral cortex resulted in a significant increase in EAA levels and inhibition with mepacrine, a global PLA2 inhibitor, significantly decreased the ischemia-evoked efflux of EAA into cortical superfusates [123]. Group IIA sPLA2 stimulates exocytosis and neurotransmitter release in pheochromocytoma-12 cells and cultured rat hippocampal neurons [124]. sPLA2-induced neuronal death was blocked by MK-801, an N-methyl-D-aspartic acid (NMDA) receptor antagonist, both in vitro and in vivo [125]. Finally, administration of the nonselective PLA2 inhibitor, 4-bromophenacyl bromide, inhibited glutamate release in the spinal cord [126]. Only one study so far has suggested that excessive EEA concentrations increase sPLA2 levels in the CNS. Interocerebroventricular injection of kainic acid (KA) resulted in an increase in both total PLA2 and sPLA2 activity and this activity could be blocked by a synthetic short inhibitor peptide for sPLA2-IIA [127].
The exact mechanism of sPLA2 action on EAA release remains unknown. It has been suggested that PLA2 disrupts an artificial planar lipid bilayer in a Ca2+-dependent manner [128]. Matsuzawa demonstrated the role of sPLA2-IIA in EEA release in a set of elegant experiments. First, sPLA2-IIA was detected in purified brain synaptosomes. Secondly, sPLA2-IIA was released upon depolarization of neurons with either high concentrations of potassium or neurotransmitters. Third, addition of sPLA2-IIA to cultures triggered neurotransmitter release. Finally, sPLA2-IIA inhibition suppressed neurotransmitter secretion [8]. The role of sPLA2 in neurotransmitter release is further supported by the fact that exogenously added sPLA2 paralyzed neuromuscular junction preparations by inducing total neurotransmitter release [129]. Interestingly, an equimolar mixture of lysophospholipids and fatty acids closely mimicked the sPLA2 paralysis [129]. These studies further suggest that changes in the local lipid composition within the synaptic buton trigger neurotransmitter release which would lead to excitotoxicity [130]. Inhibitors of total PLA2 activity such as 4-bromophenacyl bromide, a nonselective PLA2 inhibitor, 7,7-dimethyleicosadienoic, a sPLA2 specific inhibitor, AACOCF3, a cPLA2 specific inhibitor, and HELSS, an iPLA2 specific inhibitor, all reduced the efflux of both glutamate and aspartate in vivo suggesting the involvement of multiple isoforms of PLA2 in EAA release not merely sPLA2 [131].
Membrane Breakdown and Metabolites
Phospholipids are the main components of the neural cell bi-layer membrane. In addition, they provide the membrane with the necessary environment, fluidity, and ion permeability that are required for the proper function of integral membrane proteins, receptors, and ion channels. PLA2 directly hydrolyses phospholipids resulting in membrane breakdown. This results in alterations of membrane function such as fluidity and permeability, behavior of transporters and receptors, and ion homeostasis, and can eventually lead to functional failure of excitable membranes [101, 102, 132].
In addition to the direct effects of membrane breakdown on cell survival, the products of sPLA2 enzymatic activity also exhibit neurotoxic profiles. sPLA2 cleaves phospholipids into the primary metabolites free fatty acid, such as AA, and lysophospholipids such as lysophosphatidyl choline (LPC, a.k.a. lysolechithin) (see insert of Fig. 2 and Fig. 3). Both of these agents have been shown to create injury and cytotoxicity in the CNS. AA induces oxidative injury and death in cultured spinal cord neurons [133]. AA can later form epoxides via the cytochrome P450 pathway, leukotrienes via the lipoxygenase pathway, or thromboxanes or prostaglandins via the COX pathway (see insert of Fig. 2). Many of these products, such as prostaglandin E2 (PGE2) can subsequently act as potent chemoattractants increasing endogenous immune responses and subsequent secondary damage. Additionally, the expression of eicosanoids, such as thromboxane A2 (TXA2) and PGE2 following SCI have been linked to trauma induced ischemia [134]. LPC produced by sPLA2 –induced hydrolysis is also implicated in CNS damage [135]. Interestingly, direct LPC injection has been shown to create a demyelination and infiltration of macrophages in the spinal cord, brain, and peripheral nervous system with a later remyelination by Schwann cells, the myelinating cells of the peripheral nervous system [136–139]. This demyelination and remyelination by Schwann cells mimics that produced by direct injection of sPLA2, however, sPLA2 has the added cost of severe axonopathy and death of oligodendrocytes prior to remyelination [10]. It has been hypothesized that LPC may mediate the demyelinating effects of sPLA2 injections. Additionally, a recent study by Lauber, et al. found that LPC, generated by iPLA2, was the main chemoattractant for monocytic cells and primary macrophages released by apoptotic cells thus facilitating the efficient phagocytosis of cellular debris [1].
Apoptosis
In recent years, apoptosis has been identified as an important mechanism of cell death in many neurological disorders including SCI. Cells undergoing apoptosis generally release free fatty acids including AA, which parallels the reduction in cell viability [140, 141], suggesting the involvement of PLA2 in apoptosis. Recently, sPLA2-IB, IIA, and III have all been shown to induce neuronal apoptosis [142–145]. In contrast, recombinant sPLA2 appears to prevent apoptosis of mast cells [140].
ROLE OF sPLA2 IN CNS DISORDERS
Localization within the CNS
sPLA2s are present in all regions of the mammalian brain. The highest sPLA2 activities are found in medulla oblongata, pons, and hippocampus; moderate activities in the hypothalamus, thalamus, and cerebral cortex; and lowest activities in the cerebellum and olfactory bulb [127]. Molloy, et al. utilizing RT-PCR found that mRNAs for sPLA2-IIA and IIC were expressed in all regions of normal rat brain, sPLA2-V was found at low levels in most areas of the brain, but at very high levels in the hippocampus, and sPLA2-IB was not detected in the rat brain at all [146]. In contrast, Kolko, et al. reported that sPLA2-IB mRNA was detected in the rat and human brain at very high levels as well as in neurons in primary cultures using various detection methods. The distribution of sPLA2-IB seems to be mainly neuronal, with the highest abundance occurring in the cerebral cortex and hippocampus [147]. sPLA2-IIA and V were also detected in the rat cerebellum using immunostaining and in situ hybridization methods [148]. sPLA2-IIA is associated with the endoplasmic reticulum in perinuclear regions of Purkinje cell somata and sPLA2-V was localized in Bergmann glia cells [148]. Recently, Kolko, et al. found the presence of sPLA2-IIE, V, and X in the rat brain as well as in neurons in primary cultures using RT-PCR, in situ hybridization, and immunohistochemistry [149]. The distribution of sPLA2-IIE, V, and X seems to be mainly neuronal, with the highest abundance occurring in the cerebral cortex and hippocampus [149]. In the spinal cord, sPLA2 activity was detected in the normal rat spinal cord homogenate [150]. Western blots revealed that sPLA2-IIA and V are expressed in the normal rat spinal cord [150]. mRNAs of sPLA2s (IB, IIA, IIC and V) are also detected in the normal rat spinal cord with RT-PCR [151].
As stated above, the presence of sPLA2 in the CNS, particularly neurons, appears to be both constitutive (groups IB, IIA, V, and X) and inducible (group IIA). While it is assumed that the inducible sPLA2 expression is associated with inflammation, the normal physiological role of constitutively expressed sPLA2 is believed to play a crucial role in exocytosis of synaptic vesicles [8, 152]. Support for this theory arises from studies by Matsuzawa, et al., in which differentiated PC12 cells were shown to release sPLA2-IIA after depolarization and that inhibition of IIA resulted in decreased catecholamine secretion [8]. Additionally, snake PLA2 neurotoxins cause paralysis of neuromuscular junctions by triggering a massive release of all presynaptic vesicles [129]. Finally, the concentration of group V in the hippocampus and studies by Chabot, et al. indicate that sPLA2-V may play a role in the regulation of long-term potentiation and long-term depression [153].
Spinal Cord Injury
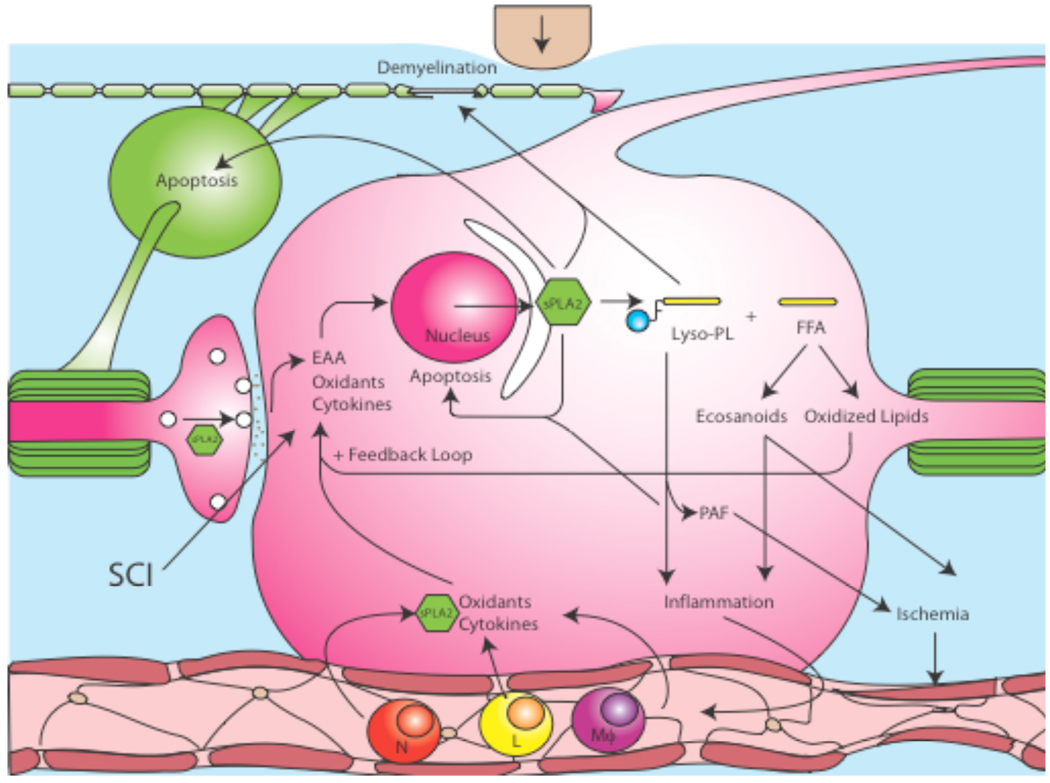
Damage from acute SCI occurs in two phases, an initial mechanical injury followed by a secondary injury mediated by multiple processes including inflammation, free radical induced cell death, cytokine production, and glutamate excitotoxicity [112, 154–156]. Following SCI, inflammatory cells such as polymorphonuclear neutrophils, macrophages, and lymphocytes quickly infiltrate into the traumatized cord, and flood the interstitial tissue with proinflammatory cytokines such as TNF-α and IL-1β, and neurotoxic factors from leukocytes such as nitric oxide, hydrogen peroxide, and myeloperoxidase [108–113]. Free radical generation and lipid peroxidation were also found to be early events subsequent to SCI [155, 157, 158]. EAAs such as glutamate and aspartate are released rapidly following SCI and their extracellular concentrations increased to neurotoxic levels within minutes post SCI [159–162]. The interplay between multiple harmful substances likely perpetuates the progressive course of secondary injury, resulting in cell death, axonal destruction, demyelination and functional loss. Evidence suggests that these harmful substances generated in the injured cord might induce sPLA2 expression which in turn exacerbates SCI. This hypothesis is supported by in vitro and in vivo experiments that indicate that increases in PLA2 activity and its metabolic products can in turn exacerbate inflammation [4, 163], oxidation [4, 163], demyelination [10], and neurotoxicity [163, 164] suggesting that sPLA2 may serve as a common mediator in the progression of secondary SCI (Fig. 4).
Fig. (4). sPLA2 activity within spinal cord injury.
Following SCI, oxidative stress, cytokines, and EAA are upregulated. These toxic factors then upregulate the synthesis of sPLA2. Subsequently sPLA2 mediates the hydrolysis of phospholipids into lysophospholipids (Lyso-PL), such as LPC, and free fatty acids (FFA), such as AA. Independent of other factors, sPLA2 and LPC demyelinate axons in the spinal cord and sPLA2 and AA have been shown to trigger apoptosis in neurons and oligodendrocytes. The metabolism of AA results in increased oxidative stress from lipid peroxidation and increased eicosanoids which have been shown to increase inflammation and ischemia. LPC also increases inflammation while its metabolite, PAF, triggers ischemia. Infiltrating polymorphonuclear neutrophils (N), lymphocytes (L), and macrophages (Mϕ) then flood the CNS with more sPLA2, oxidants, and cytokines thus exacerbating the positive feedback loop, while the upregulation in sPLA2 and LPC trigger the release of EAAs from synaptic terminals.
Recently, we showed that PLA2 activity increased following SCI, peaked at 4 h post injury, and remained elevated for one week. In the same study, we found that cPLA2 expression did not peak until 7 days post injury [105]. In vitro experiments showed that both sPLA2 and melittin, an activator of endogenous PLA2, induced spinal neuronal death in a dose-dependent manner, an effect that could be substantially reversed by mepacrine, a PLA2 inhibitor [105]. When sPLA2 was directly microinjected into the normal rat spinal cord, it induced tissue damage, demyelination, and sustained impairment in motor function [10]. Such sPLA2-induced demyelination, however, could be effectively attenuated with mepacrine, a PLA2 inhibitor [105]. Injections of sPLA2 also induced the expression of inflammatory cytokines TNF-α and IL-1β, as well as 4-hydroxynonenal, a product of lipid peroxidation and a marker for oxygen free radical-mediated membrane injury [105]. Indeed, in vivo and in vitro experiments show that exogenous administration of sPLA2 can induce neuronal death, oligodendrocyte death, and tissue damage [10, 105, 142–144, 164–166]. Importantly, to date no study has directly observed the presence of sPLA2 following SCI, which is a current focus of our lab.
The induction of sPLA2 following SCI is supported by the fact that the substrate of PLA2 metabolism, phospholipids, decreases acutely following SCI. There is a dramatic loss of membrane phospholipids following CNS trauma. During the first minute of compression trauma to the spinal cord, 10% of the plasmenylethanolamine is reduced with an overall loss of 18% found at 30 min post compression injury [167]. The hydrolysis of membrane phospholipids by PLA2 is a rate-limiting step for generation of proinflammatory mediators eicosanoids and PAF [102, 168].
Additionally, there is an increase in free fatty acids, eicosanoids, lipid peroxides, and lysophospholipids following SCI [169–171]. Severe trauma was associated with biphasic increases in free fatty acids levels, with levels peaking at 15 min and 24 hr post-trauma before declining over the next 6 days [171]. Within the first few minutes of SCI, free fatty acids have increased in the grey matter and later increase within the white matter suggesting acute PLA2 activity [170–172]. The production of free fatty acid represents a source of potentially dangerous ROS by initiating lipid peroxidation. Hydroxyl radicals can attack polyunsaturated fatty acids in membrane glycerophospholipids forming peroxyl radicals and propagating the chain reaction of lipid peroxidation products [173, 174]. The generation of free fatty acids in SCI is closely associated with increases in free radical formation observed in the lesion of the injured spinal cord [175, 176]. Application of pathophysiological concentrations of free fatty acids has been demonstrated in vitro to induce oxidative injury in spinal cord cell cultures [133]. The neurotoxic effects of AA have also been observed in hippocampal neurons and cortical neurons [177] as well as oligodendrocytes [178].
Later products of free fatty acid metabolism also increase within the injured spinal cord. COX, also known as prostaglandin G/H synthase, is the rate-limiting step in the production of prostaglandins (see insert of Fig. 2). COX-2 mRNA and protein expression are increased from 2 to 48 hr following SCI and the selective inhibition of COX-2 results in histological and functional sparing as assessed by the Basso, Beattie, and Bresnahan locomotion score [179–182]. COX-1 has also been shown to increase following SCI, persisting for as long as 4 weeks [179]. This upregulation in COX in the presence of free fatty acids, such as AA, logically progresses to an upregulation of eicosanoids. Bioactive eicosanoids, derived from PLA2-induceds production of AA, have been implicated as mediators of secondary injury via a host of mechanisms. The expression of eicosanoids, such as TXA2 and PGE2 increased in the injured cord tissue within hours of SCI and their vasoactive properties are thought to create microemboli in addition to PGE2’s well known proinflammatory effects [134, 183]. Increased production of TXA2, PGI2, LTC4 and 5-HETE have also been confirmed in experimental SCI [184, 185]. PGF2α increases three fold following SCI, and when exogenously added caused significant cell loss, increased hydroxyl radicals, and malondialdehyde - an end product of membrane lipid peroxidation [186].
The effect of lysophospholipids on spinal cord tissue has been extensively studied and lysophospholipids such as LPC and its later metabolites, such as PAF, are metabolically active in the CNS. For over 30 years it was known that injections of LPC into the spinal cord causes demyelination [133, 136, 138, 139] as well as expression of a number of chemokines and cytokines, similar to those produced following SCI [187, 188]. While lysophospholipid levels following SCI or traumatic brain injury (TBI) have not been assessed directly, their presence is strongly implied from the generous production of free fatty acids and a decrease in phospholipids. PAF, a metabolite of lysophospholipids, increases 20-fold after SCI induced by stroke [189–193]. Intrathecal administration of PAF leads to reduced spinal cord blood flow and motor deficits, an effect which can be blocked by the PAF receptor antagonist, WEB 2170 [193]. Treatment with WEB 2170 after acute spinal cord contusion resulted in significant increases in white matter sparing as well as decreases in proinflammatory cytokine mRNA levels within the lesion epicenter [192, 194]. Treatment with the PAF receptor antagonist BN52021 also improves behavioral function after SCI [195]. In vitro experiments showed that low concentrations of PAF resulted in neuronal differentiation and sprouting, while higher concentrations were neurotoxic [196]. PAF-induced death of not only cultured neuronal cells in a concentration-dependent manner [197, 198] but also that of oligodendrocytes and astrocytes [194].
Oxidative stress is well established following SCI [186, 199–202]. Work by Liu et al. has shown that H2O2 [199], iron [200], and hydroxyl radicals [200] are formed following SCI. Furthermore pathophysiological doses of these oxidants administered exogenously in vivo created significant cell death at 24 hr that could be blocked by a broad spectrum reactive species scavenger [200]. Administration of PGF2α resulted in a 3- fold increase in hydroxyl radicals and a 2-fold increase in malondialdehyde, an end product of membrane lipid peroxidation [186]. It has also been shown that H2O2 is toxic to neurons [203–207], astrocytes [208, 209], and oligodendrocytes [210–212]. Oxidative stressors, such as H2O2 administration, also increase AA release in neurons and mesanglial cells [114, 204]. It has recently been suggested that generation of ROS and polyunsaturated fatty acids, via cPLA2, following CNS injury mediates NF-κB translocation from the cytosol to the nucleus where it induces gene expression of sPLA2 and other lipid enzymes, thus potentiating a positive feedback loop [174].
High levels of EAAs such as glutamate and aspartate in experimental SCI are also an important mechanism inducing secondary injury [112]. Growing evidence indicates that sPLA2 could mediate EAA-induced neuronal death and tissue damage. Marked increases in PLA2 activity and AA release have been reported after treatments of neuronal cultures with glutamate, NMDA and KA [213, 214]. In addition, glutamate release in the spinal cord can be suppressed by PLA2 inhibitors such as indomethacin by 40%, AACOCF3 by 45%, and 4-bromophenacyl bromide by 36%, suggesting that increased PLA2-mediated EAA release is part of a positive feedback mechanism [126]. Additionally, application of sPLA2 to the ischemic rat cerebral cortex resulted in a significant increase in EAA levels and a general PLA2 inhibitor mepacrine significantly decreased the ischemia evoked efflux of EAA into cortical superfusates [123]. Thus, the excessive stimulation of NMDA receptors, as occurs in the spinal cord trauma, may result in stimulation of sPLA2 activity leading to alterations in membrane composition, permeability, and fluidity leading to neuronal and glial cell death.
In summary, sPLA2 can be induced by several key injury mediators such as inflammatory cytokines, free radicals, and EAAs that have been shown to increase following traumatic SCI. Furthermore, this increase in sPLA2 activity can further increase inflammation, oxidation, and EAA release. This indicates that sPLA2 activation may play a central role in a positive feedback loop triggered by traumatic SCI resulting in neuronal and glial cell death, tissue damage, and corresponding behavioral impairments. Thus, sPLA2 may act as a convergence molecule that mediates multiple key mechanisms of secondary spinal cord injury and blocking sPLA2 action may represent a novel and efficient strategy to block multiple injury mechanisms.
Brain Injury
Similar to SCI, TBI also triggers secondary or delayed cell death by multiple injury processes including ischemia, inflammation, generation of free radicals, and glutamate release, all of which have been showed to induce PLA2 activity [152, 215, 216]. Like SCI, there is clear evidence that TBI induces PLA2 activity resulting in membrane phospholipid degradation, generation of proinflammatory mediators, such as eicosanoids and PAF, formation of free radicals, and subsequent lipid peroxidation. Following closed head injury in rats, total PLA2 activity increased [217]. Additionally, after open traumatic brain injury, free fatty acids, such as AA, were released and membrane phospholipid degradation was found [218–220]. In humans, an increase in free fatty acids in cerebrospinal fluid (CSF) following brain injury has been reported [221].
No report to date has investigated the expression of sPLA2 following traumatic brain injury, however, cPLA2 and 4-hydroxynonenal were expressed in the transected brain [222]. Additional reports have confirmed the presence of down stream metabolites of AA. Pronounced increases in prostaglandin F2α, prostaglandin D2, leukotrienes, and thromboxane B2 have all been reported to occur in brain tissues after KA injection [223] and increases in PGE2 following closed head injury [217].
Conditions that increase sPLA2 have been shown following TBI, just as in SCI. Cerebral penetration and contusive injury both increase oxidative stress in the brain [224, 225] and blockade of oxidative stress increases learning and histological sparing [225]. Under both experimental and clinical settings, the level of extracellular EAAs such as glutamate and aspartate increased following TBI (Faden et al., 1989; Palmer et al., 1993; Globus et al., 1995; Bullock et al., 1998). Additionally, both competitive and noncompetitive NMDA and non-NMDA receptor antagonists are efficacious in the treatment of experimental brain injury [226]. Several studies showed that glutamate, NMDA, and KA result in a dose-dependent increase in AA release in hippocampal neuronal cultures [214] and PLA2 activity in neuron enriched spinal cord cultures [213]. In vivo intercerebroventricular injections of KA were shown to increase total PLA2 and sPLA2 activity in the rodent brain [127]. Increased levels of extracellular glutamate following TBI causes overstimulation of glutamate receptors that may result in secondary events such as sPLA2 release, degradation of membrane phospholipids, and accumulation of free fatty acids, leading to neuronal cell death as well as increased levels of eicosanoids and leukotrienes [112, 227]. As suggested above oxidative stress, EAA, and cytokines could induce sPLA2 release and abnormal phospholipid metabolism and may represent a common mechanism involved in traumatic spinal cord and brain injuries.
Ischemia
Ischemia is a key mechanism of secondary injury after CNS trauma [228–230]. Posttraumatic ischemia may result in energy failure that initiates a complex series of metabolic events, ultimately causing neuronal death. One such critical metabolic event is the activation of PLA2 which can result in hydrolysis of membrane phospholipids, release of free fatty acids, generation of oxygen free radicals, and formation of eicosanoids [152, 173, 231].
In both experimental models of brain [231–234] and spinal cord ischemia [235] significant increases in the level of free fatty acids, indirectly reflecting PLA2 activity, were found. Significant increases in sPLA2 activities were also reported in vivo following brain ischemia [14, 143, 236] and in astrocytes cultured under ischemic conditions such as oxygen and glucose deprivation [237]. Biphasic increased expression of sPLA2-IIA is observed in ischemic rat forebrain [14]. An early increase in sPLA2-IIA mRNA occurred at 1–6 h post-ischemia and a late phase of greater induction of sPLA2-IIA appeared between 7 and 20 days post-ischemia. Recently, increased expression of sPLA2-IIA has been confirmed at both mRNA and protein levels after brain ischemia [236, 237]. Cytokines such as TNF-α and IL-1β have been shown to mediate the ischemia induced PLA2 activation and sPLA2-IIA expression in transient focal rat cerebral ischemic model [116, 236]. Indoxam, a specific sPLA2 inhibitor, was shown to offer protection against the ischemia induced damage [143]. Quinacrine / mepacrine, a non-specific inhibitor of PLA2 activity, also showed sparing of hippocampal neurons [238] and reduced infarct size following transient focal ischemia [239]. In vitro experiments showed that increased sPLA2 activity was associated with ischemia-induced apoptosis [143]. Other studies have shown cPLA2 increases following ischemia [240–244] and other authors suggest that cPLA2 rather than sPLA2 mediates neuronal death in ischemia [245]. In summary, ischemia induces PLA2 activation which could result in deleterious effects such as the loss of membrane integrity through excessive phospholipids hydrolysis, formation of eicosanoids, cytotoxic products, ROS, and induction of apoptosis of affected cells [216, 246].
Other Degenerative Diseases
Beyond neurotrauma, sPLA2 has been suggested as a mediator of neurodegenerative disorders such as Alzheimer’s disease (AD) [247], Multiple Sclerosis [248, 249], and Parkinson’s disease [250]. AD is characterized by an increased deposition of amyloid plaques infiltrated by reactive astrocytes and microglial cells. Aggregated forms of amyloid β (Aβ) peptides, particularly A β 1–42, have been shown to elicit cytotoxic effects resulting in neuron cell death [251]. There is evidence for alterations in phospholipid levels in patients with AD [252]. In two separate studies, a decrease in PLA2 activity was found in the parietal and temporal cortices [253], as well as in the prefrontal cortex of the AD brain [254]. Contrary to these studies, immunohistochemical experiments showed increases in both sPLA2–IIA [247] and cPLA2 [255] in astrocytes of the AD brain. A recent gene array study in AD patients indicated an increase in cPLA2 and COX-2 expression, as well as upregulation of a number of apoptotic and proinflammatory genes, but no mention was made of sPLA2 [256]. These findings are in agreement with the increased oxidative and inflammatory responses and presence of reactive astrocytes associated with AD pathology [257]. In vitro studies demonstrated the ability of Aβ to enhance the activity of a number of phospholipases [258]. Nicotine, a cholinergic agonist, inhibited an Aβ-induced increase in PLA2 activation [259]. The ability of PLA2 inhibitors to attenuate Aβ-induced ROS production could indicate the involvement of PLA2 in Aβ cytotoxicity [260]. For a more thorough review please see [251].
Evidence also links sPLA2 generation to white matter disorders and their experimental equivalents. An early study by Huterer, et al., in the post mortem brains of Multiple Sclerosis patients found no difference in sPLA2-IIA activity and a decrease in cPLA2 activity within white matter lesions [261]. However, more recent studies found that cPLA2α −/− mice were more resistant to experimental autoimmune encephalomyelitis a rodent model of Multiple Sclerosis. Additionally, cPLA2α appeared to play a role in both the induction and effector phases as well as increasing inflammation in the white matter lesions [249]. Pinto, et al., found that extracellular inhibitors of sPLA2 were able to decrease CNS inflammation, prevent the induction of proinflammatory cytokines and ameliorate experimental autoimmune encephalomyelitis [248]. Finally, in the brains of patients with Krabbe Disease, a demyelinating disease of the CNS, sPLA2 was increased in post mortem human samples, and in twitcher mice, its rodent equivalent. Additionally, the use of a sPLA2 specific inhibitor reduced psychosine-induced oligodendrocyte death in vitro [262].
Studies using indirect markers for phospholipid metabolism have also suggested a role for sPLA2 in Parkinson’s disease, a degenerative disease of the CNS characterized by bradykinesia and death of dopaminergic neurons in the substantia nigra [263]. More importantly, quinacrine, a nonselective PLA2 inhibitor, significantly reduced MPTP-induced dopamine loss in an experimental model of Parkinson’s disease [250]. Mice deficient in cPLA2 were also shown to exhibit more resistance to MPTP neurotoxicity than wild-type mice, supporting a role for cPLA2 in mediating MPTP neurotoxicity [264].
CONCLUSIONS AND FUTURE DIRECTIONS
Current evidence clearly suggests that sPLA2 is present in the CNS and that its activity and metabolites exacerbate secondary SCI as well as other common CNS pathologies. Secondly, it has been shown that oxidative stress, cytokines, and EAA upregulate sPLA2 and in turn are reciprocally upregulated by sPLA2 following SCI resulting in a pathological positive feedback loop. Thus, sPLA2 clearly represents an important target for developing therapeutic interventions following SCI.
It must be noted that while this review chose to focus on sPLA2, other PLA2 subfamilies most importantly cPLA2 and iPLA2 seem to play a role in glycerophospholipid metabolism following injury as well [249, 265]. While there appears to be a reciprocal accentuation of activity among the PLA2s this issue merits further study [114]. Additionally, variations in the biological functions and species specificities of various sPLA2 isoforms complicate the issue further. Also the intracellular mechanisms utilized by oxidative stressors, cytokines, and EEA to upregulate sPLA2 after SCI remain obscure. Therefore further studies will need to assess the exact mechanism of sPLA2 –mediated CNS injury and the effect of inhibition on functional recovery following SCI.
ACKNOWLEDGEMENTS
We would like to thank Christine Nunn and Aaron Pucket for animal care. This work was supported by NIH NINDS (XMX: NS36350, NS52290, NS50253; WLT: F31 S5657401), the Kentucky Spinal Cord and Head Injury Research Trust (#4–16), the Daniel Heumann Fund for Spinal Cord Research, the James R. Petersdorf Chair (University of Louisville) and Mari Hulman George Chair (Indiana University) in Neuroscience Research. We also appreciate the use of the Center's core facility supported by NIH COBRE RR15576.
ABBREVIATIONS
- AA
Arachidonic acid
- AD
Alzheimer’s disease
- CNS
Central nervous system
- COX
Cyclooxygenase
- cPLA2
Cytosolic PLA2
- EAA
Excitatory amino acids
- HSPG
Heparan sulfate proteoglycans
- IFN-γ
Interferon gamma
- IL-1β
Interleukin-1β
- iPLA2
Independent PLA2
- KA
Kainic acid
- LPC
Lysophosphatidylcholine
- LPS
Lipopolysaccharide
- LTC4
Leukotriene C4
- Lyso-PL
Lysophospholipids
- NMDA
N-methy-D-aspartate
- PAF
Platelet activating factor
- PAF-AH
PAF acetylhydrolases
- PC
Phosphatidylcholine
- PGE2
Prostaglandin E2
- ROS
Reactive oxygen species
- SCI
Spinal cord injury
- sPLA2
Secretory phospholipases A2
- TBI
Traumatic btain injury
- TNF-α
Tumor necrosis factor-α
- TXA2
Thromboxane A2
REFERENCES
- 1.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 2.Morell P. Myelin. New York: Plenum Press; 1984. [Google Scholar]
- 3.Schaloske RH, Dennis EA. Biochim. Biophys. Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Crit. Rev. Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 5.Murakami M, Kudo I. Adv. Immunol. 2001;77:163–194. doi: 10.1016/s0065-2776(01)77017-4. [DOI] [PubMed] [Google Scholar]
- 6.Kudo I, Murakami M. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 7.Dennis EA. J. Biol. Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 8.Matsuzawa A, Murakami M, Atsumi G, Imai K, Prados P, Inoue K, Kudo I. Biochem. J. 1996;318(Pt 2):701–709. doi: 10.1042/bj3180701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kini RM, Evans HJ. J. Biol. Chem. 1987;262:14402–14407. [PubMed] [Google Scholar]
- 10.Titsworth WL, Onifer SM, Liu NK, Xu XM. Exp. Neurol. 2007;207:150–162. doi: 10.1016/j.expneurol.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Weinrauch Y, Elsbach P, Madsen LM, Foreman A, Weiss J. J. Clin. Invest. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine VJ, Gronroos JM, Nevalainen TJ. Eur. J. Clin. Chem. Clin. Biochem. 1996;34:419–422. [PubMed] [Google Scholar]
- 13.Laine VJ, Grass DS, Nevalainen TJ. Infect. Immun. 2000;68:87–92. doi: 10.1128/iai.68.1.87-92.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauritzen I, Heurteaux C, Lazdunski M. Brain Res. 1994;651:353–356. doi: 10.1016/0006-8993(94)90719-6. [DOI] [PubMed] [Google Scholar]
- 15.Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ. J. Biol. Chem. 2000;275:10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
- 16.Leitinger N, Watson AD, Hama SY, Ivandic B, Qiao JH, Huber J, Faull KF, Grass DS, Navab M, Fogelman AM, de Beer FC, Lusis AJ, Berliner JA. Arterioscler. Thromb. Vasc. Biol. 1999;19:1291–1298. doi: 10.1161/01.atv.19.5.1291. [DOI] [PubMed] [Google Scholar]
- 17.Ivandic B, Castellani LW, Wang XP, Qiao JH, Mehrabian M, Navab M, Fogelman AM, Grass DS, Swanson ME, de Beer MC, de Beer F, Lusis AJ. Arterioscler. Thromb. Vasc. Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 18.Takaku K, Sonoshita M, Sasaki N, Uozumi N, Doi Y, Shimizu T, Taketo MM. J. Biol. Chem. 2000;275:34013–34016. doi: 10.1074/jbc.C000585200. [DOI] [PubMed] [Google Scholar]
- 19.Morioka Y, Ikeda M, Saiga A, Fujii N, Ishimoto Y, Arita H, Hanasaki K. FEBS Lett. 2000;487:262–266. doi: 10.1016/s0014-5793(00)02350-4. [DOI] [PubMed] [Google Scholar]
- 20.MacPhee M, Chepenik KP, Liddell RA, Nelson KK, Siracusa LD, Buchberg AM. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 21.Cormier RT, Hong KH, Halberg RB, Hawkins TL, Richardson P, Mulherkar R, Dove WF, Lander ES. Nat. Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 22.Nevalainen TJ, Haapamèaki MM, Grèonroos JM. Biochim. Biophys. Acta. 2000;1488:83–90. doi: 10.1016/s1388-1981(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 23.Schaefers HJ, Haselmann J, Goppelt-Struebe M. Biochim. Biophys. Acta. 1996;1300:197–202. doi: 10.1016/0005-2760(96)00016-1. [DOI] [PubMed] [Google Scholar]
- 24.Reid RC. Curr. Med. Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- 25.Makela A, Sternby B, Kuusi T, Puolakkainen P, Schroder T. Scand. J. Gastroenterol. 1990;25:944–950. doi: 10.3109/00365529008997616. [DOI] [PubMed] [Google Scholar]
- 26.Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, Kloss J, Johnson LK. J. Biol. Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- 27.Kramer RM, Hession C, Johansen B, Hayes G, McGray P, Chow EP, Tizard R, Pepinsky RB. J. Biol. Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- 28.Kim DK, Fukuda T, Thompson BT, Cockrill B, Hales C, Bonventre JV. Am. J. Physiol. 1995;269:L109–L118. doi: 10.1152/ajplung.1995.269.1.L109. [DOI] [PubMed] [Google Scholar]
- 29.Andersen S, Sjursen W, Laegreid A, Volden G, Johansen B. Inflammation. 1994;18:1–12. doi: 10.1007/BF01534593. [DOI] [PubMed] [Google Scholar]
- 30.Minami T, Tojo H, Shinomura Y, Matsuzawa Y, Okamoto M. Gut. 1994;35:1593–1598. doi: 10.1136/gut.35.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otamiri T, Lindahl M, Tagesson C. Gut. 1988;29:489–494. doi: 10.1136/gut.29.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabia R, Ar'Rajab A, Willen R, Andersson R, Bengmark S. Br. J. Surg. 1993;80:1199–1204. doi: 10.1002/bjs.1800800947. [DOI] [PubMed] [Google Scholar]
- 33.Keuter M, Dharmana E, Kullberg BJ, Schalkwijk C, Gasem MH, Seuren L, Djokomoeljanto R, Dolmans WM, van den Bosch H, van der Meer JW. J. Infect. Dis. 1995;172:305–308. doi: 10.1093/infdis/172.1.305. [DOI] [PubMed] [Google Scholar]
- 34.Green JA, Smith GM, Buchta R, Lee R, Ho KY, Rajkovic IA, Scott KF. Inflammation. 1991;15:355–367. doi: 10.1007/BF00917352. [DOI] [PubMed] [Google Scholar]
- 35.Uhl W, Buchler M, Nevalainen TJ, Deller A, Beger HG. J. Trauma. 1990;30:1285–1290. doi: 10.1097/00005373-199010000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Gronroos JM, Kuttila K, Perttila J, Nevalainen TJ. Eur. J. Clin. Chem. Clin. Biochem. 1995;33:271–274. doi: 10.1515/cclm.1995.33.5.271. [DOI] [PubMed] [Google Scholar]
- 37.Crowl RM, Stoller TJ, Conroy RR, Stoner CR. J. Biol. Chem. 1991;266:2647–2651. [PubMed] [Google Scholar]
- 38.Horigome K, Hayakawa M, Inoue K, Nojima S. J. Biochem. (Tokyo) 1987;101:53–61. doi: 10.1093/oxfordjournals.jbchem.a121907. [DOI] [PubMed] [Google Scholar]
- 39.Murakami M, Kudo I, Suwa Y, Inoue K. Eur. J. Biochem. 1992;209:257–265. doi: 10.1111/j.1432-1033.1992.tb17284.x. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara H, Amabe Y, Komatsubara T, Tojo H, Okamoto M, Wakano Y, Ishida H. FEBS Lett. 1992;304:69–72. doi: 10.1016/0014-5793(92)80591-4. [DOI] [PubMed] [Google Scholar]
- 41.Hidi R, Vargaftig BB, Touqui L. J. Immunol. 1993;151:5613–5623. [PubMed] [Google Scholar]
- 42.Wightman PD, Dahlgren ME, Davies P, Bonney RJ. Biochem. J. 1981;200:441–444. doi: 10.1042/bj2000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KP, Rafter JD, Bittova L, Han SK, Snitko Y, Munoz NM, Leff AR, Cho W. J. Biol. Chem. 2001;276:11126–11134. doi: 10.1074/jbc.M004604200. [DOI] [PubMed] [Google Scholar]
- 44.Peilot H, Rosengren B, Bondjers G, Hurt-Camejo E. J. Biol. Chem. 2000;275:22895–22904. doi: 10.1074/jbc.M002783200. [DOI] [PubMed] [Google Scholar]
- 45.Kuwata H, Nakatani Y, Murakami M, Kudo I. J. Biol. Chem. 1998;273:1733–1740. doi: 10.1074/jbc.273.3.1733. [DOI] [PubMed] [Google Scholar]
- 46.Suga H, Murakami M, Kudo I, Inoue K. Eur. J. Biochem. 1993;218:807–813. doi: 10.1111/j.1432-1033.1993.tb18435.x. [DOI] [PubMed] [Google Scholar]
- 47.Oka S, Arita H. J. Biol. Chem. 1991;266:9956–9960. [PubMed] [Google Scholar]
- 48.Murakami M, Yoshihara K, Shimbara S, Lambeau G, Singer A, Gelb MH, Sawada M, Inagaki N, Nagai H, Kudo I. Biochem. Biophys. Res. Commun. 2002;292:689–696. doi: 10.1006/bbrc.2002.6716. [DOI] [PubMed] [Google Scholar]
- 49.Couturier C, Brouillet A, Couriaud C, Koumanov K, Bereziat G, Andreani M. J. Biol. Chem. 1999;274:23085–23093. doi: 10.1074/jbc.274.33.23085. [DOI] [PubMed] [Google Scholar]
- 50.Touqui L, Alaoui-El-Azher M. Curr. Mol. Med. 2001;1:739–754. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- 51.Muhl H, Geiger T, Pignat W, Marki F, van den Bosch H, Vosbeck K, Pfeilschifter J. FEBS Lett. 1991;291:249–252. doi: 10.1016/0014-5793(91)81295-j. [DOI] [PubMed] [Google Scholar]
- 52.Schalkwijk C, Pfeilschifter J, Marki F, van den Bosch H. J. Biol. Chem. 1992;267:8846–8851. [PubMed] [Google Scholar]
- 53.Bezzine S, Koduri RS, Valentin E, Murakami M, Kudo I, Ghomashchi F, Sadilek M, Lambeau G, Gelb MH. J. Biol. Chem. 2000;275:3179–3191. doi: 10.1074/jbc.275.5.3179. [DOI] [PubMed] [Google Scholar]
- 54.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, Gelb MH. J. Biol. Chem. 2004;279:25024–25038. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 55.Porcellati G. In: Neural Membranes. Sun GY, Bazan NG, Wu G, Porcellati AY, Sun, editors. New York: Humana Press; 1983. [Google Scholar]
- 56.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 57.Murakami M, Nakatani Y, Kudo I. J. Biol. Chem. 1996;271:30041–30051. doi: 10.1074/jbc.271.47.30041. [DOI] [PubMed] [Google Scholar]
- 58.Murakami M, Koduri RS, Enomoto A, Shimbara S, Seki M, Yoshihara K, Singer A, Valentin E, Ghomashchi F, Lambeau G, Gelb MH, Kudo I. J. Biol. Chem. 2001;276:10083–10096. doi: 10.1074/jbc.M007877200. [DOI] [PubMed] [Google Scholar]
- 59.Murakami M, Kambe T, Shimbara S, Yamamoto S, Kuwata H, Kudo I. J. Biol. Chem. 1999;274:29927–29936. doi: 10.1074/jbc.274.42.29927. [DOI] [PubMed] [Google Scholar]
- 60.Murakami M, Kudo I, Inoue K. J. Biol. Chem. 1993;268:839–844. [PubMed] [Google Scholar]
- 61.Murakami M, Kambe T, Shimbara S, Higasgino K, Hansaki K, Arita H, Horiguchi M, Arita M, Arai H, Inoue K, Kudo I. J. Biol. Chem. 1999;274:31435–31444. doi: 10.1074/jbc.274.44.31435. [DOI] [PubMed] [Google Scholar]
- 62.Leadbeater WE, Gonzalez AM, Logaras N, Berry M, Turn-bull JE, Logan A. J. Neurochem. 2006;96:1189–1200. doi: 10.1111/j.1471-4159.2005.03632.x. [DOI] [PubMed] [Google Scholar]
- 63.Tang X, Davies JE, Davies SJ. J. Neurosci. Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 64.Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. FASEB. J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- 65.Lambeau G, Ancian P, Barhanin J, Lazdunski M. J. Biol. Chem. 1994;269:1575–1578. [PubMed] [Google Scholar]
- 66.Ishizaki J, Hanasaki K, Higashino K, Kishino J, Kikuchi N, Ohara O, Arita H. J. Biol. Chem. 1994;269:5897–5904. [PubMed] [Google Scholar]
- 67.Hanasaki K, Arita H. Biochim. Biophys. Acta. 1992;1127:233–241. doi: 10.1016/0005-2760(92)90226-l. [DOI] [PubMed] [Google Scholar]
- 68.Lambeau G, Lazdunski M. Trends Pharmacol. Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 69.Hanasaki K, Arita H. Arch. Biochem. Biophys. 1999;372:215–223. doi: 10.1006/abbi.1999.1511. [DOI] [PubMed] [Google Scholar]
- 70.Ohara O, Ishizaki J, Arita H. Prog. Lipid Res. 1995;34:117–138. doi: 10.1016/0163-7827(94)00009-b. [DOI] [PubMed] [Google Scholar]
- 71.Ancian P, Lambeau G, Mattei MG, Lazdunski M. J. Biol. Chem. 1995;270:8963–8970. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- 72.Hanasaki K, Arita H. J. Biol. Chem. 1992;267:6414–6420. [PubMed] [Google Scholar]
- 73.Cupillard L, Mulherkar R, Gomez N, Kadam S, Valentin E, Lazdunski M, Lambeau G. J. Biol. Chem. 1999;274:7043–7051. doi: 10.1074/jbc.274.11.7043. [DOI] [PubMed] [Google Scholar]
- 74.Morioka Y, Saiga A, Yokota Y, Suzuki N, Ikeda M, Ono T, Nakano K, Fujii N, Ishizaki J, Arita H, Hanasaki K. Arch. Biochem. Biophys. 2000;381:31–42. doi: 10.1006/abbi.2000.1977. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez M, Burillo SL, Crespo MS, Nieto ML. J. Biol. Chem. 1998;273:606–612. doi: 10.1074/jbc.273.1.606. [DOI] [PubMed] [Google Scholar]
- 76.Fonteh AN, Atsumi G, LaPorte T, Chilton FH. J. Immunol. 2000;165:2773–2782. doi: 10.4049/jimmunol.165.5.2773. [DOI] [PubMed] [Google Scholar]
- 77.Arita H, Hanasaki K, Nakano T, Oka S, Teraoka H, Matsumoto K. J. Biol. Chem. 1991;266:19139–19141. [PubMed] [Google Scholar]
- 78.Kinoshita E, Handa N, Hanada K, Kajiyama G, Sugiyama M. FEBS Lett. 1997;407:343–346. doi: 10.1016/s0014-5793(97)00373-6. [DOI] [PubMed] [Google Scholar]
- 79.Kanemasa T, Hanasaki K, Arita H. Biochim. Biophys. Acta. 1992;1125:210–214. doi: 10.1016/0005-2760(92)90047-y. [DOI] [PubMed] [Google Scholar]
- 80.Yokota Y, Notoya M, Higashino K, Ishimoto Y, Nakano K, Arita H, Hanasaki K. FEBS Lett. 2001;509:250–254. doi: 10.1016/s0014-5793(01)03173-8. [DOI] [PubMed] [Google Scholar]
- 81.Hanasaki K, Yokota Y, Ishizaki J, Itoh T, Arita H. J. Biol. Chem. 1997;272:32792–32797. doi: 10.1074/jbc.272.52.32792. [DOI] [PubMed] [Google Scholar]
- 82.Hanasaki K, Arita H. Prostaglandins Other Lipid Mediat. 2002;68–69:71–82. doi: 10.1016/s0090-6980(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 83.Seilhamer JJ, Randall TL, Yamanaka M, Johnson LK. DNA. 1986;5:519–527. doi: 10.1089/dna.1.1986.5.519. [DOI] [PubMed] [Google Scholar]
- 84.Verheij HM, Slotboom AJ, de Haas GH. Rev. Physiol. Biochem. Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 85.Komada M, Kudo I, Mizushima H, Kitamura N, Inoue K. J. Biochem. (Tokyo) 1989;106:545–547. doi: 10.1093/oxfordjournals.jbchem.a122890. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA. J. Biol. Chem. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 87.Valentin E, Koduri RS, Scimeca JC, Carle G, Gelb MH, Lazdunski M, Lambeau G. J. Biol. Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 88.Ishizaki J, Suzuki N, Higashino K, Yokota Y, Ono T, Kawamoto K, Fujii N, Arita H, Hanasaki K. J. Biol. Chem. 1999;274:24973–24979. doi: 10.1074/jbc.274.35.24973. [DOI] [PubMed] [Google Scholar]
- 89.Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. J. Biol. Chem. 2000;275:7492–7496. doi: 10.1074/jbc.275.11.7492. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki N, Ishizaki J, Yokota Y, Higashino K, Ono T, Ikeda M, Fujii N, Kawamoto K, Hanasaki K. J. Biol. Chem. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Engle SJ, Seilhamer JJ, Tischfield JA. J. Biol. Chem. 1994;269:23018–23024. [PubMed] [Google Scholar]
- 92.Valentin E, Singer AG, Ghomashchi F, Lazdunski M, Gelb MH, Lambeau G. Biochem. Biophys. Res. Commun. 2000;279:223–228. doi: 10.1006/bbrc.2000.3908. [DOI] [PubMed] [Google Scholar]
- 93.Murakami M, Yoshihara K, Shimbara S, Lambeau G, Gelb MH, Singer AG, Sawada M, Inagaki N, Nagai H, Ishihara M, Ishikawa Y, Ishii T, Kudo I. J. Biol. Chem. 2002;277:19145–19155. doi: 10.1074/jbc.M112385200. [DOI] [PubMed] [Google Scholar]
- 94.Sawada H, Murakami M, Enomoto A, Shimbara S, Kudo I. Eur. J. Biochem. 1999;263:826–835. doi: 10.1046/j.1432-1327.1999.00565.x. [DOI] [PubMed] [Google Scholar]
- 95.Balboa MA, Shirai Y, Gaietta G, Ellisman MH, Balsinde J, Dennis EA. J. Biol. Chem. 2003;278:48059–48065. doi: 10.1074/jbc.M305904200. [DOI] [PubMed] [Google Scholar]
- 96.Ho IC, Arm JP, Bingham CO, 3rd, Choi A, Austen KF, Glimcher LH. J. Biol. Chem. 2001;276:18321–18326. doi: 10.1074/jbc.M008837200. [DOI] [PubMed] [Google Scholar]
- 97.Cupillard L, Koumanov K, Mattâei MG, Lazdunski M, Lambeau G. J. Biol. Chem. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- 98.Hanasaki K, Ono T, Saiga A, Morioka Y, Ikeda M, Kawamoto K, Higashino K, Nakano K, Yamada K, Ishizaka J, Arita H. J. Biol. Chem. 1999;274:34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- 99.Gelb MH, Valentin E, Ghomashchi F, Lazdunski M, Lambeau G. J. Biol. Chem. 2000;275:39823–39826. doi: 10.1074/jbc.C000671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Regan MH, Smith-Barbour M, Perkins LM, Phillis JW. Neurosci. Lett. 1995;185:191–194. doi: 10.1016/0304-3940(95)11259-y. [DOI] [PubMed] [Google Scholar]
- 101.Klein J. J. Neural. Transm. 2000;107:1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- 102.Farooqui AA, Yang HC, Rosenberger TA, Horrocks LA. J. Neurochem. 1997;69:889–901. doi: 10.1046/j.1471-4159.1997.69030889.x. [DOI] [PubMed] [Google Scholar]
- 103.Adibhatla RM, Hatcher JF. Free Radic. Biol. Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 104.Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. Curr. Top. Med. Chem. 2007;7:765–777. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- 105.Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. Anal. Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- 106.Nethery D, Stofan D, Callahan L, DiMarco A, Supinski G. J. Appl. Physiol. 1999;87:792–800. doi: 10.1152/jappl.1999.87.2.792. [DOI] [PubMed] [Google Scholar]
- 107.Neuzil J, Upston JM, Witting PK, Scott KF, Stocker R. Biochem. 1998;37:9203–9210. doi: 10.1021/bi9730745. [DOI] [PubMed] [Google Scholar]
- 108.Jones TB, Hart RP, Popovich PG. J. Neurosci. 2005;25:6576–6583. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang XF, Huang LD, Yu PP, Hu JG, Yin L, Wang L, Xu XM, Lu PH. Acta Neuropathol. (Berl) 2006;111:220–228. doi: 10.1007/s00401-005-0016-x. [DOI] [PubMed] [Google Scholar]
- 110.Blight AR. Cent. Nerv. Syst. Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 111.Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 112.Park E, Velumian AA, Fehlings MG. J. Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 113.Popovich PG, Wei P, Stokes BT. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 114.Han WK, Sapirstein A, Hung CC, Alessandrini A, Bonventre JV. J. Biol. Chem. 2003;278:24153–24163. doi: 10.1074/jbc.M300424200. [DOI] [PubMed] [Google Scholar]
- 115.Farooqui AA, Litsky ML, Farooqui T, Horrocks LA. Brain Res. Bull. 1999;49:139–153. doi: 10.1016/s0361-9230(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 116.Adibhatla RM, Hatcher JF. Brain Res. 2007;1134:199–205. doi: 10.1016/j.brainres.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Tong W, Hu ZY, Sun GY. Mol. Chem. Neuropathol. 1995;25:1–17. doi: 10.1007/BF02815083. [DOI] [PubMed] [Google Scholar]
- 118.Li W, Xia J, Sun GY. J. Interfer. Cytokine Res. 1999;19:121–127. doi: 10.1089/107999099314261. [DOI] [PubMed] [Google Scholar]
- 119.Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandins Leukotr. Essent. Fatty Acids. 2003;69:437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 120.Morioka N, Takeda K, Kumagai K, Hanada T, Ikoma K, Hide I, Inoue A, Nakata Y. J. Neurochem. 2002;80:989–997. doi: 10.1046/j.0022-3042.2002.00722.x. [DOI] [PubMed] [Google Scholar]
- 121.Kolko M, Rodriguez de Turco EB, Diemer NH, Bazan NG. Neurosci. Lett. 2003;338:164–168. doi: 10.1016/s0304-3940(02)01385-x. [DOI] [PubMed] [Google Scholar]
- 122.Dorandeu F, Pernot-Marino I, Veyret J, Perrichon C, Lallement G. J. Neurosci. Res. 1998;54:848–862. doi: 10.1002/(SICI)1097-4547(19981215)54:6<848::AID-JNR13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 123.O'Regan MH, Smith-Babour M, Perkins LM, Phillis JW. Neurosci. Lett. 1995;185:191–194. doi: 10.1016/0304-3940(95)11259-y. [DOI] [PubMed] [Google Scholar]
- 124.Wei S, Ong WY, Thwin MM, Fong CW, Farooqui AA, Gopalakrishnakone P, Hong W. Neuroscience. 2003;121:891–898. doi: 10.1016/s0306-4522(03)00525-6. [DOI] [PubMed] [Google Scholar]
- 125.Kolko M, de Turco EB, Diemer NH, Bazan NG. Neuroreport. 2002;13:1963–1966. doi: 10.1097/00001756-200210280-00026. [DOI] [PubMed] [Google Scholar]
- 126.Sundstrom E, Mo LL. J. Neurotrauma. 2002;19:257–266. doi: 10.1089/08977150252806992. [DOI] [PubMed] [Google Scholar]
- 127.Thwin MM, Ong WY, Fong CW, Sato K, Kodama K, Farooqui AA, Gopalakrishnakone P. Exp.. Brain Res. Experimentelle Hirnforschung. Expâerimentation câerâebrale. 2003;150:427–433. doi: 10.1007/s00221-003-1476-7. [DOI] [PubMed] [Google Scholar]
- 128.O'Regan MH, Alix S, Woodbury DJ. Neurosci. Lett. 1996;202:201–203. doi: 10.1016/0304-3940(95)12238-9. [DOI] [PubMed] [Google Scholar]
- 129.Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- 130.Piomelli D, Astarita G, Rapaka R. Nat. Rev. Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- 131.Phillis JW, O'Regan MH. Brain Res. 1996;730:150–164. doi: 10.1016/0006-8993(96)00434-9. [DOI] [PubMed] [Google Scholar]
- 132.Farooqui AA, Ong WY, Horrocks LA. Neurochem. Res. 2004;29:1961–1977. doi: 10.1007/s11064-004-6871-3. [DOI] [PubMed] [Google Scholar]
- 133.Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. J. Neurochem. 1999;73:684–692. doi: 10.1046/j.1471-4159.1999.0730684.x. [DOI] [PubMed] [Google Scholar]
- 134.Tonai T, Taketani Y, Ueda N, Nishisho T, Ohmoto Y, Sakata Y, Muraguchi M, Wada K, Yamamoto S. J. Neurochem. 1999;72:302–309. doi: 10.1046/j.1471-4159.1999.0720302.x. [DOI] [PubMed] [Google Scholar]
- 135.Dutta J, Das AK, Biswas A. J. Chromatography. 1979;173:379–387. doi: 10.1016/s0021-9673(00)92307-0. [DOI] [PubMed] [Google Scholar]
- 136.Blakemore WF. Neuropathol. Appl. Neurobiol. 1982;8:365–375. doi: 10.1111/j.1365-2990.1982.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 137.Jeffery ND, Blakemore WF. J. Neurocytol. 1995;24:775–781. doi: 10.1007/BF01191213. [DOI] [PubMed] [Google Scholar]
- 138.Blakemore WF. Neuropathol. Appl. Neurobiol. 1978;4:47–59. doi: 10.1111/j.1365-2990.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 139.Blakemore WF, Eames RA, Smith KJ, McDonald WI. J. Neurol. Sci. 1977;33:31–43. doi: 10.1016/0022-510x(77)90179-4. [DOI] [PubMed] [Google Scholar]
- 140.Taketo MM, Sonoshita M. Biochim. Biophys. Acta. 2002;1585:72–76. doi: 10.1016/s1388-1981(02)00326-8. [DOI] [PubMed] [Google Scholar]
- 141.Balsinde J, Perez R, Balboa MA. Biochim. Biophys. Acta. 2006;1761:1344–1350. doi: 10.1016/j.bbalip.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 142.Yagami T, Ueda K, Asakura K, Hayasaki-Kajiwara Y, Nakazato H, Sakaeda T, Hata S, Kuroda T, Takasu N, Hori Y. J. Neurochem. 2002;81:449–461. doi: 10.1046/j.1471-4159.2002.00800.x. [DOI] [PubMed] [Google Scholar]
- 143.Yagami T, Ueda K, Asakura K, Hata S, Kuroda T, Sakaeda T, Takasu N, Tanaka K, Gemba T, Hori Y. Mol. Pharmacol. 2002;61:114–126. doi: 10.1124/mol.61.1.114. [DOI] [PubMed] [Google Scholar]
- 144.Yagami T, Ueda K, Asakura K, Hata S, Kuroda T, Sakaeda T, Kishino J, Sakaguchi G, Itoh N, Hori Y. Brain Res. 2002;949:197–201. doi: 10.1016/s0006-8993(02)03144-x. [DOI] [PubMed] [Google Scholar]
- 145.DeCoster MA. Brain Res. 2003;988:20–28. doi: 10.1016/s0006-8993(03)03326-2. [DOI] [PubMed] [Google Scholar]
- 146.Molloy GY, Rattray M, Williams R. J. Neurosci. Lett. 1998;258:139–142. doi: 10.1016/s0304-3940(98)00838-6. [DOI] [PubMed] [Google Scholar]
- 147.Kolko M, Christoffersen NR, Varoqui H, Bazan NG. Cell Mol. Neurobiol. 2005;25:1107–1122. doi: 10.1007/s10571-005-8221-7. [DOI] [PubMed] [Google Scholar]
- 148.Shirai Y, Ito M. J. Neurocytol. 2004;33:297–307. doi: 10.1023/B:NEUR.0000044191.83858.f7. [DOI] [PubMed] [Google Scholar]
- 149.Kolko M, Christoffersen NR, Barreiro SG, Miller ML, Pizza AJ, Bazan NG. J. Neurosci. Res. 2006;83:874–882. doi: 10.1002/jnr.20773. [DOI] [PubMed] [Google Scholar]
- 150.Svensson CI, Lucas KK, Hua XY, Powell HC, Dennis EA, Yaksh TL. Neuroscience. 2005;133:543–553. doi: 10.1016/j.neuroscience.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 151.Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Br. J. Pharmacol. 2005;144:940–952. doi: 10.1038/sj.bjp.0706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phillis JW, O'Regan MH. Brain Res. Brain Res. Rev. 2004;44:13–47. doi: 10.1016/j.brainresrev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 153.Chabot C, Gagne J, Giguere C, Bernard J, Baudry M, Massicotte G. Hippocampus. 1998;8:299–309. doi: 10.1002/(SICI)1098-1063(1998)8:3<299::AID-HIPO11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 154.Young W. J. Emerg. Med. 1993;11 Suppl 1:13–22. [PubMed] [Google Scholar]
- 155.Hall ED, Braughler JM. Cent. Nerv. Syst. Trauma. 1986;3:281–294. doi: 10.1089/cns.1986.3.281. [DOI] [PubMed] [Google Scholar]
- 156.Buki A, Okonkwo DO, Wang KK, Povlishock JT. J. Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Springer JE, Azbill RD, Mark RJ, Begley JG, Waeg G, Mattson MP. J. Neurochem. 1997;68:2469–2476. doi: 10.1046/j.1471-4159.1997.68062469.x. [DOI] [PubMed] [Google Scholar]
- 158.Hall ED, Yonkers PA, Andrus PK, Cox JW, Anderson DK. J. Neurotrauma. 1992;9 Suppl 2:S425–S442. [PubMed] [Google Scholar]
- 159.Panter SS, Yum SW, Faden AI. Ann. Neurol. 1990;27:96–99. doi: 10.1002/ana.410270115. [DOI] [PubMed] [Google Scholar]
- 160.Farooque M, Hillered L, Holtz A, Olsson Y. J. Neurochem. 1996;66:1125–1130. doi: 10.1046/j.1471-4159.1996.66031125.x. [DOI] [PubMed] [Google Scholar]
- 161.Liu D, Xu GY, Pan E, McAdoo DJ. Neuroscience. 1999;93:1383–1389. doi: 10.1016/s0306-4522(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 162.McAdoo DJ, Xu GY, Robak G, Hughes MG. Exp. Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- 163.Bonventre JV. J. Lipid Mediat. Cell Signal. 1996;14:15–23. doi: 10.1016/0929-7855(96)00503-2. [DOI] [PubMed] [Google Scholar]
- 164.Clapp LE, Klette KL, DeCoster MA, Bernton E, Petras JM, Dave JR, Laskosky MS, Smallridge RC, Tortella FC. Brain Res. 1995;693:101–111. doi: 10.1016/0006-8993(95)00720-b. [DOI] [PubMed] [Google Scholar]
- 165.Kolko M, DeCoster MA, Rodriguez de Turco EB, Bazan NG. J. Biol. Chem. 1996;271:32722–32728. doi: 10.1074/jbc.271.51.32722. [DOI] [PubMed] [Google Scholar]
- 166.Kolko M, Bruhn J, Schweitz H, Qar J, Lazdunski M, Lambeau G, Bazan NG, Diemer NH. Neurosci. Lett. 1999;274:167–170. doi: 10.1016/s0304-3940(99)00709-0. [DOI] [PubMed] [Google Scholar]
- 167.Horrocks LA, Demediuk P, Saunders RD, Dugan L, Clendenon NR, Means ED, Anderson DK. Cent. Nerv. Syst. Trauma. 1985;2:115–120. doi: 10.1089/cns.1985.2.115. [DOI] [PubMed] [Google Scholar]
- 168.Farooqui AA, Litsky ML, Farooqui T, Horrocks LA. Brain Res. Bull. 1999;49:139–153. doi: 10.1016/s0361-9230(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 169.Lukacova N, Halat G, Chavko M, Marsala J. Neurochem. Res. 1996;21:869–873. doi: 10.1007/BF02532334. [DOI] [PubMed] [Google Scholar]
- 170.Demediuk P, Saunders RD, Clendenon NR, Means ED, Anderson DK, Horrocks LA. Prog. Brain Res. 1985;63:211–226. doi: 10.1016/S0079-6123(08)61985-8. [DOI] [PubMed] [Google Scholar]
- 171.Demediuk P, Daly MP, Faden AI. J. Neurosci. Res. 1989;23:95–106. doi: 10.1002/jnr.490230113. [DOI] [PubMed] [Google Scholar]
- 172.Faden AI, Chan PH, Longar S. J. Neurochem. 1987;48:1809–1816. doi: 10.1111/j.1471-4159.1987.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 173.Muralikrishna Adibhatla R, Hatcher JF. Free Radic. Biol. Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 174.Sun GY, Horrocks LA, Farooqui AA. J. Neurochem. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 175.Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, Fukuzawa K. Free Radic. Biol. Med. 1996;20:1–9. doi: 10.1016/0891-5849(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 176.Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Brain Res. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- 177.Li Y, Maher P, Schubert D. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 178.Wang H, Li J, Follett PL, Zhang Y, Cotanche DA, Jensen FE, Volpe JJ, Rosenberg PA. Eur. J. Neurosci. 2004;20:2049–2058. doi: 10.1111/j.1460-9568.2004.03650.x. [DOI] [PubMed] [Google Scholar]
- 179.Schwab JM, Brechtel K, Nguyen TD, Schluesener HJ. J. Neuroimmunol. 2000;111:122–130. doi: 10.1016/s0165-5728(00)00372-6. [DOI] [PubMed] [Google Scholar]
- 180.Resnick DK, Graham SH, Dixon CE, Marion DW. J. Neurotrauma. 1998;15:1005–1013. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- 181.Hoffmann C. Curr. Med. Chem. 2000;7:1113–1120. doi: 10.2174/0929867003374282. [DOI] [PubMed] [Google Scholar]
- 182.Hains BC, Yucra JA, Hulsebosch CE. J. Neurotrauma. 2001;18:409–423. doi: 10.1089/089771501750170994. [DOI] [PubMed] [Google Scholar]
- 183.Resnick DK, Nguyen P, Cechvala CF. Spine J. 2001;1:432–436. doi: 10.1016/s1529-9430(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 184.Mitsuhashi T, Ikata T, Morimoto K, Tonai T, Katoh S. Paraplegia. 1994;32:524–530. doi: 10.1038/sc.1994.84. [DOI] [PubMed] [Google Scholar]
- 185.Jacobs TP, Shohami E, Baze W, Burgard E, Gunderson C, Hallenbeck J, Feuerstein G. Cent. Nerv. Syst. Trauma. 1987;4:95–118. doi: 10.1089/cns.1987.4.95. [DOI] [PubMed] [Google Scholar]
- 186.Liu D, Li L, Augustus L. J. Neurochem. 2001;77:1036–1047. doi: 10.1046/j.1471-4159.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 187.Ousman SS, David S. J. Neurosci. 2001;21:4649–4656. doi: 10.1523/JNEUROSCI.21-13-04649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ousman SS, David S. Glia. 2000;30:92–104. [PubMed] [Google Scholar]
- 189.Lindsberg PJ, Yue TL, Frerichs KU, Hallenbeck JM, Feuerstein G. Stroke. 1990;21:1452–1457. doi: 10.1161/01.str.21.10.1452. [DOI] [PubMed] [Google Scholar]
- 190.Xiao J, Zhao D, Jia L. Zhonghua Yi Xue Za Zhi. 1996;76:120–123. [PubMed] [Google Scholar]
- 191.Xiao J, Zao D, Zhen H. Zhonghua Wai Ke Za Zhi. 1995;33:715–718. [PubMed] [Google Scholar]
- 192.Hostettler ME, Carlson SL. Neuroreport. 2002;13:21–24. doi: 10.1097/00001756-200201210-00009. [DOI] [PubMed] [Google Scholar]
- 193.Faden AI, Halt P. J. Pharmacol. Exp. Ther. 1992;261:1064–1070. [PubMed] [Google Scholar]
- 194.Hostettler ME, Knapp PE, Carlson SL. Glia. 2002;38:228–239. doi: 10.1002/glia.10065. [DOI] [PubMed] [Google Scholar]
- 195.Xiao J, Zhao D, Hou T, Wu K, Zeng H. Chin. Med. J. (Engl.) 1998;111:443–446. [PubMed] [Google Scholar]
- 196.Kornecki E, Ehrlich YH. Science. 1988;240:1792–1794. doi: 10.1126/science.3381103. [DOI] [PubMed] [Google Scholar]
- 197.Xu Y, Tao YX. Neuroreport. 2004;15:263–266. doi: 10.1097/00001756-200402090-00010. [DOI] [PubMed] [Google Scholar]
- 198.Bate C, Rumbold L, Williams A. J. Neuroinflamm. 2007;4:5. doi: 10.1186/1742-2094-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu D, Liu J, Wen J. Free Radic. Biol. Med. 1999;27:478–482. doi: 10.1016/s0891-5849(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 200.Liu D, Liu J, Sun D, Wen J. J. Neurotrauma. 2004;21:805–816. doi: 10.1089/0897715041269650. [DOI] [PubMed] [Google Scholar]
- 201.Liu D, Liu J, Sun D, Alcock NW, Wen J. Free Radic. Biol. Med. 2003;34:64–71. doi: 10.1016/s0891-5849(02)01184-x. [DOI] [PubMed] [Google Scholar]
- 202.Bao F, Liu D. Neuroscience. 2004;126:285–295. doi: 10.1016/j.neuroscience.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 203.Whittemore ER, Loo DT, Cotman CW. Neuroreport. 1994;5:1485–1488. doi: 10.1097/00001756-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 204.Samanta S, Perkinton MS, Morgan M, Williams RJ. J. Neurochem. 1998;70:2082–2090. doi: 10.1046/j.1471-4159.1998.70052082.x. [DOI] [PubMed] [Google Scholar]
- 205.Lim CS, Lee JC, Kim SD, Chang DJ, Kaang BK. Brain Res. 2002;941:137–145. doi: 10.1016/s0006-8993(02)02646-x. [DOI] [PubMed] [Google Scholar]
- 206.Hoyt KR, Gallagher AJ, Hastings TG, Reynolds IJ. Neurochem. Res. 1997;22:333–340. doi: 10.1023/a:1022403224901. [DOI] [PubMed] [Google Scholar]
- 207.Fatokun AA, Stone TW, Smith RA. Brain Res. 2007;1132:193–202. doi: 10.1016/j.brainres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 208.Rouach N, Calvo CF, Duquennoy H, Glowinski J, Giaume C. Glia. 2004;45:28–38. doi: 10.1002/glia.10300. [DOI] [PubMed] [Google Scholar]
- 209.Vieira de Almeida LM, Pineiro CC, Leite MC, Brolese G, Leal RB, Gottfried C, Goncalves CA. Neurochem. Res. 2008;33(1):8–15. doi: 10.1007/s11064-007-9399-5. [DOI] [PubMed] [Google Scholar]
- 210.Mronga T, Stahnke T, Goldbaum O, Richter-Landsberg C. Glia. 2004;46:446–455. doi: 10.1002/glia.20022. [DOI] [PubMed] [Google Scholar]
- 211.Vollgraf U, Wegner M, Richter-Landsberg C. J. Neurochem. 1999;73:2501–2509. doi: 10.1046/j.1471-4159.1999.0732501.x. [DOI] [PubMed] [Google Scholar]
- 212.Richter-Landsberg C, Vollgraf U. Exp. Cell Res. 1998;244:218–229. doi: 10.1006/excr.1998.4188. [DOI] [PubMed] [Google Scholar]
- 213.Farooqui AA, Yi Ong W, Lu XR, Halliwell B, Horrocks LA. Brain Res. Brain Res. Rev. 2001;38:61–78. doi: 10.1016/s0169-328x(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 214.Dumuis A, Sebben M, Haynes L, Pin JP, Bockaert J. Nature. 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- 215.Mattson MP. Annu. Rev. Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 216.Farooqui AA, Ong WY, Horrocks LA. Pharmacol. Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 217.Shohami E, Shapira Y, Yadid G, Reisfeld N, Yedgar S. J. Neurochem. 1989;53:1541–1546. doi: 10.1111/j.1471-4159.1989.tb08550.x. [DOI] [PubMed] [Google Scholar]
- 218.Homayoun P, Rodriguez de Turco EB, Parkins NE, Lane DC, Soblosky J, Carey ME, Bazan NG. J. Neurochem. 1997;69:199–205. doi: 10.1046/j.1471-4159.1997.69010199.x. [DOI] [PubMed] [Google Scholar]
- 219.Dhillon HS, Donaldson D, Dempsey RJ, Prasad MR. J. Neurotrauma. 1994;11:405–415. doi: 10.1089/neu.1994.11.405. [DOI] [PubMed] [Google Scholar]
- 220.Homayoun P, Parkins NE, Soblosky J, Carey ME, Rodriguez de Turco EB, Bazan NG. Neurochem. Res. 2000;25:269–276. doi: 10.1023/a:1007583806138. [DOI] [PubMed] [Google Scholar]
- 221.Pilitsis JG, Coplin WM, O'Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Brain Res. 2003;985:198–201. doi: 10.1016/s0006-8993(03)03044-0. [DOI] [PubMed] [Google Scholar]
- 222.Lu XR, Ong WY, Halliwell B. Exp. Brain Res. 2001;138:500–508. doi: 10.1007/s002210100737. [DOI] [PubMed] [Google Scholar]
- 223.Baran H, Heldt R, Hertting G. Brain Res. 1987;404:107–112. doi: 10.1016/0006-8993(87)91360-6. [DOI] [PubMed] [Google Scholar]
- 224.Layton ME, Pazdernik TL, Samson FE. Neurosci. Lett. 1997;236:63–66. doi: 10.1016/s0304-3940(97)00765-9. [DOI] [PubMed] [Google Scholar]
- 225.Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. J. Neurotrauma. 2004;21:1712–1722. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- 226.Bullock R, Fujisawa H. J. Neurotrauma. 1992;9 Suppl 2:S443–S462. [PubMed] [Google Scholar]
- 227.McIntosh TK, Saatman KE, Raghupathi R, Graham DI, Smith DH, Lee VM, Trojanowski JQ. Neuropathol. Appl. Neurobiol. 1998;24:251–267. doi: 10.1046/j.1365-2990.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- 228.Tator CH, Fehlings MG. J. Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 229.Tator CH. Neurochirurgie. 1991;37:291–302. [PubMed] [Google Scholar]
- 230.Gennarelli TA. J. Emerg. Med. 1993;11 Suppl 1:5–11. [PubMed] [Google Scholar]
- 231.Nakano S, Kogure K, Abe K, Yae T. J. Neurochem. 1990;54:1911–1916. doi: 10.1111/j.1471-4159.1990.tb04890.x. [DOI] [PubMed] [Google Scholar]
- 232.Yoshida S, Inoh S, Asano T, Sano K, Shimasaki H, Ueta N. J. Neurochem. 1983;40:1278–1286. doi: 10.1111/j.1471-4159.1983.tb13567.x. [DOI] [PubMed] [Google Scholar]
- 233.Narita K, Kubota M, Nakane M, Kitahara S, Nakagomi T, Tamura A, Hisaki H, Shimasaki H, Ueta N. Neurol. Res. 2000;22:393–400. doi: 10.1080/01616412.2000.11740689. [DOI] [PubMed] [Google Scholar]
- 234.Abe K, Yuki S, Kogure K. Stroke. 1988;19:480–485. doi: 10.1161/01.str.19.4.480. [DOI] [PubMed] [Google Scholar]
- 235.Halat G, Lukacova N, Chavko M, Marsala J. Gen. Physiol. Biophys. 1987;6:387–399. [PubMed] [Google Scholar]
- 236.Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao FH. J. Biol. Chem. 2006;281:6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- 237.Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY. J. Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 238.Phillis JW. Life Sci. 1996;58:PL97–PL101. doi: 10.1016/0024-3205(95)02320-8. [DOI] [PubMed] [Google Scholar]
- 239.Estevez AY, Phillis JW. Brain Reseach. 1997;752:203–208. doi: 10.1016/s0006-8993(96)01450-3. [DOI] [PubMed] [Google Scholar]
- 240.Owada Y, Tominaga T, Yoshimoto T, Kondo H. Brain Res. Mol. Brain Res. 1994;25:364–368. doi: 10.1016/0169-328x(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 241.Saluja I, Song D, O'Regan MH, Phillis JW. Neurosci. Lett. 1997;233:97–100. doi: 10.1016/s0304-3940(97)00646-0. [DOI] [PubMed] [Google Scholar]
- 242.Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 243.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 244.Tabuchi S, Uozumi N, Ishii S, Shimizu Y, Watanabe T, Shimizu T. Acta. Neurochir. Suppl. 2003;86:169–172. doi: 10.1007/978-3-7091-0651-8_36. [DOI] [PubMed] [Google Scholar]
- 245.Arai K, Ikegaya Y, Nakatani Y, Kudo I, Nishiyama N, Matsuki N. Eur. J. Neurosci. 2001;13:2319–2323. doi: 10.1046/j.0953-816x.2001.01623.x. [DOI] [PubMed] [Google Scholar]
- 246.Sapirstein A, Bonventre JV. Neurochem. Res. 2000;25:745–753. doi: 10.1023/a:1007583708713. [DOI] [PubMed] [Google Scholar]
- 247.Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. J. Neuroinflamm. [Electronic Resource] 2006;3:28. doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pinto F, Brenner T, Dan P, Krimsky M, Yedgar S. GLIA. 2003;44:275–282. doi: 10.1002/glia.10296. [DOI] [PubMed] [Google Scholar]
- 249.Marusic S, Leach MW, Pelker JW, Azoitei ML, Uozumi N, Cui J, Shen MW, DeClercq CM, Miyashiro JS, Carito BA, Thakker P, Simmons DL, Leonard JP, Shimizu T, Clark JD. J. Exp. Med. 2005;202:841–851. doi: 10.1084/jem.20050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tariq M, Khan HA, Al Moutaery K, Al Deeb S. Brain Res. Bull. 2001;54:77–82. doi: 10.1016/s0361-9230(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 251.Sun Gy, Xu J, Jensen MD, Simonyi A. J. Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 252.Farooqui AA, Rapoport SI, Horrocks LA. Neurochem. Res. 1997;22:523–527. doi: 10.1023/a:1027380331807. [DOI] [PubMed] [Google Scholar]
- 253.Ross BM, Moszczynska A, Erlich J, Kish SJ. J. Neurochem. 1998;70:786–793. doi: 10.1046/j.1471-4159.1998.70020786.x. [DOI] [PubMed] [Google Scholar]
- 254.Talbot K, Young RA, Jolly-Tornetta C, Lee VM, Trojanowski JQ, Wolf BA. Neurochem. Int. 2000;37:17–31. doi: 10.1016/s0197-0186(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 255.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Neurobiol. Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 256.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. J. Neurosci. Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 257.Butterfield DA, Griffin S, Munch G, Pasinetti GM. J. Alzheimers Dis. 2002;4:193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- 258.Kanfer JN, Sorrentino G, Sitar DS. Neurosci. Lett. 1998;257:93–96. doi: 10.1016/s0304-3940(98)00806-4. [DOI] [PubMed] [Google Scholar]
- 259.Singh IN, Sorrentino G, Sitar DS, Kanfer JN. Brain Res. 1998;800:275–281. doi: 10.1016/s0006-8993(98)00532-0. [DOI] [PubMed] [Google Scholar]
- 260.Andersen JM, Myhre O, Fonnum F. Neurochem. Res. 2003;28:319–326. doi: 10.1023/a:1022389503105. [DOI] [PubMed] [Google Scholar]
- 261.Huterer SJ, Tourtellotte WW, Wherrett JR. Neurochem. Res. 1995;20:1335–1343. doi: 10.1007/BF00992509. [DOI] [PubMed] [Google Scholar]
- 262.Giri S, Khan M, Rattan R, Singh I, Singh AK. J. Lipid Res. 2006;47:1478–1492. doi: 10.1194/jlr.M600084-JLR200. [DOI] [PubMed] [Google Scholar]
- 263.Hayakawa T, Chang MC, Rapoport SI, Appel NM. J. Pharmacol. Exp. Ther. 2001;296:1074–1084. [PubMed] [Google Scholar]
- 264.Klivenyi P, Beal MF, Ferrante RJ, Andreassen OA, Wermer M, Chin MR, Bonventre JV. J. Neurochem. 1998;71:2634–2637. doi: 10.1046/j.1471-4159.1998.71062634.x. [DOI] [PubMed] [Google Scholar]
- 265.Kalyvas A, David S. Neuron. 2004;41:323–335. doi: 10.1016/s0896-6273(04)00003-0. [DOI] [PubMed] [Google Scholar]