Abstract
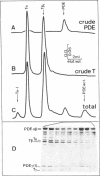
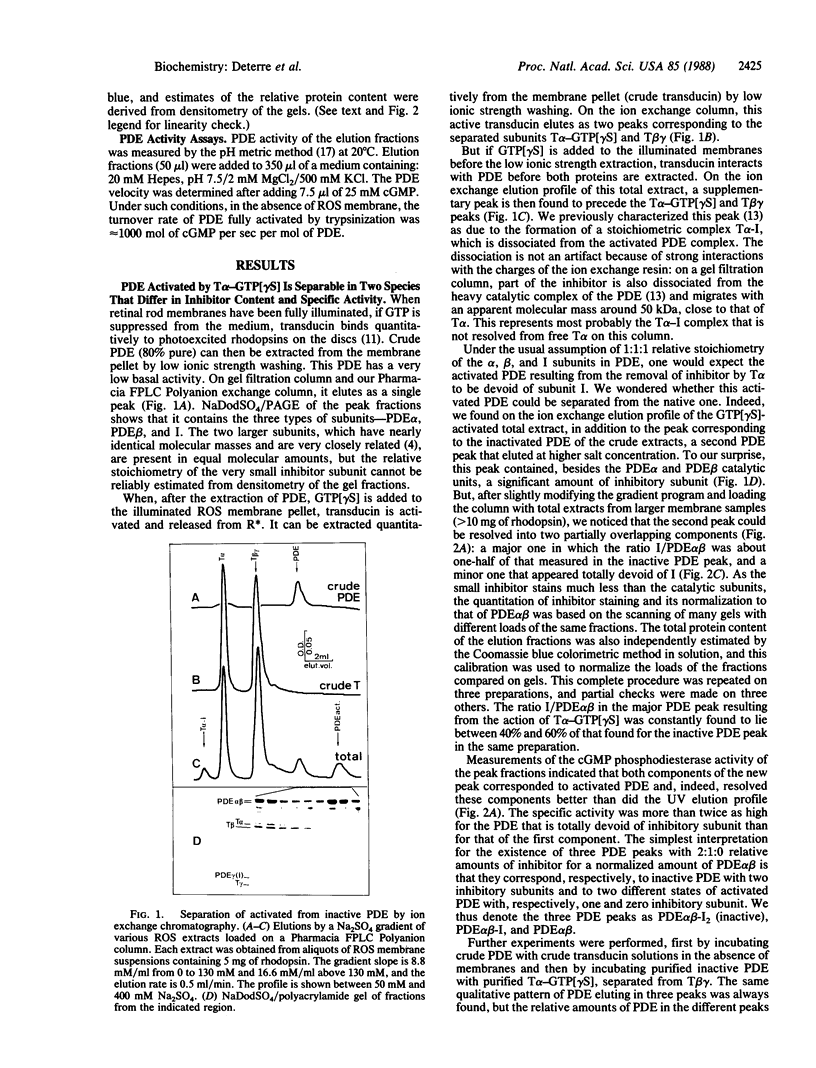
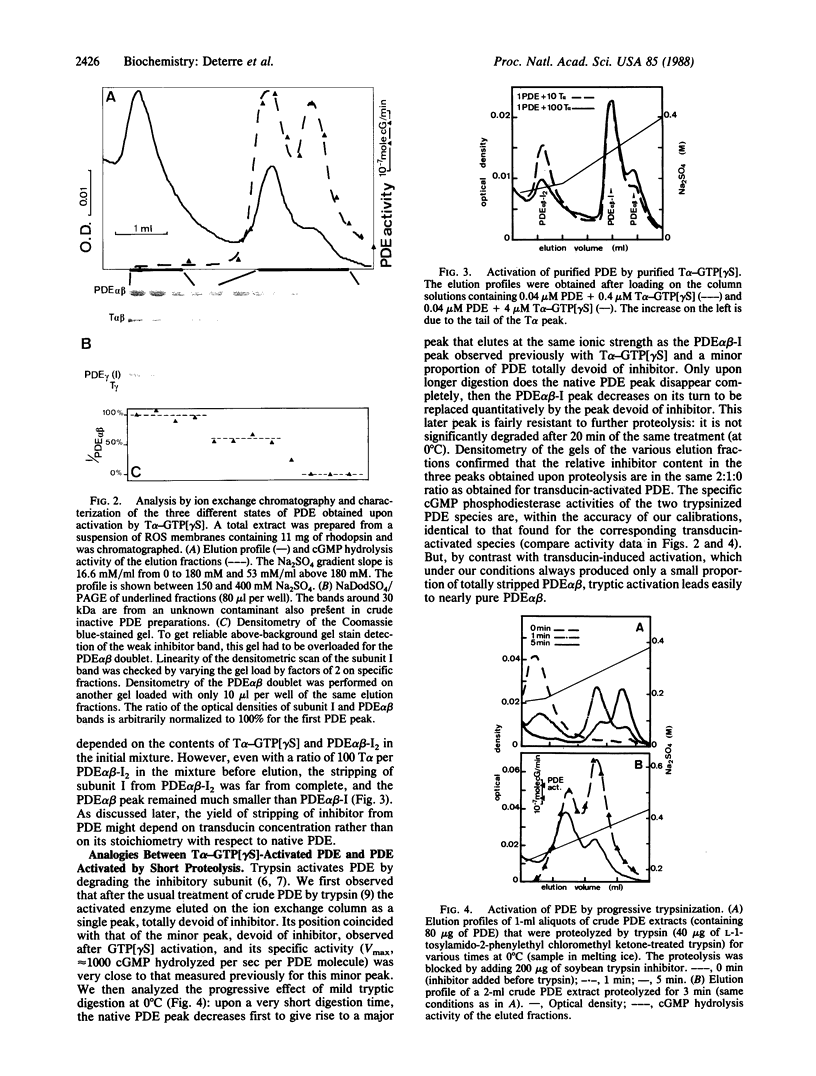
The cGMP phosphodiesterase (PDE) of cattle retinal rod outer segments comprises three types of subunits: the two heavy catalytic ones, PDE alpha and PDE beta, each around 85 kDa, and the light inhibitory one, PDE gamma or I (11 kDa). The relative stoichiometry is usually assumed to be 1:1:1. PDE activation in the visual transduction cascade results from removal of the inhibitor by the alpha subunit of transducin (T alpha). The stoichiometric complex T alpha-I, separated from activated PDE, has been isolated and characterized. Analyzing now the activated PDE, we find that it still contains some inhibitor and is resolvable into two species, one with 50% of the inhibitor content of the native enzyme and the other totally devoid of it. The same two species are observed upon activation of PDE by very short tryptic proteolysis, which specifically degrades the inhibitor. This leads us to conclude that the composition of the native enzyme is PDE alpha beta-I2. The two inhibitory subunits are differentially bound, sequentially removable, and exchangeable between the native complex PDE alpha beta-I2 and the fully active PDE alpha beta. The possibility of this exchange precludes as yet an unambiguous estimate of the actual activity of the intermediate complex PDE alpha beta-I. The differential binding and the exchangeability of the inhibitors raises the possibility of a fast, diffusion controlled, switch-off mechanism of PDE activity after a flash, which would shortcut the inactivation resulting from the slow GTPase rate of transducin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Pfister C., Chabre M. Guanine nucleotides and magnesium dependence of the association states of the subunits of transducin. FEBS Lett. 1984 Dec 10;178(2):228–232. doi: 10.1016/0014-5793(84)80606-7. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin alpha-subunit. Proteins. 1986 Oct;1(2):188–193. doi: 10.1002/prot.340010210. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
- Hurley J. B. Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol. 1987;49:793–812. doi: 10.1146/annurev.ph.49.030187.004045. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Kohnken R. E., Eadie D. M., Revzin A., McConnell D. G. The light-activated GTP-dependent cyclic GMP phosphodiesterase complex of bovine retinal rod outer segments. Dark resolution of the catalytic and regulatory proteins. J Biol Chem. 1981 Dec 10;256(23):12502–12509. [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Parker K. R., Dratz E. A. The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment. Annu Rev Physiol. 1987;49:765–791. doi: 10.1146/annurev.ph.49.030187.004001. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Sitaramayya A., Harkness J., Parkes J. H., Gonzalez-Oliva C., Liebman P. A. Kinetic studies suggest that light-activated cyclic GMP phosphodiesterase is a complex with G-protein subunits. Biochemistry. 1986 Feb 11;25(3):651–656. doi: 10.1021/bi00351a021. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]