Abstract
The miR-17∼92 cluster is frequently amplified or overexpressed in human cancers and has emerged as the prototypical oncogenic polycistron microRNA (miRNA). miR-17∼92 is a direct transcriptional target of c-Myc, and experiments in a mouse model of B-cell lymphomas have shown cooperation between these two oncogenes. However, both the molecular mechanism underlying this cooperation and the individual miRNAs that are responsible for it are unknown. By using a conditional knockout allele of miR-17∼92, we show here that sustained expression of endogenous miR-17∼92 is required to suppress apoptosis in Myc-driven B-cell lymphomas. Furthermore, we show that among the six miRNAs that are encoded by miR-17∼92, miR-19a and miR-19b are absolutely required and largely sufficient to recapitulate the oncogenic properties of the entire cluster. Finally, by combining computational target prediction, gene expression profiling, and an in vitro screening strategy, we identify a subset of miR-19 targets that mediate its prosurvival activity.
Keywords: microRNAs, Myc, miR-17∼92, cancer, mouse
The miR-17∼92 cluster encodes six distinct microRNAs (miRNAs) that are processed from a common primary transcript (Fig. 1A; for review, see Mendell 2008). A growing body of evidence points to an important role of this cluster of miRNAs in the pathogenesis of human cancers (for review, see Ventura and Jacks 2009). Overexpression of miR-17∼92 is observed in a large fraction of human cancers, including carcinomas of the breast, lung, and colon; medulloblastomas; neuroblastomas; and B-cell lymphomas (Hayashita et al. 2005; He et al. 2005; Tagawa and Seto 2005; Fontana et al. 2008; Uziel et al. 2009). In addition, a substantial fraction of diffuse large B-cell lymphomas harbors recurrent genomic amplification of the miR-17∼92 locus (Ota et al. 2004).
Figure 1.
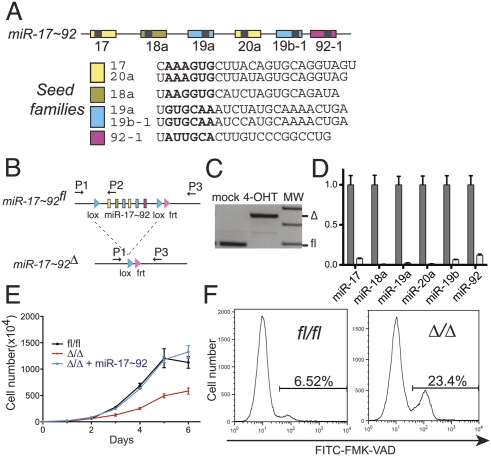
miR-17∼92 suppresses cell death in Eμ-Myc lymphomas. (A) Schematic representation of the miR-17∼92 cluster. Each miRNA is represented by a colored box and is color-coded based on the seed family to which it belongs. The sequence of each mature miRNA is also shown. (B) Schematic of the conditional miR-17∼92 knockout allele. Arrows represent the primers used to detect the floxed and the deleted (Δ) alleles. (C) PCR on genomic DNA extracted from Eμ-Myc;miR-17∼92fl/fl;Cre-ER lymphoma cells mock-treated or after 4 d of 4-OHT treatment. (D) Quantitative RT–PCR analysis of the expression of miR-17∼92 in lymphoma cells before (gray bars) and after (white bars) 4-OHT treatment. Each component of miR-17∼92 was detected independently, and the results were normalized relative to the expression observed in mock-treated cells. Each experiment was performed in quadruplicate. Error bar, standard deviation (SD). (E) Growth curves of miR-17∼92fl/fl cells (black line), miR-17∼92Δ/Δ cells (red line), and miR-17∼92Δ/Δ cells infected with a retrovirus expressing the entire miR-17∼92 cluster (blue line). Error bars, SD of three replicates. The plot is representative of three independent experiments. (F) Caspase activity in exponentially growing miR-17∼92fl/fl and miR-17∼92Δ/Δ lymphoma cells as detected by flow cytometry using FITC-conjugated VAD-FMK. The percent of VAD-FMK+ cells is shown.
The evidence for a causal link between miR-17∼92 overexpression and tumorigenesis is strengthened by the observation that transgenic expression of this cluster in mice leads to a lymphoproliferative disorder (Xiao et al. 2008), while its genetic ablation impairs normal B-cell development (Ventura et al. 2008). In addition, ectopic expression of miR-17∼92 cooperates with the c-Myc oncogene in a mouse model of B-cell lymphomas (He et al. 2005). The functional interplay between miR-17∼92 and c-Myc is further underlined by the finding that c-Myc itself is a potent and direct transcriptional activator of miR-17∼92 (O'Donnell et al. 2005), thus suggesting that miR-17∼92 may contribute to the oncogenic properties of c-Myc.
The experiments presented in this study were designed to examine the role of the endogenous miR-17∼92 allele in Myc-driven lymphomas, and to determine the relative contribution of each of the six constituent miRNAs to the overall oncogenic potential of the cluster.
Our results show that, in the context of Myc-driven B-cell lymphomas, genetic ablation of the endogenous miR-17∼92 locus leads to a dramatic reduction of tumor cell growth in vitro and suppresses tumorigenicity in vivo, two effects that are largely the consequence of increased cell death. We also demonstrate that, among the six miRNAs encoded by the miR-17∼92 cluster, the members of the miR-19 family (miR-19a and miR-19b) are essential to mediate the oncogenic activity of the entire cluster, and that they do so at least in part by modulating the expression of the tumor suppressor gene Pten (phosphatase and tensin homologous).
Results and Discussion
Generation of miR-17∼92flox/flox;Eμ-Myc mice
To investigate the role of miR-17∼92 in Myc-induced cancers, we employed the Eμ-Myc mouse model of B-cell lymphomas (Adams et al. 1985). Eμ-Myc mice express a c-Myc transgene under the control of the B-cell-specific Eμ enhancer and develop B-cell lymphomas within 4–6 mo of age (Adams et al. 1985). Eμ-Myc mice were crossed to mice carrying a conditional miR-17∼92 knockout allele (miR-17∼92fl) (Fig. 1B; Ventura et al. 2008). To temporally control the deletion of the floxed miR-17∼92 allele, these mice were further crossed to mice carrying a 4-hydroxytamoxifen (4-OHT)-inducible Cre-recombinase estrogen receptor-T2 (Cre-ERT2) knock-in allele targeted to the ubiquitously expressed ROSA26 locus (R26-Cre-ERT2 mice, hereafter referred to as Cre-ER) (Ventura et al. 2007).
As expected, Eμ-Myc; miR-17∼92fl/fl; Cre-ER mice developed B-cell lymphomas with similar latency and phenotype as the parental Eμ-Myc strain (data not shown). From these mice, we derived two independent lymphoma lines (AV4174 and AV4182) that could be propagated easily in culture and readily formed tumors when injected into immunocompromised mice. Both lymphoma lines exhibited similar behavior in vitro and in vivo. Unless otherwise specified, the experiments discussed here were performed using the AV4182 cell line.
To determine the efficiency of miR-17∼92 deletion, miR-17∼92fl/fl lymphoma cells were treated with 250 nM 4-OHT. This treatment lead to the efficient deletion of both endogenous miR-17∼92 alleles (Fig. 1C), with concomitant loss of expression of the corresponding miRNAs (Fig. 1D).
We next examined the phenotypic consequences of deleting miR-17∼92 in B-lymphoma cells. Because sustained Cre expression has been reported to negatively affect the growth of Eμ-Myc lymphoma cells (Schmidt-Supprian and Rajewsky 2007), 4-OHT was administered for 4 d, after which the lymphoma cells were allowed to recover for a minimum of 4 d before being examined. As shown in Figure 1E, deletion of miR-17∼92 dramatically reduced the proliferation of Eμ-Myc lymphoma cells. Importantly, this phenotype was fully rescued by reintroduction of the entire miR-17∼92 cluster (Fig. 1E).
The different growth kinetics between the miR-17∼92fl/fl and miR-17∼92Δ/Δ lymphoma cells could be due to the latter displaying reduced proliferation, increased spontaneous cell death, or a combination of both. While cell cycle distribution and BrdU incorporation were similar between miR-17∼92fl/fl and miR-17∼92Δ/Δ cells (Supplemental Fig. 1A), the fraction of cells undergoing apoptosis, as determined by detecting caspase activation, was approximately fourfold higher in the absence of miR-17∼92 (Fig. 1F). Increased apoptosis was confirmed by measuring the DNA fragmentation using the TUNEL assay (Supplemental Fig. 1B). These results demonstrate that expression of the endogenous miR-17∼92 locus is required for the optimal survival of Myc-driven B-lymphoma cells. In addition, they are consistent with the finding by He et al. (2005) that ectopic expression of miR-17∼92 cooperates with c-Myc by reducing spontaneous apoptosis.
miR-19a and miR-19b mediate the oncogenic properties of miR-17∼92
This observation provides a rationale and an opportunity to genetically dissect the functions of this cluster and to identify its relevant target mRNAs. The six miRNAs encoded by miR-17∼92 can be grouped into four “seed families,” based on sequence identity at positions 2–7 (Fig. 1A): the miR-17 family (miR-17 and miR-20a), the miR-18 family (miR-18a), the miR-19 family (miR-19a and miR-19b-1), and the miR-92 family (miR-92-1). miRNAs belonging to the same seed family are predicted to target highly overlapping sets of mRNAs, and thus are expected to exert similar biological functions (Bartel 2009). To examine the role of each seed family in the context of Eμ-Myc lymphomas, we generated a series of miR-17∼92 mutant alleles, each lacking expression of the miRNA(s) belonging to one of the four seed families (Supplemental Fig. 2A). The wild-type and the mutant alleles of miR-17∼92 were cloned into MSCV-Puro-IRES-GFP (PIG), a retroviral vector encoding the green fluorescent protein (GFP) and the Puromycin resistance gene, and the resulting constructs were transduced into miR-17∼92Δ/Δ lymphoma cells. First, we verified that these constructs correctly expressed the desired miRNAs (Supplemental Fig. 2B). This was an essential control because deletion of even a single miRNA from the miR-17∼92 cluster could, in principle, negatively affect the processing and expression of the remaining ones, thus compromising our experimental approach.
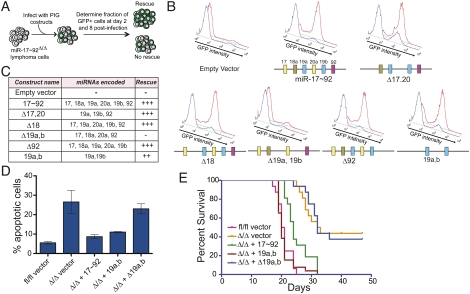
To determine the ability of each construct to rescue the phenotype caused by miR-17∼92 deletion, we titrated the viral preparations to achieve an infection efficiency of 5%–30%, as judged by GFP expression. We reasoned that, if reintroduction of miR-17∼92 or one of its derivatives is sufficient to suppress the increased cell death observed in miR-17∼92Δ/Δ cells, it will provide the infected cells with a growth advantage that will in turn be reflected by an increase in the fraction of GFP-positive cells over time (see schematic in Fig. 2A). As predicted, reintroduction of the full-length miR-17∼92 cluster resulted in a rapid increase of GFP+ cells that quickly outcompeted the uninfected cells (Fig. 2B). Interestingly, among the four mutant constructs, only the one lacking the miR-19 seed family (Δ19a, 19b) failed to provide a growth advantage (Fig. 2B,C) and to suppress the increased apoptosis caused by deletion of miR-17∼92 (Fig. 2D), suggesting that this seed family is necessary for the oncogenic properties of the cluster. This was further confirmed by showing that reintroduction of miR-19a and miR-19b alone was largely sufficient to rescue the growth defect caused by deletion of the entire miR-17∼92 cluster and to suppress apoptosis (Fig. 2B,D, miR-19a,b construct).
Figure 2.
miR-19a and miR-19b mediate the prosurvival and oncogenic functions of miR-17∼92 in Eμ-Myc B-cell lymphomas. (A) Schematic of the experimental design. (B) Histogram overlays of miR-17∼92Δ/Δ cells transduced with PIG retroviruses expressing the indicated miR-17∼92 derivatives. The cells were assayed by flow cytometry to detect GFP expression at day 2 (blue plot) and day 8 (red plot) post-infection. A schematic of the miR-17∼92 derivative used is shown below each overlay. (C) Table summarizing the results of the experiments shown in B. (D) Caspase activity in miR-17∼92fl/fl and miR-17∼92Δ/Δ cells transduced with the indicated PIG constructs. Error bar, 1 SD deviation. (E) Survival analysis of mice injected with miR-17∼92fl/fl and miR-17∼92Δ/Δ lymphoma cells transduced with the indicated PIG constructs. N = 16 mice for each construct, over three independent experiments.
Deletion of miR-19 affects tumorigenicity in vivo
To determine whether the miR-19 seed family is required for the tumorigenicity of Eμ-Myc-driven B-cell lymphomas in vivo, we injected a cohort of nude mice with miR-17∼92fl/fl and miR-17∼92Δ/Δ lymphoma cells. While miR-17∼92fl/fl cells invariably lead to the formation of lymphomas that lead to death within 2–3 wk, the miR-17∼92Δ/Δ cells produced lymphomas with a significantly (P < 0.0001) longer latency (Fig. 2E). Tumorigenicity was fully restored by ectopic expression of the full-length miR-17∼92 cluster (P < 0.0001), but not by expression of the miR-17∼92 mutant lacking miR-19a and miR-19b (P = 0.9816) (Fig. 2E). Re-expression of miR-19a and miR-19b also largely rescued tumorigenicity, although it did so somewhat less efficiently than the full-length miR-17∼92 (P = 0.0002 for the comparison between Δ/Δ and Δ/Δ + miR-19a,b; P = 0.0013 for the comparison between fl/fl and Δ/Δ + miR-19a,b).
Identification of miR-19 targets in B-cell lymphomas
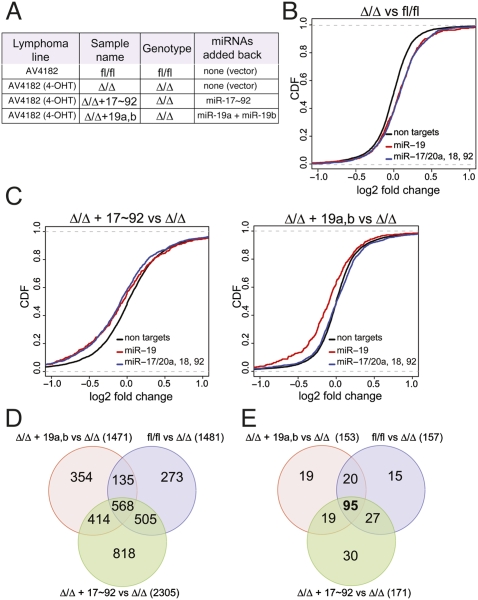
Having demonstrated a critical role of miR-19a and miR-19b in Myc-driven B-cell lymphomas, we next sought to identify their functionally relevant target mRNAs. miRNA target prediction algorithms (TargetScan, Miranda, and Pictar) (John et al. 2004; Krek et al. 2005; Grimson et al. 2007; Betel et al. 2008) identify several hundreds of potential targets of miR-19; however, only a fraction of these mRNAs will likely be functionally relevant in any particular cellular context. To identify the genes whose expression is effectively modulated by miR-17∼92 in B-cell lymphomas, we compared the gene expression profile of the AV4182 cell line before and after deletion of miR-17∼92 (fl/fl vs. Δ/Δ). We also included miR-17∼92Δ/Δ lymphoma cells that had been transduced with either PIG-miR-17∼92wt or PIG-miR-19a/19b (Fig. 3A). In choosing this approach, we were supported by a number of recent reports showing that mRNA destabilization contributes to miRNA-mediated regulation of gene expression (Bagga et al. 2005; Lim et al. 2005; Baek et al. 2008; Selbach et al. 2008), which can be detected by conventional mRNA expression arrays. As predicted, deletion of miR-17∼92 led to the preferential up-regulation of genes whose 3′ untranslated regions (UTRs) contain seed matches for the miRNAs encoded by this cluster (P-value < 2.22e-16, KS test) (Fig. 3B; Supplemental Fig. 3). Accordingly, ectopic expression of miR-17∼92 in miR-17∼92Δ/Δ cells led to the preferential down-regulation of miR-17∼92 targets (P-value < 2.22e-16, KS test) (Fig. 3C; Supplemental Fig. 3). Finally, reintroduction of a mutant version of the miR-17∼92 cluster expressing only miR-19a and miR-19b selectively affected mRNAs carrying binding sites for these two miRNAs (P-value = 6.35e-15), but not genes with binding sites for the other members of the miR-17∼92 cluster.
Figure 3.
Gene expression profiling identifies miR-19 targets in Eμ-Myc lymphoma cells. (A) Description of the various lymphoma cells used. (B) Differences in mRNA levels between miR-17∼92fl/fl and miR-17∼92Δ/Δ lymphoma cells transduced with the empty PIG vector were monitored with microarrays. Cumulative distribution function (CDF) plots are shown for mRNAs that do not contain miR-17∼92 seed matches in their 3′UTRs (black line), mRNAs containing one or more seed matches for miR-19 in their 3′UTR (red line), and mRNAs containing one or more seed matches for either miR-17, miR-20a, miR-18a, or miR-92 (blue line). In the absence of endogenous miR-17∼92 expression, a statistically significant up-regulation (P-value < 2.22e-16, KS test) is observed for the predicted miR-17∼92 targets relative to the background gene population. (C) CDF plots of the changes in mRNA expression levels between miR-17∼92Δ/Δ + PIG-miR-17∼92 and miR-17∼92Δ/Δ lymphoma cells (left panel) and between miR-17∼92Δ/Δ + PIG-miR-19a,b and miR-17∼92Δ/Δ lymphoma cells (right panel). (D) Venn diagram summarizing the overlap in gene expression changes observed between the various transduction experiments. (E) As in D, but the analysis was restricted to mRNAs whose 3′UTR contains at least one predicted binding site for miR-19.
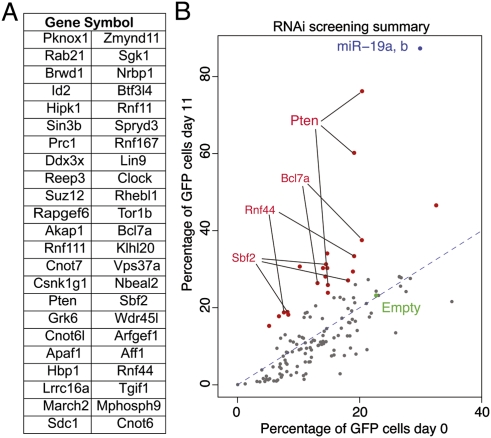
By comparing the four gene expression profiles, we identified a total of 568 genes whose expression was up-regulated (log2 expression change >0.20) by deletion of the endogenous miR-17∼29 locus (fl/fl vs. Δ/Δ comparison) and down-regulated by the reintroduction of the full-length miR-17∼92 cluster (miR-17∼92 vs. Δ/Δ) and of miR-19a and miR-19b only (miR-19a/b vs. Δ/Δ; log2 expression change <−0.20) (Fig. 3D). Ninety-five of them contained in their 3′UTR one or more conserved binding sites for miR-19, according to TargetScan 5.1 (Fig. 3E; Supplemental Table 1), and were analyzed further. Guided by our findings that miR-19 suppresses apoptosis in Eμ-Myc lymphoma cells, we inspected the list of 95 genes and selected a subset of 46 of them for functional validation (Fig. 4A; Supplemental Table 2). We reasoned that, if miR-19 promotes survival by repressing the expression of one or more of these genes, this effect should be at least partially phenocopied by RNAi-mediated knockdown of the relevant targets. To test this hypothesis, for each of the 46 genes selected for validation we designed three shRNAs. The shRNAs were cloned into the MLP vector, a retroviral vector also expressing GFP, and each construct was individually transduced into miR-17∼92Δ/Δ lymphoma cells. Analogous to the experiments described in Figure 2A, the viral preparations were titrated in order to achieve a transduction efficiency of 5%–30%, and the fraction of GFP+ cells was measured 2 d after infection (day 0) and again 11 d later. The results of this experiment are summarized in Figure 4B. For the majority of shRNAs, the fraction of GFP+-positive cells did not change over time or, for a small number of them, was lower at day 11 compared with day 0, indicating that expression of the shRNA did not provide any growth advantage to the infected cells or was detrimental, respectively (Fig. 4B; Supplemental Table 2). However, for a subset of shRNAs, we observed a significant increase in the fraction of GFP+ cells at day 11 compared with day 0 (Fig. 4B; Supplemental Fig. 5). Among them, two out of three shRNAs targeting the Pten tumor suppressor gene had the largest effect, largely phenocopying ectopic expression of miR-19a/b. A third shRNA directed against the Pten mRNA had a more modest, but still significant, effect. In addition, a number of the other shRNAs provided a significant growth advantage, although the effect was more modest than that observed with Pten shRNAs (Fig. 4B; Supplemental Table 2). In particular, all three shRNAs directed against Sbf2 and two out of three directed against Bcl7a and Rnf44 scored positive in this screening, suggesting that these three genes may contribute to the prosurvival functions of miR-19.
Figure 4.
An in vitro RNAi screen to identify functionally relevant miR-19 targets. (A) List of the genes assayed in the in vitro shRNA screen. (B) Scatter plot summarizing the result of the screen. Each dot represents an individual shRNA construct. The X-axis is the percentage of GFP cells at the beginning of the experiment (2 d after infection) and the Y-axis is the percentage of GFP cells 11 d later. The green dot identifies the empty vector control. shRNAs that scored positive in the screen are highlighted in red and labeled. Dots corresponding to genes for which at least two out of three shRNAs provided significant growth advantage are labeled. PIG-miR-19a/b was included in the screen as positive control (blue dot).
PTEN is one of the most frequently mutated tumor suppressor genes in human cancers, and monoallelic mutations at this locus are observed in >50% of sporadic tumors (for review, see Salmena et al. 2008). In mice, homozygous deletion of Pten leads to early embryonic lethality, while heterozygotes show greatly increased incidence of a variety of tumors, including T-cell lymphomas, as well as tumors of endometrium, liver, prostate, gastrointestinal tract, and thyroid (Di Cristofano et al. 1998; Suzuki et al. 1998; Podsypanina et al. 1999). Functionally, PTEN is a phospholipid phosphatase that acts as a negative regulator of cell survival and protein synthesis via inhibition of the AKT/mammalian target of rapamycin (mTOR) pathway. Studies in mouse models and mutational analysis of human tumors indicate that Pten is an haploinsufficient tumor suppressor gene (Salmena et al. 2008), suggesting that even modest modulation of its levels by miRNAs may have functional consequences.
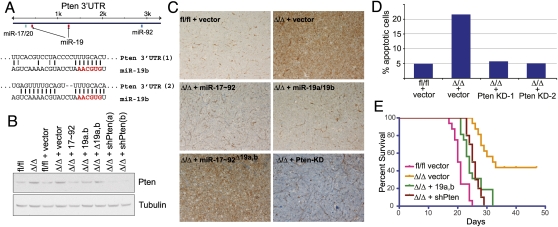
The Pten 3′UTR contains two conserved binding sites for miR-19a and miR-19b (Fig. 5A), and it has been shown previously to be a direct miR-19 target (O'Donnell et al. 2005; Xiao et al. 2008). We first confirmed that miR-19 directly acts on the Pten 3′UTR via binding to the two predicted sites by performing reporter assays in human cancer cells and in miR-17∼92+/+ and miR-17∼92Δ/Δ mouse embryo fibroblasts (Supplemental Fig. 4). We next determined the extent to which Pten expression is modulated by miR-19 in Myc-driven B-cell lymphomas. Western blotting and immunohistochemistry analysis of miR-17∼92fl/fl and miR-17∼92Δ/Δ lymphomas confirmed a consistent up-regulation of Pten expression in the latter (Fig. 5B,C). Reintroduction of full-length miR-17∼92 or of miR-19a and miR-19b alone, but not of miR-17∼92Δ19a,b, in miR-17∼92Δ/Δ lymphomas was sufficient to restore Pten expression to levels similar to those observed in miR-17∼92fl/fl cells (Fig. 5B,C). In addition, analogous to reintroduction of miR-19, RNAi-mediated knockdown of Pten in miR-17∼92Δ/Δ lymphoma cells was sufficient to reduce spontaneous apoptosis to the levels observed in miR-17∼92fl/fl cells (Fig. 5D). We next examined whether Pten knockdown was also sufficient to restore the tumorigenicity of miR-17∼92Δ/Δ lymphoma cells. Mice injected with miR-17∼92Δ/Δ; sh-Pten developed aggressive lymphomas that led to death with a latency similar to that observed in mice injected with miR-17∼92Δ/Δ cells ectopically expressing miR-19a/b (Fig. 5D). Also in this case, survival was slightly longer compared with mice injected with miR-17∼92fl/fl cells (P = 0.0002), indicating the existence of additional functionally relevant targets.
Figure 5.
Pten is a functionally relevant miR-19 target in B-cell lymphomas. (A) Schematic representation of the Pten 3′UTR with the location of the predicted binding sites for members of the miR-17∼92 cluster and sequence alignments between miR-19b and its two predicted binding sites. (B) Pten Western blot on lysates of B-lymphoma cells transduced with the indicated PIG constructs. (Lanes 8,9) For comparison, lysates from miR-17∼92Δ/Δ cell expressing the two Pten shRNAs that scored positive in the in vitro screen were also assayed. (C) Pten immunohistochemistry on lymphoma sections obtained from mice injected with miR-17∼92fl/fl and miR-17∼92Δ/Δ B-lymphoma cells transduced with the indicated miR-17∼92 derivatives (objective, 20×). Brown staining indicates Pten signal. (D) Knockdown of Pten suppresses apoptosis in miR-17∼92Δ/Δ cells. Apoptosis was measured by detecting caspase activity in miR-17∼92fl/fl and miR-17∼92Δ/Δ cells transduced with the indicated retroviruses. (E) Kaplan-Meier survival curve of mice injected with miR-17∼92Δ/Δ lymphoma cells transduced with retroviruses expressing shRNAs against Pten. N = 10 (five mice for shPTEN-1 and five mice for shPTEN-2). For comparison, the survival curves of mice injected with miR-17∼92fl/fl, miR-17∼92Δ/Δ, and miR-17∼92Δ/Δ + miR-19a,b from Figure 2C are included.
In summary, the results presented here provide a mechanistic explanation for the functional cooperation between c-Myc and miR-17∼92, identify the miR-19 seed family as the primary oncogenic determinant of this cluster, and pave the way for the development of novel anti-cancer strategies based on the pharmacological inhibition of miR-19 function.
Material and methods
Mouse husbandry
Animal studies and procedures were approved by the Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee. Mice were maintained in a mixed 129SvJae and C57/B6 background. The Rosa26-Cre-ERT2 and miR-17∼92fl/fl mice have been described previously (Ventura et al. 2007, 2008). The Eμ-Myc mice were generated and described by Adams et al. (1985).
For the in vivo tumorigenicity studies, 4- to 8-wk-old athymic (nu/nu) mice were injected intravenously with 105 lymphoma cells and monitored daily. Mice were euthanized when moribund. Kaplan-Meier curves were plotted using PRISM software, and the log-rank Mantel-Cox test was used to determine statistical significance.
Antibodies and immunohistochemistry
Antibodies and experimental conditions for Western blotting and immunohistochemistry are described in the Supplemental Material.
Cell culture and retroviral transduction
The Eμ-Myc;miR-17∼92fl/fl;Cre-ERT2 lymphoma lines were cultured on a feeder of irradiated NIH-3T3 cells in a medium composed of 50% DMEM and 50% IMDM, supplemented with 10% fetal bovine serum.
To induce deletion of the miR-17∼92 cluster, cells were incubated for 4 d with 250 nM 4-OHT. During our initial set of experiments with 4-OHT-treated lymphoma cells, we noticed that, upon prolonged passages, the few cells that had escaped full miR-17∼92 deletion (miR-17∼92fl/fl and miR-17∼92fl/Δ) invariably outcompeted the miR-17∼92Δ/Δ cells, eventually becoming the majority within a couple of weeks. To avoid this limitation and allow the execution of long-term in vivo experiments, 4 d after 4-OHT treatment, subclones were isolated by plating 10 cells per well into a 96-well plate using a MoFlo fluorescence-activated cell sorter. After expansion, clones composed solely of fully recombined cells were isolated and used for further manipulation.
Retroviruses were generated in Phoenix packaging cells. When required, transduced cells were selected by adding puromycin (2 μg/mL) to the culture medium for 4 d.
Plasmids and shRNA library
A 1.2-kb fragment encompassing the entire miR-17∼92 cluster was PCR-amplified from mouse genomic DNA and cloned into the MSCV-PIG retroviral vector (a gift from Mike Hemann, Massachusetts Institute of Technology). Deletion mutants were by site-directed PCR and verified by sequencing. Primers and sequences are available on request.
The shRNA library was cloned in the MLP retroviral vector (a gift from Michael Hemann, Massachusetts Institute of Technology). For each gene, three shRNA directed against the coding sequence were designed using the RNAi Central resource created by the laboratory of Greg Hannon (http://katahdin.cshl.org:9331/siRNA/RNAi.cgi?type=shRNA). Each construct was sequence-verified.
Apoptosis assays
Apoptosis was measured using the Caspase Detection Kit (Red-VAD-FMK or FITC-VAD-FMK, Calbiochem) and confirmed using the TUNEL assay (In Situ Cell Death Detection Kit, TMR red, Roche) following the manufacturer's instructions.
Gene expression analysis
Total RNA extracted from three technical replicates was hybridized to the Affymetrix 430 A2.0 gene chip, following the manufacturer's instruction. Gene expression was normalized using the GCRMA Bioconductor package, and log expression values were computed using the limma package. For genes with multiple probes, the probe with lowest adjusted P-value was selected. Genes with a log expression change of <−0.2 in all three comparisons and with an adjusted P-value < 0.05 in at least one comparison were considered for subsequent overlap analysis.
miRNA target predictions
miRNA targets were predicted using miRanda (http://www.microrna.org) and TargetScan (http://www.targetscan.org). For the cumulative distribution function (CDF) plots, target sites were restricted to perfect seed complementarity between positions 2 and 7 of the miRNA. Empirical cumulative distributions were computed using R ecdf function for the set of predicted gene of the transduced miRNAs and for the genes with no target sites (background). P-values were computed using the KS two-sample test.
Acknowledgments
We thank Jane Qiu for technical assistance. A.V. is grateful to Tyler Jacks and Robert Benezra for their generosity and support. This work was funded by the Sidney Kimmel Cancer Research Foundation and the Geoffrey Beene Cancer Research Foundation.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1872909.
Supplemental material is available at http://www.genesdev.org.
References
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau N, Garrett-Engele P, Grimson A, Schelter J, Castle J, Bartel DP, Linsley PS, Johnson J. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Seto M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia. 2005;19:2013–2016. doi: 10.1038/sj.leu.2403942. [DOI] [PubMed] [Google Scholar]
- Uziel T, Karginov FV, Xie S, Parker JS, Wang YD, Gajjar A, He L, Ellison D, Gilbertson RJ, Hannon G, et al. The miR-17∼92 cluster collaborates with the Sonic Hedgehog pathway in medulloblastoma. Proc Natl Acad Sci. 2009;106:2812–2817. doi: 10.1073/pnas.0809579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Jacks T. MicroRNAs and cancer: Short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]