Abstract
The kinetochore is a macromolecular complex that controls chromosome segregation and cell cycle progression. When sister kinetochores make bioriented attachments to microtubules from opposite poles, the spindle checkpoint is silenced. Biorientation and the spindle checkpoint are regulated by a balance between the Ipl1/Aurora B protein kinase and the opposing activity of protein phosphatase I (PP1). However, little is known about the regulation of PP1 localization and activity at the kinetochore. Here, we developed a method to purify centromere-bound kinetochores and used quantitative proteomics to identify the Fin1 protein as a PP1 regulatory subunit. The Fin1/PP1 complex is regulated by phosphorylation and 14–3–3 protein binding. When Fin1 is mislocalized, bipolar spindles fail to assemble but the spindle checkpoint is inappropriately silenced due to PP1 activity. These data suggest that Fin1 is a PP1 regulatory subunit whose spatial and temporal activity must be precisely controlled to ensure genomic stability.
Keywords: Kinetochore, Ipl1/Aurora B protein kinase, PP1/Glc7 protein phosphatase I, Fin1, 14–3–3, SILAC quantitative mass spectrometry
Faithful chromosome segregation is essential to avoid the aneuploidy that is associated with cancer and birth defects (Kops et al. 2005). Segregation is directed by the kinetochore, the macromolecular protein complex that assembles onto centromeric chromatin. The simplest kinetochore characterized to date is in budding yeast, where 38 structural proteins in various subcomplexes assemble onto the ∼125-base-pair (bp) centromere to form a single microtubule-binding site (for reviews, see Westermann et al. 2007; Santaguida and Musacchio 2009). Inner kinetochore proteins assemble directly onto centromeric DNA, while outer kinetochore proteins mediate attachment to spindle microtubules. In all organisms, segregation requires sister kinetochores to biorient and attach to spindle microtubules emanating from opposite poles. Once all chromosomes biorient, segregation is initiated by the anaphase-promoting complex (APC/C)-mediated destruction of the anaphase inhibitor Pds1/securin. Defects in kinetochore biorientation are monitored by the spindle checkpoint that delays the onset of anaphase by inhibiting the APC/C (for reviews, see Musacchio and Salmon 2007; Westermann et al. 2007; Tanaka and Desai 2008).
Errors in kinetochore–microtubule attachment must be corrected prior to anaphase. A variety of evidence suggests that mono-oriented attachments are destabilized by the phosphorylation of kinetochore proteins by the conserved Ipl1/Aurora B protein kinase (for reviews, see Ruchaud et al. 2007; Kelly and Funabiki 2009). Ipl1/Aurora B activity is also required for the spindle checkpoint, and this may be coupled to its role in generating unattached kinetochores in response to biorientation defects (Pinsky et al. 2006b). Although a number of Ipl1/Aurora B substrates have been identified, it is not clear whether the phosphorylation of these targets occurs specifically in response to defective kinetochore–microtubule attachments or whether additional substrates exist. To fully understand the process of kinetochore biorientation, all of the Ipl1/Aurora B-mediated phosphorylation events that occur on misoriented kinetochores must be identified. However, the lack of a method to selectively purify chromatin-bound kinetochore complexes has made it difficult to detect phosphorylation specific to misoriented kinetochores.
It is also critical to understand the mechanisms that counteract Ipl1/Aurora B-dependent phosphorylation to stabilize proper attachments and silence the spindle checkpoint. To date, the only activity known to oppose Ipl1/Aurora B is dephosphorylation by protein phosphatase I (PP1) (Francisco et al. 1994; Sassoon et al. 1999; Hsu et al. 2000; Pinsky et al. 2006a; Emanuele et al. 2008). PP1 is a ubiquitous serine/threonine phosphatase that regulates numerous cellular processes at various intracellular locations (for review, see Cohen 2002). Because the catalytic subunit of PP1 has little substrate specificity, these processes are controlled by regulatory (or targeting) subunits that direct PP1 localization and activity (Egloff et al. 1997; Hendrickx et al. 2009). Although PP1 localizes to kinetochores (Bloecher and Tatchell 2000; Trinkle-Mulcahy et al. 2003) where it opposes Ipl1/Aurora B (Sassoon et al. 1999; Pinsky et al. 2006a), a regulatory subunit that targets PP1 to kinetochores has not been identified in any organism. It is therefore unclear how PP1 activity is regulated to stabilize proper bioriented attachments, yet still allow efficient phosphorylation of inappropriately attached kinetochores.
To identify key proteins and post-translational modifications required for kinetochore biorientation, we established a method to enrich for centromere-bound kinetochores by isolating centromeric minichromosomes from budding yeast. Mass spectrometry (MS) on the purified samples allowed us to identify the majority of known kinetochore proteins as well as to detect novel phosphorylation events on the centromere-bound kinetochores. Strikingly, quantitative MS analysis of purified kinetochores identified Fin1. Here, we show that Fin1 is a PP1 regulatory subunit that helps mediate PP1 binding to kinetochore proteins. The activity of the Fin1/PP1 complex is tightly controlled by phosphorylation, 14–3–3 protein binding, and PP1 itself. When Fin1 is prematurely targeted to microtubules, bipolar spindles do not form. However, the balance between PP1 and Ipl1/Aurora B activity is lost and the spindle checkpoint is silenced. Our results identify a Fin1/PP1 complex that must be tightly controlled to ensure that the Ipl1/Aurora B kinase and PP1 phosphatase activities are balanced.
Results
Yeast kinetochores can be purified by isolating centromeric minichromosomes
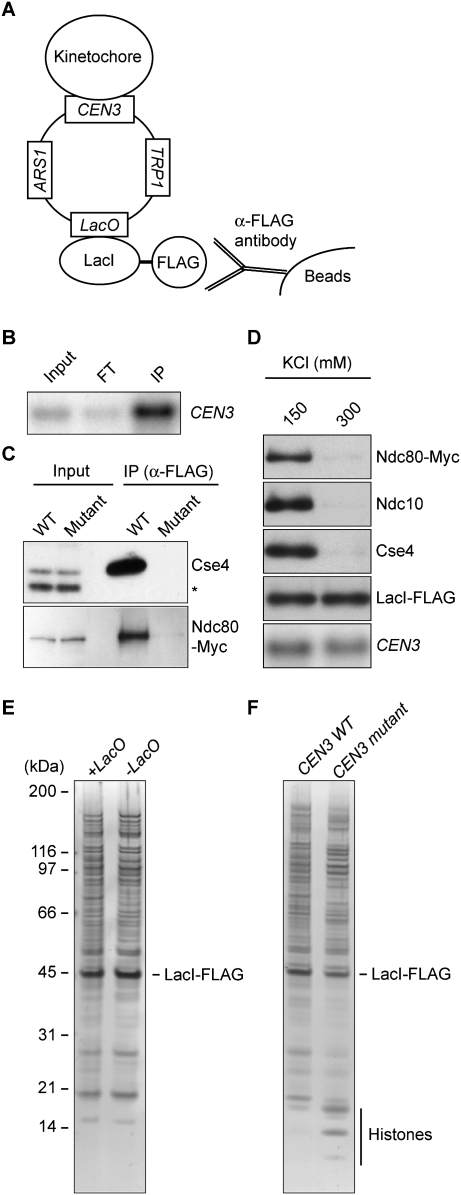
We sought to establish a method to comprehensively characterize the composition and post-translational modification status of intact kinetochores. Because chromosomes contain a single kinetochore relative to an abundance of other chromosomal proteins (e.g., histones and transcription factors), we anticipated that it would be difficult to obtain sufficiently pure kinetochores for MS analysis. In addition, although stable kinetochore subcomplexes have been isolated, the properties of an intact kinetochore are unknown (De Wulf et al. 2003). We therefore chose to purify circular minichromosomes from budding yeast for a number of reasons. First, minichromosomes containing the yeast centromere assemble kinetochores and segregate faithfully during cell division (Clarke and Carbon 1980). Second, the small size of a minichromosome (∼2 kb) ensures a high ratio of kinetochore proteins relative to other chromatin-associated proteins. Third, minichromosomes have previously aided studies of other chromatin-based processes (Ducker and Simpson 2000; Ivanov and Nasmyth 2005). We therefore optimized a lactose operon purification system (LacI–LacO) to isolate minichromosomes containing kinetochores (Ducker and Simpson 2000; A Unnikrishnan, PR Gafken, and T Tsukiyama, in prep.). The key elements of the minichromosome include a selectable marker (TRP1), an origin of replication (ARS1), tandem repeats of lactose operators (LacO), and the centromere from Chromosome III (CEN3) (Fig. 1A). We introduced the minichromosome into a host strain expressing high levels of a LacI-Flag fusion protein from a constitutive promoter. To isolate the minichromosomes, we lysed cells and captured the minichromosomes with beads conjugated to anti-Flag antibodies in a buffer containing a physiological concentration of salt (150 mM KCl). Under these conditions, the majority of LacI-Flag from the extract was immunoprecipitated (data not shown), and Southern blot analysis confirmed that the minichromosome copurified with LacI-Flag (Fig. 1B).
Figure 1.
Yeast kinetochores can be purified by isolating centromeric minichromosomes. (A) Minichromosome purification scheme. Centromeric minichromosomes containing LacO repeats are captured by LacI-Flag affinity purification. (B) Purified minichromosome DNA (SBY5218) was detected by Southern blot analysis using a probe specific to CEN3. Note that the immunoprecipitation (IP) lane contains 30 times more sample equivalent than input and flow-through (FT). (C) Kinetochore proteins remain associated with purified minichromosomes. Wild-type (SBY5218) or mutant (SBY5248) CEN3 minichromosomes were purified from strains expressing Ndc80-Myc. Immunoblots were performed using anti-Cse4 (asterisk indicates background band) and anti-Myc antibodies. (D) Kinetochores dissociate from CEN minichromosomes in the presence of 300 mM KCl. Minichromosomes (SBY5218) were washed with buffer containing 150 or 300 mM KCl. Purified samples were analyzed by immunoblots using antibodies to the indicated kinetochore proteins and by Southern blot analysis using a CEN3 probe to detect minichromosome DNA. (E) The LacI-Flag-purified samples have high levels of proteins that copurify independently of the lacO sequence. Proteins that copurified with minichromosomes that contained LacO repeats (SBY6107) or lacked the repeats (SBY6037) were analyzed by SDS-PAGE followed by silver staining to compare the sample purity. (F) The higher cellular copy number of mutant CEN minichromosomes increases the yield of mutant minichromosomes. Proteins that copurified with minichromosomes containing a wild-type (SBY6107) or a mutant (SBY6114) CEN were analyzed as in E. Note that histone bands were easily detected in the mutant CEN minichromosome purification.
We next tested whether kinetochore proteins remain associated with the minichromosomes by performing immunoblots. As a control, we purified minichromosomes that cannot assemble kinetochores due to mutations in the conserved centromere domain CDEIII (Ortiz et al. 1999). Because these mutant minichromosomes lack kinetochores, the intracellular copy number is ∼20-fold higher than the wild-type (WT) centromeric minichromosome (Tschumper and Carbon 1983; data not shown). Despite this copy number difference, the centromeric histone H3 variant (Cse4) and Ndc80 outer kinetochore component specifically copurified with minichromosomes containing functional centromeres (Fig. 1C). Because Ndc80 depends on other inner kinetochore proteins for its kinetochore association (Westermann et al. 2003), this result suggests that the majority of kinetochore proteins stay bound to minichromosomes. Indeed, Ndc10, Mif2, and Ctf19 also copurified with centromeric minichromosomes (Supplemental Fig. S1).
We next determined the stability of the kinetochore structure under more stringent wash conditions. Neither inner nor outer kinetochore proteins copurified with minichromosomes in the presence of 300 mM KCl, suggesting that the kinetochore structure is disrupted (Fig. 1D). Because we used mild wash conditions (150 mM KCl) to maintain the kinetochore structure, we analyzed the level of nonspecific binding by comparing LacI-Flag purifications from strains containing minichromosomes with or without LacO repeats. Although kinetochore proteins copurified specifically with LacO-containing minichromosomes (data not shown), both purifications exhibited similar amounts and patterns of proteins on a silver-stained SDS-PAGE gel, indicating a high level of nonspecific binding (Fig. 1E). The majority of these proteins were not detected when the purification was performed from cells lacking LacI-Flag (data not shown), indicating that they are LacI-Flag-binding proteins. Because LacI has affinity for DNA (Lin and Riggs 1975), we assume that the majority of these proteins are associated with chromatin in the extract (see below).
Our ability to detect kinetochore proteins specifically in the centromeric minichromosome sample prompted us to test whether they could be identified by MS. We purified minichromosomes with wild-type or mutant centromeres from 10 L of mitotic cells. As expected due to the higher copy number, we isolated more mutant centromeric minichromosomes than wild type (data not shown). Consistent with this, histone bands were detectable in the mutant centromere sample by silver-stained SDS-PAGE, although the overall protein amounts in the two samples were relatively similar due to the nonspecific LacI-Flag-binding proteins (Fig. 1F). The purified samples were digested with site-specific proteases, and the resulting peptides were separated by strong cation exchange (SCX) chromatography. Fractionated samples were analyzed by reverse-phase liquid chromatography electrospray ionization tandem MS (LC-MS/MS). Sequence database searching identified 329 proteins in the wild-type centromeric minichromosome sample (Supplemental Table S1), and 249 proteins in the mutant centromere sample (Supplemental Table S2). As expected from our observation that the bulk of purified proteins nonspecifically associate with LacI-Flag, the majority of proteins identified in both purifications interact with DNA (e.g., histones and chromatin remodeling factors). However, the majority (35 out of 38) of known constitutive kinetochore components were detected specifically in the wild-type centromeric minichromosome sample (Supplemental Table S3); only one kinetochore protein (two peptides derived from Mif2) was detected in the mutant centromere sample. These results suggest that kinetochore proteins are below the limit of detection unless wild-type centromeric minichromosomes are purified. This is consistent with the low abundance of kinetochore proteins in the cell, and their specific association with centromeric DNA. In agreement with estimates of multiple copies of the KMN (KNL1–Mis12–Ndc80 complex) network at the kinetochore (Joglekar et al. 2006), the percentage of sequence coverage of proteins in the Ndc80, Mis12, and Spc105 subcomplexes was generally higher than other kinetochore proteins (Supplemental Table S3). There are also likely to be kinetochore proteins that we did not detect due to their weak association throughout the purification and/or difficulty in detecting solely by MS techniques.
Various post-translational modifications play important roles in chromosome segregation (Kotwaliwale and Biggins 2008), and phosphorylation is critical because many essential protein kinases and phosphatases regulate segregation (Taylor and Peters 2008; De Wulf et al. 2009). However, the identification of phosphorylation sites by MS remains a challenge, in part due to their low stoichiometry and the labile nature of some phospho-modifications during MS analysis. We searched the MS data for phosphorylated peptides and identified 10 phosphorylation events (four novel phosphorylations) on seven kinetochore proteins (Supplemental Table S4). Taken together, these data show that the minichromosome purification substantially enriches kinetochore proteins to allow phosphorylation on centromere-bound kinetochore proteins to be detected.
Quantitative MS analysis identifies Fin1 as a kinetochore protein
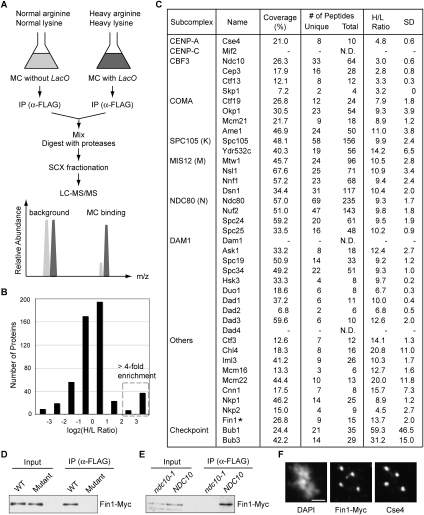
To identify previously undetected kinetochore proteins, we employed a quantitative proteomics approach that is based on the use of the stable isotope labeling by amino acids in cell culture (SILAC) technique (Mann 2006) to compare the relative enrichment of proteins derived from a specific complex purification versus nonspecific purification (Ranish et al. 2003). Because it is beneficial for samples to have similar levels of protein, we compared proteins that copurified with mitotic centromeric minichromosomes with or without LacO repeats (Fig. 1E). The proteins in the test strain (with LacO) were labeled with isotopically heavy lysine and arginine during cell growth, and then minichromosomes were purified and subsequently mixed with proteins purified from a reference strain (without LacO) grown in the presence of isotopically normal lysine and arginine (Fig. 2A). The protein mixture was processed and analyzed by LC-MS/MS as above, except that the high-resolution linear ion trap-Orbitrap (LTQ-Orbitrap) instrument was used to facilitate high-throughput peptide identification and improved quantification of peptide abundances (Hu et al. 2005). The enrichment ratios were determined by comparing the relative ion intensities of peptide pairs derived from the two samples (Han et al. 2001).
Figure 2.
Quantitative MS analysis identifies the Fin1 kinetochore protein. (A) Quantitative proteomics strategy. Centromeric minichromosomes (MCs) with LacO repeats (SBY6107) were grown in media containing isotopically heavy arginine and lysine (dark gray), while those without LacO (SBY6037) were grown in normal media (light gray). The graph shows that proteins specific to the heavy LacO-containing sample will be more abundant than proteins derived from the light sample. Background proteins will have abundance ratios that are similar or higher in the sample derived from the strain lacking the LacO sites. (B) Distribution of proteins detected by MS based on the enrichment ratio (H/L indicates heavy/light ratio). The dotted box indicates the proteins greater than fourfold-enriched in the heavy sample containing the LacO minichromosome. (C) List of kinetochore proteins with percentage of sequence coverage, number of unique and total peptides (N.D. indicates not detected), and H/L ratio with standard deviation (SD). (D) Fin1 associates with the centromere. Wild-type (SBY6010) or mutant (SBY6013) CEN3 minichromosomes were purified from cells that express Fin1-Myc and immunoblotted with anti-Myc antibodies. (E) The association of Fin1 with centromeric minichromosomes depends on kinetochore assembly. Wild-type CEN3 minichromosomes were purified from ndc10-1 (SBY6593) or NDC10 (SBY6594) strains and immunoblotted as in D. (F) Fin1 localizes to endogenous kinetochores during metaphase. Fin1-Myc cells (SBY8301) were arrested in metaphase by nocodazole treatment, and chromosome spreads were immunostained with anti-Cse4 and anti-Myc antibodies. Bar, 2 μm.
Five-hundred-eighteen proteins were identified and quantified (Supplemental Table S5). Most proteins showed an enrichment ratio close to 1 since the bulk of them copurify with LacI-Flag (Fig. 2B). However, 44 proteins were highly enriched (greater than fourfold) in the minichromosome sample containing LacO repeats, and the majority were kinetochore proteins (Supplemental Table S5). Strikingly, all detected constitutive kinetochore (35 out of 38) proteins exhibited enrichment ratios >2.7-fold (Fig. 2C). Replication factors (MCM2–7 complex) and other DNA-binding factors (Gal4 and Gcn4) also showed a high enrichment ratio, consistent with the presence of ARS1 and other chromosomal elements on the minichromosome (Supplemental Table S5). Unexpectedly, the Fin1 protein that has no known role in chromosome function also showed a high enrichment ratio (13.7-fold). Fin1 is a cell cycle-regulated protein that accumulates during S phase and is degraded at the end of anaphase (van Hemert et al. 2002; Woodbury and Morgan 2007). During metaphase, Fin1 is diffusely nuclear, and then it translocates onto spindles and spindle poles at anaphase, where it stabilizes the spindle. To determine whether Fin1 also associates with the kinetochore, we purified wild-type and mutant centromeric minichromosomes from cells containing Myc epitope-tagged Fin1. Fin1-Myc copurified with minichromosomes in a centromere-dependent manner, suggesting that Fin1 is a kinetochore protein (Fig. 2D). Consistent with this, Fin1 no longer associated with centromeric minichromosomes purified from ndc10-1 mutant cells that disrupt kinetochore function at the restrictive temperature (Fig. 2E; Goh and Kilmartin 1993). To test for cell cycle regulation of Fin1 localization to kinetochores, we also purified minichromosomes from cells arrested in metaphase versus anaphase and found that Fin1 is associated at both cell cycle stages (Supplemental Fig. S2). To ensure that Fin1 is present on endogenous kinetochores, we performed chromosome spreads to remove soluble nuclear material and allow kinetochore visualization by immunofluorescence microscopy. Spreads prepared from nocodazole-arrested cells showed that Fin1-Myc colocalizes with the centromeric histone variant Cse4 (Fig. 2F). Taken together, these results show that Fin1 is a previously unidentified kinetochore protein.
Fin1 associates with 14–3–3 proteins, outer kinetochore proteins, and PP1
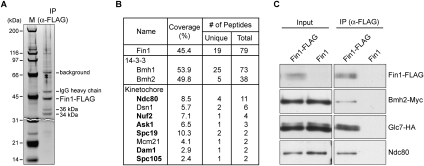
To gain insight into a potential kinetochore function for Fin1, we identified interacting proteins by purifying Fin1-Flag protein from asynchronously growing cells. Silver staining of the sample detected two major bands (34 kDa and 36 kDa) in addition to Fin1-Flag and commonly found contaminants (Fig. 3A; data not shown). We performed LC-MS/MS analysis on the sample and detected a number of kinetochore proteins (Fig. 3B; Supplemental Table S6). The majority are outer kinetochore proteins, suggesting that Fin1 may localize to the outer kinetochore. In addition, the budding yeast 14–3–3 proteins (Bmh1 and Bmh2) that were shown previously to interact with Fin1 (Mayordomo and Sanz 2002; van Hemert et al. 2003) were detected with the highest sequence coverage (Fig. 3B). We confirmed that the 36-kDa protein is Bmh2 (Supplemental Fig. S3), so the 34-kDa protein is likely Bmh1. Although it was reported previously that Fin1 interacts with Glc7 (Mayordomo and Sanz 2002), the sole budding yeast PP1 catalytic subunit (Stark 1996), we did not detect Glc7 by MS. The inability to detect Glc7 by MS may be due to substoichiometric association with Fin1, so we tested whether Glc7 copurifies with Fin1 by immunoprecipitating Fin1-Flag from cells that contained Glc7-HA. In addition to verifying that Glc7 associates with Fin1, we confirmed that Bmh2 and the outer kinetochore protein Ndc80 also copurify (Fig. 3C).
Figure 3.
Fin1 associates with 14–3–3, outer kinetochore proteins, and PP1. (A,B) MS analysis identified Fin1-binding proteins. Fin1-Flag protein was purified from 5 L of asynchronously growing cells (SBY5962). (A) Sample was analyzed by SDS-PAGE followed by silver staining. Two proteins (36 kDa and 34 kDa) specifically copurified with Fin1-Flag. (B) Purified sample was analyzed by LC-MS/MS, and a summary is shown in the table. Outer kinetochore proteins are shown in bold. (C) Coimmunoprecipitation experiments confirmed that Fin1 specifically associates with 14–3–3, Ndc80, and the Glc7 phosphatase. Proteins were purified with anti-Flag antibodies from cells containing Bmh2-Myc and Glc7-HA that express either Fin1-Flag (SBY6368) or untagged Fin1 (SBY6370). Samples were analyzed by immunoblots with the corresponding antibodies.
Fin1 mediates PP1 binding to kinetochore components
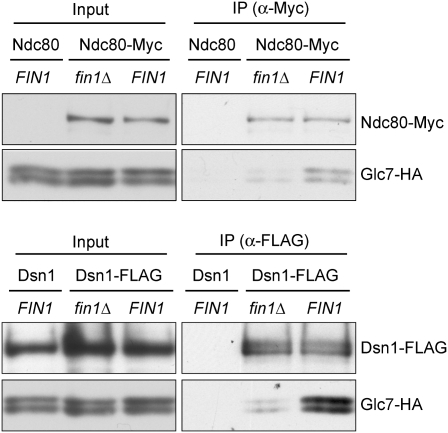
Because Fin1 binds to Glc7, we considered the possibility that it might be a PP1 regulatory subunit that recruits Glc7 onto kinetochores. However, the global localization of Glc7 to chromatin (Hsu et al. 2000) prevented us from using the minichromosome purification technique, chromatin immunoprecipitation, or chromosome spread assays to analyze Glc7 association specifically with kinetochores (data not shown). We therefore asked whether Glc7 copurifies with kinetochore proteins in a Fin1-dependent manner. Using mild immunoprecipitation conditions (150 mM KCl), we detected an interaction between two kinetochore proteins (Ndc80 and Dsn1) and Glc7 (Fig. 4). Although we cannot determine how much of the interaction we detect is occurring on centromere-bound kinetochores, the bulk of the interaction depended on Fin1. Therefore, Fin1 helps to mediate the association between Glc7 and kinetochore proteins. However, the interaction was not completely disrupted in the absence of Fin1, indicating that additional factors recruit Glc7 to bind to kinetochore proteins. This may explain why fin1Δ cells do not exhibit any strong defect in chromosome segregation (Woodbury and Morgan 2007) or major genetic interactions with ipl1-321 (Supplemental Fig. S4).
Figure 4.
Fin1 partially mediates the interaction between Glc7 and kinetochore proteins. (Top) Ndc80-Myc was immunoprecipitated from fin1Δ (SBY7895) and FIN1 (SBY7897) cells expressing Glc7-HA. Purified samples were analyzed by immunoblots using anti-Myc and anti-HA antibodies. Glc7-HA cells with untagged Ndc80 (SBY625) were used as negative control. (Bottom) Dsn1-Flag was purified with anti-Flag antibodies from fin1Δ (SBY7899) and FIN1 (SBY7900) cells expressing Glc7-HA. Purified samples were analyzed by immunoblots using anti-Flag and anti-HA antibodies. Note that the Dsn1-Flag band overlaps with a background signal in the input.
Mislocalization of Fin1 silences the checkpoint via PP1
Although fin1Δ cells do not exhibit significant growth defects or sensitivity to the microtubule-destabilizing drug benomyl (data not shown), Fin1 mislocalization is lethal (Woodbury and Morgan 2007). Cdk1-dependent phosphorylation of Fin1 prevents its premature localization to the spindle and poles, and the overexpression of a Fin1-5A phospho-deficient mutant is toxic (Woodbury and Morgan 2007). We analyzed the corresponding phenotype by arresting cells containing GFP-tubulin and galactose-inducible FIN1-WT or fin1-5A in G1 and then releasing them into galactose media to induce Fin1 expression. Although both strains exhibited similar kinetics of bud emergence, the cells expressing Fin1 assembled bipolar spindles, while most Fin1-5A cells had monopolar spindles (Fig. 5A,B). Consistent with this, 94% of wild-type cells eventually segregated DNA to opposite poles, compared with 29% of Fin1-5A cells (Supplemental Fig. S5). Instead, the majority of Fin1-5A-overexpressing cells moved the entire nucleus into the bud without segregating chromosomes (Supplemental Fig. S5). Because we rarely detected bipolar spindles in the Fin1-5A cells, we do not know whether spindle pole body duplication or spindle assembly is defective. Although monopolar spindles were not detected previously within the first cell cycle in a similar experiment (possibly due to differences in Fin1-5A protein levels), Fin1-5A overexpression in metaphase-arrested cells caused spindles to collapse (Woodbury and Morgan 2007). Taken together, these data suggest that high levels of Fin1-5A destabilize bipolar spindles.
Figure 5.
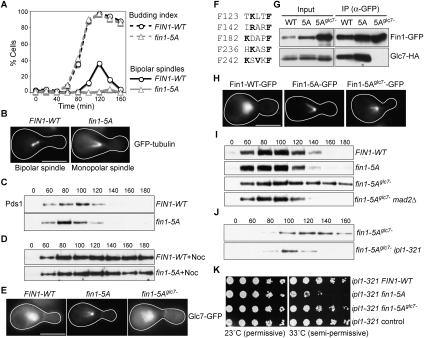
Fin1 regulates Glc7 and antagonizes the Ipl1 kinase. (A,B) Fin1-5A overexpression blocks bipolar spindle assembly. Cells with galactose-inducible FIN1-WT (SBY6825) or fin1-5A (SBY6809) expressing GFP-Tub1 were released from G1 into galactose and fixed every 20 min after release. Budding index and spindle morphology (bipolar or monopolar) were analyzed. Bar, 5 μm. (C) Fin1-5A cells do not activate the spindle checkpoint in response to spindle defects. Cells containing Pds1-Myc and pGAL-FIN1-WT (SBY6458) or pGAL-fin1-5A (SBY6459) were synchronized as in A. Lysates were prepared at the indicated time points and monitored for Pds1-Myc by immunoblot. (D) Cells expressing Fin1-5A activate the spindle checkpoint in response to microtubule depolymerization. The experiment in C was repeated by releasing cells into nocodazole. (E) Glc7 is mistargeted by Fin1-5A. Glc7-GFP localization was monitored in cells with galactose-inducible FIN1-WT (SBY6483), fin1-5A (SBY6484), or fin1-5Aglc7− (SBY6685) as in A. At 110 min after release, 89% of Fin1-5A cells showed Glc7 signal specifically on spindles and poles, while 100% of Fin1-WT and Fin1-5Aglc7− cells exhibited diffuse nuclear Glc7 staining in metaphase. (F) Fin1 possesses five potential Glc7-binding motifs. Residues that match the consensus PP1-binding sequence are highlighted in bold. (G) Fin1-5Aglc7− fails to bind Glc7. Asynchronously growing cells containing Glc7-HA and galactose-inducible FIN1-WT-GFP (SBY6497), fin1-5A-GFP (SBY6498), or fin1-5Aglc7−-GFP (SBY6662) were induced with galactose for 3 h. Fin1-GFP was immunoprecipitated with anti-GFP antibodies, and the samples were analyzed by immunoblots with anti-GFP and anti-HA antibodies. (H) Fin1-5Aglc7− prematurely localizes onto spindles. Cells expressing galactose-inducible FIN1-WT-GFP (SBY6915), fin1-5A-GFP (SBY6916), or fin1-5Aglc7−-GFP (SBY6917) were treated as in E. One-hundred percent of the metaphase cells expressing Fin1-5A-GFP or Fin1-5Aglc7−-GFP cells showed premature spindle localization of Fin1 compared with 0% for Fin1-WT-GFP. (I) The spindle defect in Fin1-5Aglc7− cells triggers the spindle checkpoint. Cells containing Pds1-Myc and galactose-inducible FIN1-WT-GFP (SBY6573), fin1-5A-GFP (SBY6574), fin1-5Aglc7−-GFP (SBY6686), or fin1-5Aglc7−-GFP mad2Δ (SBY6824) were used to analyze checkpoint activation as in C. (J) Ipl1 activity is required for spindle checkpoint activation in Fin1-5Aglc7− cells. IPL1 (SBY6857) or ipl1-321 (SBY6858) cells containing Pds1-Myc and galactose-inducible Fin1-5Aglc7−-GFP were used to analyze checkpoint activation as in C, except that cells were released to 37°C. (K) Endogenous levels of Fin1-5A exhibit genetic interactions with an ipl1 mutant. Serial dilutions (fivefold) of ipl1-321 cells expressing FIN1-WT-GFP (SBY7352), fin1-5A-GFP (SBY7547), or fin1-5Aglc7−-GFP (SBY7548) from the endogenous promoter were plated at 23°C (permissive temperature) and 33°C (semipermissive temperature for ipl1-321). SBY7356 contains a control vector.
The spindle checkpoint halts the cell cycle in response to defects in spindle assembly (Weiss and Winey 1996), but it was reported previously that cells overexpressing Fin1-5A do not exhibit any cell cycle arrest (Woodbury and Morgan 2007). We therefore examined the levels of the anaphase inhibitor Pds1 as cells overexpressing Fin1-WT or Fin1-5A were released from G1. Despite the lack of bipolar spindle formation, Pds1 cycled normally in cells expressing Fin1-5A (Fig. 5C). These data suggested that the spindle checkpoint may not be functional, so we tested this by releasing Fin1-WT or Fin1-5A cells from G1 into nocodazole to depolymerize the microtubules and create unattached kinetochores. Pds1 levels were stabilized in both strains (Fig. 5D), indicating that Fin1-5A cells are capable of activating the checkpoint in response to unattached kinetochores. Although there is a slight decrease in Pds1 levels in Fin1-5A cells at later time points, we did not detect a corresponding increase in sister chromatid separation when we monitored a fluorescently marked chromosome (Supplemental Fig. S6). Therefore, the checkpoint appears to be functional in response to unattached kinetochores in cells overexpressing Fin1-5A.
The defect in spindle checkpoint activation in the fin1-5A mutant cells was similar to our previous observations that ipl1 kinase mutants can trigger the spindle checkpoint in the absence of microtubules but not in their presence (Biggins and Murray 2001). In addition, PP1 activity is required to silence the spindle checkpoint (Pinsky et al. 2009; Vanoosthuyse and Hardwick 2009). We therefore considered the possibility that Fin1-5A prevented checkpoint activation in response to spindle defects by misregulating Glc7. To test this, we first determined whether Fin1-5A expression altered Glc7 localization. As reported previously (Bloecher and Tatchell 2000), Glc7-GFP was localized throughout the nucleus as well as to the bud neck in both wild-type cells and those overexpressing Fin1-WT at metaphase (Fig. 5E; data not shown). In contrast, Glc7 localized to the spindle pole and spindle microtubules in 89% of metaphase cells overexpressing Fin1-5A, confirming that Fin1 is a Glc7 regulatory subunit (Fig. 5E). The bud neck staining appeared weaker in Fin1-5A cells, consistent with the observation that the overexpression of PP1 regulatory subunits can alter its localization (Pinsky et al. 2006a).
To determine whether the defects in spindle assembly and spindle checkpoint activation associated with Fin1-5A were due to Glc7 misregulation, we mutated the five PP1 consensus binding motifs [R/K-(X)0-1-V/I-X-F/W] (Egloff et al. 1997; Hendrickx et al. 2009) in Fin1-5A to create Fin1-5Aglc7− (Fig. 5F). As expected, the control Fin1 proteins copurified with Glc7, but the Fin1-5Aglc7− mutant did not (Fig. 5G). We therefore characterized the phenotypes associated with Fin1-5Aglc7− and found that it prematurely associated with spindle microtubules and blocked spindle assembly in a manner comparable with Fin1-5A cells (Fig. 5H; data not shown). However, Glc7 localization was no longer altered in cells expressing Fin1-5Aglc7− (Fig. 5E). Therefore, Fin1-5A expression blocks spindle assembly regardless of whether it can bind to the Glc7 phosphatase, likely due to its premature loading onto the spindle and poles.
We next tested whether the spindle assembly defect in Fin1-5A cells could trigger the checkpoint when Glc7 was no longer bound to Fin1. We released FIN1-WT, fin1-5A, fin1-5Aglc7-, or fin1-5Aglc7− mad2Δ cells from G1 into galactose and analyzed Pds1 levels (Fig. 5I). Although Pds1 cycled in the Fin1-WT and Fin1-5A cells, it was stabilized in Fin1-5Aglc7− cells in a Mad2 spindle checkpoint protein-dependent manner. Consistent with the role of Glc7 in opposing Ipl1, the checkpoint delay in Fin1-5Aglc7− cells requires Ipl1 kinase activity (Fig. 5J). Taken together, these data strongly suggest that Fin1-5A expression silences the checkpoint by misregulating Glc7, thus leading to an altered kinase/phosphatase balance. However, we did not detect a defect in checkpoint silencing in fin1Δ cells (data not shown), consistent with the possibility that additional regulatory subunits control PP1.
Mutations that lead to Glc7 hyperactivation should exhibit genetic interactions with decreased Ipl1 activity, so we expressed fin1-5A from its endogenous promoter and tested interactions with the ipl1-321 mutant. Although fin1-5A expressed from its endogenous promoter did not cause any obvious growth or spindle assembly defects (Supplemental Fig. S7; data not shown), fin1-5A ipl1-321 cells exhibited a synthetic growth defect (Fig. 5K). Growth was restored when the Glc7-binding motif in Fin1-5A was mutated, supporting the idea that a Fin1–Glc7 complex can antagonize Ipl1 function when it is misregulated.
Regulation of Fin1 by 14–3–3 proteins and PP1
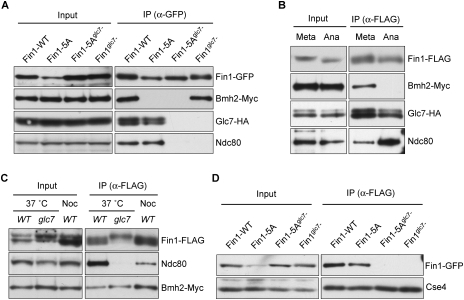
Because Fin1 can cause severe mitotic defects when misregulated, it is crucial to understand how its activity is normally restrained. The identification of 14–3–3 proteins as major Fin1-binding partners suggested that they could be key Fin1 regulators. Because 14–3–3 proteins often interact via phosphorylation (for reviews, see Dougherty and Morrison 2004; van Heusden and Steensma 2006), we tested whether Fin1 can bind to Bmh2 when the Cdk1 phosphorylation sites are mutated. Fin1-5A and Fin1-5Aglc7− no longer interacted with Bmh2 (Fig. 6A), suggesting that phosphorylation creates one or more 14–3–3-binding sites. To confirm this, we also tested whether they interact in anaphase-arrested cells when the Cdk1 sites on Fin1 are dephosphorylated (Woodbury and Morgan 2007). Cells containing galactose-inducible nondegradable Clb2 were arrested in either metaphase with nocodazole treatment or anaphase by the overexpression of nondegradable Clb2 (Surana et al. 1993). Fin1 no longer interacted with Bmh2 in anaphase, supporting the role of Cdk1-dependent phosphorylation in mediating the Fin1/14–3–3 interaction (Fig. 6B). Because Cdk1-dependent phosphorylation also prevents Fin1 from prematurely loading onto spindles at metaphase (Woodbury and Morgan 2007), it is likely that 14–3–3 binding restrains the bulk of Fin1 from localizing to spindles until it gets dephosphorylated at anaphase.
Figure 6.
Regulation of Fin1 by phosphorylation, 14–3–3 proteins, and the PP1 phosphatase. (A) Phosphorylation is required for the Fin1 interaction with 14–3–3 proteins, and Glc7 binding is required for Fin1 to bind the Ndc80 kinetochore protein. Fin1-GFP proteins were immunoprecipitated with anti-GFP antibodies from cells containing Bmh2-Myc, Glc7-HA, and either FIN1-WT-GFP (SBY7609), fin1-5A-GFP (SBY7610), fin1-5Aglc7−-GFP (SBY7611), or fin1glc7−-GFP (SBY7627) expressed from the endogenous FIN1 promoter. The samples were analyzed by immunoblots with the indicated antibodies. (B) 14–3–3 proteins require phosphorylation to bind to Fin1. Cells containing Fin1-Flag, Bmh2-Myc, Glc7-HA ,and galactose-inducible nondegradable clb2 (SBY6452) were grown and then split into two cultures that were treated with either nocodazole (metaphase arrest) or galactose (late anaphase arrest) for 2.5 h. Fin1-Flag was immunoprecipitated, and samples were analyzed by immunoblots. (C) Glc7 is required for the Fin1-Ndc80 interaction. Fin1-Flag was purified from wild-type (SBY6373) or glc7-12 (SBY7841) strains that had been shifted to the restrictive temperature for 2.5 h, and copurifying proteins were analyzed by immunoblots. As a metaphase arrest control, wild-type cells treated with nocodazole were analyzed. (D) Glc7-binding mutants of Fin1 do not localize to kinetochores. Centromeric minichromosomes were purified from cells containing FIN1-WT-GFP (SBY7590), fin1-5A-GFP (SBY7591), fin1-5Aglc7−-GFP (SBY7592), or fin1glc7−-GFP (SBY7628) expressed from the endogenous FIN1 promoter. Samples were analyzed by immunoblots with anti-GFP and anti-Cse4 (loading control) antibodies.
Although our results suggest that 14–3–3 binding inhibits Fin1 localization to spindle microtubules prior to anaphase, we detected Fin1 at kinetochores in both metaphase and anaphase (Supplemental Fig. S2). These data suggest that there is a pool of kinetochore-bound Fin1 that is not bound to the 14–3–3 proteins, so we wanted to understand how this population of Fin1 is regulated. When we characterized Fin1 mutant proteins that could not bind to Glc7, we found that they also could not bind to Ndc80 (Fig. 6A). These data suggested that Glc7 might be required for Fin1 to associate with the kinetochore, so we tested whether Fin1 requires Glc7 activity to associate with kinetochore proteins. We used a temperature-sensitive glc7 mutant that arrests in mitosis (Hisamoto et al. 1994) and compared it with nocodazole-arrested mitotic cells as a control. The association between Fin1 and Ndc80 was abolished in glc7-12 cells (Fig. 6C), confirming that Fin1 requires Glc7 to interact with Ndc80. To directly test whether Fin1 requires Glc7 to bind to kinetochores, we purified centromeric minichromosomes from strains expressing Fin1-GFP proteins containing intact or mutated Glc7-binding sites. Although Fin1 proteins that retain Glc7-binding sites copurify in equivalent amounts with minichromosomes, the Fin1-5Aglc7− and Fin1glc7− proteins that cannot bind to Glc7 no longer copurified (Fig. 6D). These data strongly support the idea that Fin1 requires Glc7 activity to associate with kinetochores. Consistent with this, Fin1 migrates more slowly in glc7 mutant cells (see Fig. 6C). Therefore, the Glc7 phosphatase is required to promote the binding of one of its regulatory subunits to kinetochores, thereby establishing an additional level of control over PP1 activity at kinetochores.
Discussion
As a step toward understanding changes in kinetochore composition and modification state associated with microtubule attachment state, we established a method to purify and detect chromatin-bound kinetochores by isolating centromeric minichromosomes. Using this approach, we found that Fin1 is a previously unidentified kinetochore protein that regulates PP1. Fin1 is inhibited by 14–3–3 binding via Cdk1-dependent phosphorylation, and it requires PP1 for its kinetochore localization. When Fin1 is misregulated, it leads to defects in spindle assembly and causes premature silencing of the spindle checkpoint. Taken together, our data identify a PP1 regulatory subunit at kinetochores and demonstrate that its activity must be carefully controlled to maintain proper kinetochore and spindle function.
A method to purify the kinetochore complex
The kinetochore plays essential roles in chromosome segregation by mediating microtubule attachment and signaling to the spindle checkpoint. It is therefore essential that the components and post-translational modifications that regulate kinetochore functions are elucidated. Although substantial progress has been made in isolating chromosomes (e.g., Mitchison and Kirschner 1985; Dejardin and Kingston 2009; Kulukian et al. 2009) and partially assembling kinetochores in vitro (Sandall et al. 2006), kinetochores have not been purified in chromatin-bound form with sufficient purity for general biochemical analyses. Previous studies have therefore analyzed total soluble protein, making it unclear what specific modifications occur on kinetochores throughout the cell cycle and in response to various microtubule attachments. We therefore modified a LacO–LacI minichromosome purification scheme (Ducker and Simpson 2000; Ivanov and Nasmyth 2005; A Unnikrishnan, PR Gafken, and T Tsukiyama, in prep.) to enrich for centromere-bound kinetochores, and obtained sufficient purity to detect the majority of known kinetochore components by MS. Although it was necessary to perform mild washes to maintain kinetochore structure, more stringent washes should allow similar studies on more stably bound proteins such as histones or replication factors. Indeed, a modified version of the purification method has successfully identified numerous modifications of minichromosome-bound histones (A Unnikrishnan, PR Gafken, and T Tsukiyama, in prep.). This method should therefore be readily adapted to study other chromatin-based processes, as well as facilitate structural studies and other in vitro assays that require purified kinetochores.
Our technique allowed us to identify phosphorylation of centromere-bound kinetochore proteins by MS. Interestingly, six out of 10 phosphorylation sites identified match the Cdk1 consensus sequence. Because Cdk1 mutants have pleiotropic effects, it has been difficult to study the role of Cdk1 phosphorylation in kinetochore function. Our data suggest that Cdk1 directly regulates kinetochores, and the precise functions may be elucidated by studying these specific phosphorylation events. We also identified unique modifications by isolating minichromosomes under different cell cycle states (Akiyoshi et al. 2009), so additional phosphopeptide enrichment and analytical techniques as well as relevant inhibitors should allow the identification of other modifications associated with specific changes in kinetochore–microtubule attachment in the future.
Fin1 is a PP1 regulatory subunit
Our quantitative MS analysis of purified minichromosomes also identified Fin1, a kinetochore protein that had not been detected previously because it appears to be diffuse throughout the nucleus at metaphase (van Hemert et al. 2002; Woodbury and Morgan 2007). Fin1 is degraded by APCCdh1-dependent proteolysis in G1 (Woodbury and Morgan 2007), and we found that it associates with kinetochores in both metaphase and anaphase. Here, we present evidence that Fin1 is a Glc7 regulatory subunit that targets the phosphatase to spindles and kinetochores (for model, see Fig. 7). Fin1 binds to Glc7 via consensus PP1-binding motifs and partially mediates the interaction between Glc7 and kinetochore proteins. The overexpression of Fin1-WT causes Glc7 to redistribute from being diffusely nuclear to strongly spindle-associated in anaphase cells (Supplemental Fig. S8), and Fin1-5A overexpression causes Glc7 to prematurely localize to spindles prior to anaphase. Strikingly, Fin1-5A expression prematurely silenced the checkpoint in a Glc7-dependent manner, supporting recent data showing that PP1 regulates spindle checkpoint exit (Pinsky et al. 2009; Vanoosthuyse and Hardwick 2009). However, due to technical issues, we do not know if Fin1-5A expression recruits additional Glc7 onto kinetochores at metaphase. Therefore, we cannot determine whether the premature silencing of the checkpoint is due to Glc7 binding to kinetochores, spindles, or both structures. Regardless, these results establish Fin1 as a PP1 regulatory subunit on kinetochores and spindles that can silence the checkpoint when misregulated. Because Glc7 overexpression bypasses a nocodazole arrest but Fin1-5A overexpression does not, Fin1 misregulation may lead to Glc7 hyperactivation toward a subset of targets. It is also possible that microtubules are required for Fin1-5A to direct Glc7 activity to silence the checkpoint, or that Fin1-5A cannot hyperactivate Glc7 to the same level as overexpression of the phosphatase alone.
Figure 7.
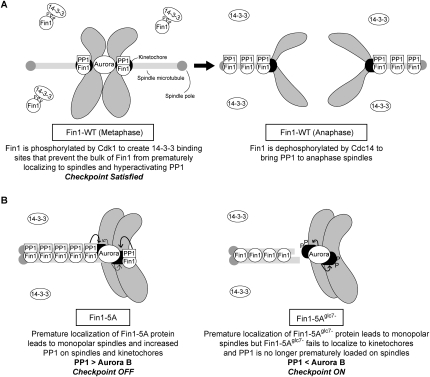
Models for wild-type and mutant Fin1 protein function. (A) Wild-type Fin1 protein: (Left) In metaphase, Cdk1-dependent phosphorylation of Fin1 leads to 14–3–3 binding that prevents the bulk of Fin1 from prematurely localizing to spindles, thereby maintaining appropriate levels of the Aurora B kinase and PP1 phosphatase on kinetochores. There is also a pool of Fin1/PP1 complex at kinetochores that can oppose Aurora B. (Right) Once all kinetochores biorient, the checkpoint is satisfied and cells enter anaphase. During anaphase, the bulk of Fin1 is dephosphorylated by Cdc14 phosphatase to recruit Fin1 and PP1 to anaphase spindles. (B) Mutant Fin1 proteins: The premature localization of the Fin1-5A proteins (Fin1-5A and Fin1-5Aglc7−) to microtubules results in monopolar spindles in a PP1-independent manner. (Left) The increased Fin1-5A protein on spindles and kinetochores inappropriately silences the spindle checkpoint. (Right) The Fin1-5Aglc7− fails to bind PP1 and kinetochores, so the spindle checkpoint remains active due to monopolar spindle formation.
We hypothesize that high levels of Fin1-5A lead to an increased local concentration of Glc7 on kinetochores that can cause dephosphorylation of key Ipl1 targets. Because Fin1-5A prevented spindle assembly, we were not able to determine whether Fin1-5A silenced the checkpoint by restoring microtubule attachments or whether it led to biorientation defects. Consistent with a role in opposing Ipl1 phosphorylation, endogenous levels of Fin1-5A are toxic to ipl1 mutants in a manner that depends on the Glc7-binding sites in Fin1. However, Fin1-5A expression did not alter the phosphorylation of Dam1, a known Ipl1 kinetochore target (data not shown), indicating that the Fin1/Glc7 complex silences the checkpoint by dephosphorylating a subset of unidentified targets. It will therefore be critical to identify the Glc7 substrates that lead to spindle checkpoint inactivation and determine which ones are regulated by Fin1.
We presume that Fin1 is nonessential because there are one or more redundant factors that recruit Glc7 to kinetochores, consistent with our data showing that some Glc7 still associated with kinetochores in the absence of Fin1 and that fin1Δ cells are not delayed in checkpoint exit (data not shown). In addition, there is no apparent Fin1 homolog, so additional proteins must contribute to the regulation of PP1 at kinetochores in both budding yeast and other organisms. The kinetochore protein Spc105 has a conserved PP1-binding motif (residues 74–78: RRVSF) that is essential (data not shown), and Spc105 binds to PP1 in vitro (Hendrickx et al. 2009). It will therefore be important to determine the relative contributions of Spc105, Fin1, and other unidentified PP1 regulatory subunits in opposing Ipl1 function in the future.
Regulation of the Fin1/PP1 phosphatase complex
Although fin1Δ cells are viable, the misregulation of Fin1 causes severe mitotic defects. The spindle defect caused by Fin1-5A is not due to Glc7 hyperactivation, because we observed a similar defect with the Fin1-5Aglc7− mutant that does not bind Glc7. Because both mutant proteins load prematurely onto metaphase spindles, this likely reflects a role for Fin1 in directly regulating microtubules or altering the activity or binding of other microtubule-associated proteins. Consistent with this, we found that ase1 fin1 double mutants are inviable due to defects in spindle assembly (data not shown). The Fin1 purification suggested that the bulk of Fin1 is bound to 14–3–3 proteins via the Cdk1 consensus sites. Because the phosphorylation of these sites is also required to prevent Fin1 from localizing to spindles, we propose that 14–3–3 binding restrains Fin1 from hyperactivating Glc7 in metaphase. This may also explain why the overexpression of Fin1-WT does not affect the localization of Glc7 at metaphase, but alters it at anaphase. We note that many PP1 regulatory subunits copurify with 14–3–3 (Kakiuchi et al. 2007), raising the possibility that 14–3–3 binding may be another general mechanism that controls PP1 subunits.
We also found that Fin1 requires Glc7 activity to associate with kinetochores, and Fin1 is hyperphosphorylated in a glc7 mutant, suggesting that Glc7-dependent dephosphorylation of Fin1 increases its affinity toward kinetochores. Fin1 may only bind to kinetochores when it is bound to Glc7, although our MS data suggest that Fin1 is stably bound to the kinetochore, while Glc7 may associate in a dynamic fashion. We favor a positive feedback model in which Glc7 increases the affinity of Fin1 for the kinetochore and therefore promotes the binding of one of its regulatory subunits to kinetochores (for model, see Supplemental Fig. S9). One possibility is that Glc7 can reverse the Cdk1 phosphorylation (Wu et al. 2009), creating a small pool of active Fin1. Glc7 could then bind to this pool of Fin1 at kinetochores to promote spindle checkpoint silencing at metaphase when Cdk1 activity is high. During anaphase, the additional recruitment of Glc7 to spindles may help to keep the spindle checkpoint inactive. Interestingly, we also found that Fin1 is a substrate of Ipl1 in vitro (Supplemental Fig. S10), raising the possibility that Fin1 is regulated by Ipl1/Glc7 phosphorylation and dephosphorylation to modulate the activity of the phosphatase at kinetochores.
In summary, our work established a chromatin-bound kinetochore enrichment method that should be generally applicable to studying spatial and temporal regulation of kinetochores in the future. Using this method, we identified a PP1 regulatory subunit at spindles and kinetochores that is subject to an exquisite level of control, underscoring the critical nature of phosphatases in ensuring faithful chromosome segregation. In the future, it will be critical to identify additional regulatory subunits that control PP1 function, as well as to isolate the key substrates of the phosphatase that lead to checkpoint silencing.
Materials and methods
Yeast strains, plasmids, and microbial techniques
Yeast strains and plasmids used in this study are listed in Supplemental Tables S7 and S8 and were constructed by standard techniques (see the Supplemental Material). Media and genetic and microbial techniques were essentially as described (Rose et al. 1990). Plasmids were maintained by growing cells in synthetic media lacking appropriate amino acids. Isotopically heavy lysine [13C6 15N2] and arginine [13C6 15N4] (Sigma-Aldrich) were used at 20 mg/L. All experiments in which cells were released from G1 arrest were carried out by adding 1 μg/mL α-factor for 2.5 h and releasing cells as described previously (Biggins and Murray 2001). Nocodazole was used at 10 μg/mL for 3 h. For ndc10-1 experiments, cells were shifted for 3 h to 37°C. Galactose induction was performed by growing cells in 2% raffinose and adding galactose to a final concentration of 2%.
Protein purification, immunological, and Southern blot techniques
Whole-cell extracts were made and immunoblotted as described (Biggins et al. 1999). Commercial antibodies used for immunoblotting were 9E10 (Covance) at a 1:10,000 dilution for the Myc tag, 12CA5 (Roche) at 1:10,000 for the HA tag, anti-Flag antibodies (Sigma-Aldrich) at 1:3000, and anti-GFP (Roche) antibodies at 1:1000. Anti-Cse4 antibodies were used at 1:500 (Pinsky et al. 2003). Anti-Ndc80 (OD4, 1:10,000), anti-Ndc10 (OD1, 1:5000), anti-Mif2 (OD2, 1:6000), and anti-Ctf19 (OD10, 1:15,000) antibodies were kind gifts from Arshad Desai. For all time-course experiments, equal protein loading was confirmed by anti-tubulin immunoblotting (data not shown). Silver staining was performed using a SilverQuest silver staining kit according to instructions (Invitrogen). Southern blot analyses were performed using a CEN3 probe as described (Furuyama and Biggins 2007). Ipl1 kinase assays were performed as described (Buvelot et al. 2003).
The large-scale minichromosome purification technique and associated MS preparation and data analysis are described in depth in the Supplemental Material. For small-scale purification experiments, immunoprecipitations were performed as described in the Supplemental Material, except that cell extracts were prepared with glass beads in a beater (Biospec Products, Inc.) for 35 sec, three times, with 1 min on ice in between. Copurifying proteins were eluted by boiling the beads in SDS sample buffer. Mouse monoclonal anti-Flag antibodies and anti-GFP antibodies (Roche) were conjugated to protein G dynabeads, whereas rabbit polyclonal anti-Myc antibodies (A14, Santa Cruz Biotechnologies) were conjugated to protein A dynabeads (Dynal).
Microscopy
Analysis of GFP-Tub1, Glc7-GFP, and Fin1-GFP in fixed cells as well as chromosome spreads were performed as described (Biggins et al. 1999). For spreads, 9E10 and Cse4 antibodies were used at a 1:250 dilution. At least 100 cells were analyzed for all reported experiments.
Acknowledgments
We are especially grateful to Toshio Tsukiyama and Ashwin Unnikrishnan for advice and help developing the kinetochore purification method. We are very thankful to Arshad Desai and Dave Morgan for reagents and valuable discussions, as well as Linda Wordeman and Steve Hahn for advice. We thank J. Kilmartin, D. Pellman, M. Stark, and T. Tsukiyama for strains and plasmids, and members of the Biggins laboratory and Chip Asbury, Ben Pinsky, Andy Powers, Andrew Franck, Krishna Sarangapani, Jason Stumpff, and Toshio Tsukiyama for comments on the manuscript. We also thank Min Yuan in the ISB Proteomics Facility for help with MS analyses. This work was supported by a Beckman Young Investigator grant and NIH grants (GM078079 and GM064386) to S.B., and an NIGMS grant (PM50 GM076547/Center for Systems Biology) to J.A.R. S.B. is a Scholar of the Leukemia and Lymphoma Society.
Footnotes
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.1865909.
Supplemental material is available at http://www.genesdev.org.
References
- Akiyoshi B, Nelson CR, Ranish JA, Biggins S. Analysis of Ipl1-mediated phosphorylation of the Ndc80 kinetochore protein in Saccharomyces cerevisiae. Genetics. 2009 doi: 10.1534/genetics.109.109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes & Dev. 2001;15:3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Severin FF, Bhalla N, Sassoon I, Hyman AA, Murray AW. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes & Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloecher A, Tatchell K. Dynamic localization of protein phosphatase type 1 in the mitotic cell cycle of Saccharomyces cerevisiae. J Cell Biol. 2000;149:125–140. doi: 10.1083/jcb.149.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvelot S, Tatsutani SY, Vermaak D, Biggins S. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J Cell Biol. 2003;160:329–339. doi: 10.1083/jcb.200209018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980;287:504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1—Targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Dejardin J, Kingston RE. Purification of proteins associated with specific genomic loci. Cell. 2009;136:175–186. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P, McAinsh AD, Sorger PK. Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes & Dev. 2003;17:2902–2921. doi: 10.1101/gad.1144403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P, Montani F, Visintin R. Protein phosphatases take the mitotic stage. Curr Opin Cell Biol. 2009 doi: 10.1016/j.ceb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Morrison DK. Unlocking the code of 14–3–3. J Cell Sci. 2004;117:1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- Ducker CE, Simpson RT. The organized chromatin domain of the repressed yeast a cell-specific gene STE6 contains two molecules of the corepressor Tup1p per nucleosome. EMBO J. 2000;19:400–409. doi: 10.1093/emboj/19.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff MP, Johnson DF, Moorhead G, Cohen PT, Cohen P, Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CS, Stukenberg PT. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco L, Wang W, Chan CS. Type 1 protein phosphatase acts in opposition to Ipl1 protein kinase in regulating yeast chromosome segregation. Mol Cell Biol. 1994;14:4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama S, Biggins S. Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc Natl Acad Sci. 2007;104:14706–14711. doi: 10.1073/pnas.0706985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh PY, Kilmartin JV. NDC10: A gene involved in chromosome segregation in Saccharomyces cerevisiae. J Cell Biol. 1993;121:503–512. doi: 10.1083/jcb.121.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DK, Eng J, Zhou H, Aebersold R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nat Biotechnol. 2001;19:946–951. doi: 10.1038/nbt1001-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx A, Beullens M, Ceulemans H, Abt TD, Van Eynde A, Nicolaescu E, Lesage B, Bollen M. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem Biol. 2009;16:365–371. doi: 10.1016/j.chembiol.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Hisamoto N, Sugimoto K, Matsumoto K. The Glc7 type 1 protein phosphatase of Saccharomyces cerevisiae is required for cell cycle progression in G2/M. Mol Cell Biol. 1994;14:3158–3165. doi: 10.1128/mcb.14.5.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Graham Cooks R. The Orbitrap: A new mass spectrometer. J Mass Spectrom. 2005;40:430–443. doi: 10.1002/jms.856. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Nasmyth K. A topological interaction between cohesin rings and a circular minichromosome. Cell. 2005;122:849–860. doi: 10.1016/j.cell.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Joglekar AP, Bouck DC, Molk JN, Bloom KS, Salmon ED. Molecular architecture of a kinetochore–microtubule attachment site. Nat Cell Biol. 2006;8:581–585. doi: 10.1038/ncb1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi K, Yamauchi Y, Taoka M, Iwago M, Fujita T, Ito T, Song SY, Sakai A, Isobe T, Ichimura T. Proteomic analysis of in vivo 14–3–3 interactions in the yeast Saccharomyces cerevisiae. Biochemistry. 2007;46:7781–7792. doi: 10.1021/bi700501t. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Funabiki H. Correcting aberrant kinetochore microtubule attachments: An Aurora B-centric view. Curr Opin Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Kotwaliwale CV, Biggins S. Post-translational modifications that regulate kinetochore activity. In: De Wulf P, Earnshaw WC, editors. The kinetochore: From molecular discoveries to cancer therapy. Springer; New York: 2008. pp. 293–343. [Google Scholar]
- Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell. 2009;16:105–117. doi: 10.1016/j.devcel.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Riggs AD. The general affinity of lac repressor for E. coli DNA: Implications for gene regulation in procaryotes and eucaryotes. Cell. 1975;4:107–111. doi: 10.1016/0092-8674(75)90116-6. [DOI] [PubMed] [Google Scholar]
- Mann M. Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- Mayordomo I, Sanz P. The Saccharomyces cerevisiae 14–3–3 protein Bmh2 is required for regulation of the phosphorylation status of Fin1, a novel intermediate filament protein. Biochem J. 2002;365:51–56. doi: 10.1042/BJ20020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW. Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol. 1985;101:755–765. doi: 10.1083/jcb.101.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Stemmann O, Rank S, Lechner J. A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes & Dev. 1999;13:1140–1155. doi: 10.1101/gad.13.9.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky BA, Tatsutani SY, Collins KA, Biggins S. An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev Cell. 2003;5:735–745. doi: 10.1016/s1534-5807(03)00322-8. [DOI] [PubMed] [Google Scholar]
- Pinsky BA, Kotwaliwale CV, Tatsutani SY, Breed CA, Biggins S. Glc7/Protein phosphatase-1 regulatory subunits can oppose the Ipl1/Aurora protein kinase by redistributing Glc7. Mol Cell Biol. 2006a;26:2648–2680. doi: 10.1128/MCB.26.7.2648-2660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006b;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- Pinsky BA, Nelson CR, Biggins S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr Biol. 2009;19:1182–1187. doi: 10.1016/j.cub.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranish JA, Yi EC, Leslie DM, Purvine SO, Goodlett DR, Eng J, Aebersold R. The study of macromolecular complexes by quantitative proteomics. Nat Genet. 2003;33:349–355. doi: 10.1038/ng1101. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Heiter P. Methods in yeast genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1990. [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. The chromosomal passenger complex: One for all and all for one. Cell. 2007;131:230–231. doi: 10.1016/j.cell.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Sandall S, Severin F, McLeod IX, Yates JR, III, Oegema K, Hyman A, Desai A. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 2006;127:1179–1191. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Musacchio A. The life and miracles of kinetochores. EMBO J. 2009;28:2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon I, Severin FF, Andrews PD, Taba MR, Kaplan KB, Ashford AJ, Stark MJ, Sorger PK, Hyman AA. Regulation of Saccharomyces cerevisiae kinetochores by the type 1 phosphatase Glc7p. Genes & Dev. 1999;13:545–555. doi: 10.1101/gad.13.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MJ. Yeast protein serine/threonine phosphatases: Multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU, Desai A. Kinetochore-microtubule interactions: The means to the end. Curr Opin Cell Biol. 2008;20:53–63. doi: 10.1016/j.ceb.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Peters JM. Polo and Aurora kinases: Lessons derived from chemical biology. Curr Opin Cell Biol. 2008;20:77–84. doi: 10.1016/j.ceb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L, Andrews PD, Wickramasinghe S, Sleeman J, Prescott A, Lam YW, Lyon C, Swedlow JR, Lamond AI. Time-lapse imaging reveals dynamic relocalization of PP1γ throughout the mammalian cell cycle. Mol Biol Cell. 2003;14:107–117. doi: 10.1091/mbc.E02-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumper G, Carbon J. Copy number control by a yeast centromere. Gene. 1983;23:221–232. doi: 10.1016/0378-1119(83)90054-9. [DOI] [PubMed] [Google Scholar]
- van Hemert MJ, Lamers GE, Klein DC, Oosterkamp TH, Steensma HY, van Heusden GP. The Saccharomyces cerevisiae Fin1 protein forms cell cycle-specific filaments between spindle pole bodies. Proc Natl Acad Sci. 2002;99:5390–5393. doi: 10.1073/pnas.072556099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert MJ, Deelder AM, Molenaar C, Steensma HY, van Heusden GP. Self-association of the spindle pole body-related intermediate filament protein Fin1p and its phosphorylation-dependent interaction with 14–3–3 proteins in yeast. J Biol Chem. 2003;278:15049–15055. doi: 10.1074/jbc.M212495200. [DOI] [PubMed] [Google Scholar]
- van Heusden GP, Steensma HY. Yeast 14–3–3 proteins. Yeast. 2006;23:159–171. doi: 10.1002/yea.1338. [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse V, Hardwick KG. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Winey M. The S. cerevisiae SPB duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Cheeseman IM, Anderson S, Yates JR, III, Drubin DG, Barnes G. Architecture of the budding yeast kinetochore reveals a conserved molecular core. J Cell Biol. 2003;163:215–222. doi: 10.1083/jcb.200305100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Drubin DG, Barnes G. Structures and functions of yeast kinetochore complexes. Annu Rev Biochem. 2007;76:563–591. doi: 10.1146/annurev.biochem.76.052705.160607. [DOI] [PubMed] [Google Scholar]
- Woodbury EL, Morgan DO. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat Cell Biol. 2007;9:106–112. doi: 10.1038/ncb1523. [DOI] [PubMed] [Google Scholar]
- Wu JQ, Guo JY, Tang W, Yang CS, Freel CD, Chen C, Nairn AC, Kornbluth S. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]