Abstract
Reaching to grasp an object of interest requires complex sensorimotor coordination involving eye, head, hand and trunk. While numerous studies have demonstrated deficits in each of these systems individually, little is known about how children with cerebral palsy (CP) coordinate multiple motor systems for functional tasks. Here we used kinematics, remote eye tracking and a trunk support device to examine the functional coupling of the eye, head and hand and the extent to which it was constrained by trunk postural control in 10 children with CP (6–16 years). Eye movements in children with CP were similar to typically developing (TD) peers, while hand movements were significantly slower. Postural support influenced initiation of hand movements in the youngest children (TD & CP) and execution of hand movements in children with CP differentially depending on diagnosis. Across all diagnostic categories, the most robust distinction between TD children and children with CP was in their ability to isolate eye, head and hand movements. Results of this study suggest that deficits in motor coordination for accurate reaching in children with CP may reflect coupled eye, head, and hand movements. We have previously suggested that coupled activation of effectors may be the default output for the CNS during early development.
Keywords: Eye hand coordination, development, cerebral palsy, posture
Reaching deficits in children with CP contribute to disability and interfere with development of independent life skills (van der Heide et al. 2005). Such movements require a complex sensorimotor transformation that takes into account the visual attributes of the object (i.e., location, size, shape, etc), the initial direction of gaze, and the initial position of the head, hand and trunk (Jeannerod 1990).
Accurate reaching behavior typically develops in children between 5 and 13 months of age (von Hofsten & Ronnqvist 1988; von Hofsten 2007). This skill is then refined over a period of more than 8 years such that most children achieve adult like timing and accuracy by 9 years of age (von Hofsten 2007; Favilla 2006). By contrast, children with cerebral palsy (CP) often have difficulty making accurate reaching movements to point at or grasp objects of interest (van Thiel et al 2000; Mackey et al. 2006; Ronnqvist & Rosblad 2007; Chang et al. 2005).
Reaching deficits observed in children with CP could be due to primary deficits in oculomotor (Katayama & Tamas 1987; Fedrizzi et al. 1998; Kozeis et al. 2007; Jacobson & Dutton 2000; Salati et al. 2002), manual motor control (van Thiel et al 2000; Mackey et al. 2006; Ronnqvist & Rosblad 2007; Chang et al. 2005), or anticipatory postural responses (van der Heide et al. 2004; 2005). However, it is the coordination between the eye, trunk, and hand that ultimately drives performance. The ability to isolate the eye, head and hand develops in typical children between 5 and 9 years of age (Saavedra et al. 2007). Although several studies have evaluated the timing and coordination within (Chang et al. 2005; Utley & Sugden 1998; Mutsaarts et al. 2006; Ricken et al. 2005; Chen & Yang 2007), or between (Hung et al. 2004; Chang et al. 2005, Steenbergen et al 1996) hands during reaching in children with hemiplegia, and the interactions between trunk postural control and reaching in children with hemiplegia (Mackey et al. 2006; Ricken et al. 2005; Steenbergen & Meulenbroek 2006, van Roon et al. 2005), diplegia (van der Heide et al. 2004, Hadders-Algra et al. 2007), and quadriplegia (van Roon et al. 2004; 2005a & b), to our knowledge no research has been completed that examines the coordination between the eye, trunk, and hand during reaching in children with CP.
Tight temporal coordination or coupling between the hands is present in subjects with hemiplegia as well as control subjects during bimanual activities (Steenbergen et al 1996). This has been interpreted to indicate that the limbs are constrained to act as a single functionally specific unit, with the central nervous system simultaneously activating homologous muscle groups (Steenbergen et al 1996, Kelso et al 1983). Theoretically, this simplifies the control problem by reducing the number of degrees of freedom which must be actively controlled (Bernstein 1967). Similar simplification of control mechanisms for the eye and hand during reaching have been shown during reaching in adults based on the simultaneous onset of EMG activity underlying the movement of each effector (Biguer et al., 1982). More recently, Verrel and coworkers (2008) examined temporal coupling between eye and hand movements during an object prehension and transport task and concluded that individuals with hemiplegic CP adapted their eye movements when using the affected hand compared to the unaffected hand. They suggested that longer time with eyes on the target was evidence of increased visual attentiveness.
Increased visual attentiveness has been reported during reaching in people with CP (Steenbergen & van der Kamp 2004) and this has been predicted to be a compensatory mechanism to deal with sensory-motor deficits (Verrel et al 08, van Roon et al 2005b). However, the benefit of visual attentiveness has not been explicitly demonstrated. Removing vision of the hand during reaching in individuals with mild quadriplegic CP was not detrimental to accuracy (van Roon et al 2005a). Moreover, movement time was longer during high accuracy tasks with vision present than when it was removed (van Roon et al 2005a). In healthy adults learning complex eye hand tasks such as juggling, it has been shown that vision adapts to changing sensory-motor needs by creating different temporal coupling patterns between the eyes and hands as the skill improves (Huys et al 2004a; 2004b). Therefore, it is reasonable to assume that individuals with CP may adapt the various effectors differently depending on which systems are concurrently active. Thus, we predicted that the children with CP would show delays in motor performance when required to use multiple systems in a coordinated manner rather than in isolation.
Individuals with CP have been shown to use increased trunk movement during reaching tasks compared to controls. Whether this is the result of compensatory action due to limitations of range-of-motion or strength in the arm (Van Thiel & Steenbergen 2001), adaptive activity to assist with task demands for accuracy (van Roon et al 2004), an inertial by-product related to the speed of arm movement (van Roon et al 2005b), or the result of variability or delay in anticipatory postural reactions (van der Heide et al 2004) is the subject of ongoing debate. However, van Roon et al (2005b) demonstrated that when TD subjects were asked to reach within easy range at movement speeds resembling those of the subjects with CP differences in trunk displacement between the groups disappeared.
Surprisingly few studies have explicitly examined the effects of altering trunk postural demands by comparing tasks with and without external fixation of the trunk. In healthy adults it was shown that providing trunk fixation decreased postural demands below the level of support and increased the speed of reaching (Cordo and Nashner 1982). Van der Heide et al. (2004) noted improved reaching performance in children with severe diplegic CP who received additional postural support compared to children who did not receive support. Van Roon et al. (2005b) compared reaching dynamics in subjects with hemiplegia, quadriplegia and controls with and without external trunk fixation and found differential effects depending on group. It is unknown whether the improvements with postural fixation in these studies were the result of reduced degrees of freedom, reduced demands for concurrent postural control or the effect of improved vertical alignment of the trunk. Vertical trunk alignment has been shown to reduce trunk extensor tone (Nwaobi et al 1983, Nwaobi 1986) and improve reach in children with CP (Nwaobi et al 1987). It has also been shown to improve upper extremity function in healthy adults (Gillen et al 2007).
The current study used 3 postural conditions, sitting on a bench (postural control needed, child’s natural alignment), sitting with a hip/pelvic strapping system (postural control needed, trunk aligned vertically) and a third condition using hip strapping plus support at the upper thorax (reduction of postural demands, trunk aligned vertically). We predicted that all children would have improved performance when the trunk was aligned vertically and that children with CP would gain additional benefits from reduction in postural demands in the trunk fixation condition.
Thus, the primary purpose of the current study was to examine the functional coupling of the eye, head and hand during reaching in children in the various diagnostic groups of CP (spastic, ataxic, dyskinetic) and the secondary purpose was to determine the extent to which it was constrained by concurrent demands for trunk postural control. For this purpose children aged 6–16 years with a diagnosis of CP made eye and hand movements either together or in isolation with different levels of external postural support. Results were compared with those from a previous study of TD children (Saavedra et al. 2007). Comparison groups included age matched TD peers as well as younger TD children (4–6 years). The younger group was included to evaluate potential developmental deficits in children with CP.
Methods
Subjects
Ten children with CP referred by clinicians and teachers in response to flyers distributed to regional schools and pediatric clinics participated in the study. Eligibility criteria included: a diagnosis of CP, ability to sit independently on a bench, and ability to follow simple directions like look or don’t look, touch or don’t touch. Additional inclusion criteria for head free eye tracking included the absence of visual field deficits and the ability to sustain visual fixation for at least 3 seconds. All children with CP selected for the study were assessed using a complete neurologic and musculoskeletal exam by a board certified neuro-developmental pediatrician (Table 1 demographics). All children who completed the study had parental report of normal or corrected to normal visual acuity. The study was conducted in accord with the declaration of Helsinki guidelines and had ethical approval from the Human Subjects Committee at University of Oregon. Written consent was obtained from participants and/or their legal guardians prior to beginning the data collection.
Table 1.
Demographic data: (F) female; (M) male; (GMFCS) Gross Motor Functional Classification System; (MACS) Manual Ability Classification System
| Subject | Age (yrs) | Sex | Diagnosis | GMFCS | MACS |
|---|---|---|---|---|---|
| 1 | 16 | F | ataxia | III | II |
| 2 | 14 | F | spastic diplegia | II | III |
| 3 | 7 | F | spastic hemiplegia | I | II |
| 4 | 11 | F | spastic diplegia | III | II |
| 5 | 11 | F | mixed spastic hemiplegia & ataxia | I | III |
| 6 | 11 | M | spastic diplegia | I | I |
| 7 | 7 | M | spastic hemiplegia | I | III |
| 8 | 9 | M | spastic hemiplegia | I | III |
| 9 | 12 | F | spastic diplegia | II | I |
| 10 | 6 | F | ataxia | I | II |
Experimental Tasks
Kinematics and point of gaze eye tracking
The experimental protocol has been described previously (Saavedra et al. 2007). Head free remote eye tracking offered many advantages; postural support effects could be accurately evaluated, the dynamics of head movements were not altered by weight to the head, and the children were not encumbered by restrictions to head movement. Overall, this allowed more natural less restrained movements as well as excellent compliance from the children. An ASL remote eye tracker [Applied Science Laboratories, Bedford, MA, USA] with two magnetic sensors (Minibird system) was used to collect simultaneous eye, head, and hand kinematic data at 60 Hz while the children performed blocks of 4 eye-hand tasks. The magnetic tracking system had a recording volume of 1 m3 with a spatial accuracy of 1.8mm. One sensor was attached to the center of the forehead just above the eyes using a headband, while the other sensor was taped firmly to the fingernail of the index finger on the dominant hand. Corneal and pupil reflections were recorded by the remote eye tracker camera and transformed into horizontal and vertical point of gaze coordinates. The children were positioned so that they could easily reach the targets on the computer screen. Several practice reaches were completed to determine the child’s comfortable reach and return positions. A foam pad was adjusted on the tabletop to mark the starting position of the hand prior to each trial. Calibration of eye point of gaze and finger touch was completed by having the subject look at and touch each of the targets.
Eye-hand coordination tasks
Children sat on a bench facing a computer screen with hands resting on a table. In all tasks the child began with fixation on a central cue and subsequently looked and/or pointed to a target appearing in the periphery (3.5, 7.5 and 10 cm to the dominant side (affected side in children with hemiplegia) or 3.5 cm to the non-dominant side). During the “Control” task children maintained central fixation when a peripheral target appeared. They quickly looked at the target during the “Eye Only” task, concurrently looked at and touched the target during the “Eye Hand” task and quickly touched the target while maintaining central fixation during the “Hand Only” task. These tasks allowed us to evaluate the child’s ability to isolate individual motor systems and to assess the influence of eye movements on hand movements or hand movements on eye movements.
Postural support conditions
The 4 tasks were completed under 3 different levels of external trunk support. In the “No Support” condition, participants sat on the bench with no additional support, this allowed evaluation of the child’s most natural, well practiced responses. Reduced postural demand was evaluated by providing an external brace at the level of the xiphoid process in the “Upper Torso Support” condition. In order to differentiate between reduced degrees of freedom and changes in reach due to upright alignment, we added a 3rd condition, “Hip Support”, in which the pelvis was stabilized in vertical alignment with straps thus providing improved alignment without reducing the postural demands of reaching. The support conditions allowed us to examine the influence of postural control and alignment on oculomotor and manual motor performance.
Total number of trials
A total of 198 trials were collected for each subject, 12 Control trials and 18 trials each of Eye Only, Eye-Hand, and Hand Only were collected for each level of support. Of these trials, 66% were positioned 7.5 cm to the dominant side (affected side for children with hemiplegia) and these were submitted for further analysis, the remaining trials served to decrease anticipation and prevent preplanned responses. The 3 support levels and 4 task sets were completed in a counterbalanced order across subjects.
Data Reduction
Head, hand and eye movements were digitized for off-line analysis using custom Matlab programs. Manual selection of primary and secondary saccade start and end times were determined from plots of horizontal eye position for each trial. Saccades were processed in a repetitive manner through all conditions (4 tasks, 3 levels of support) for each subject. The condition being analyzed was not immediately apparent to the coder. This manual procedure was carried out because of the frequent artifacts induced in the data by blinks and head motion in the children with CP. Only trials in which the primary saccade covered at least 90% of the distance to the target were considered for further analysis. Head azimuth minimum and maximum and hand start and stop times were marked automatically using a custom computer algorithm, verified by inspection and adjusted if necessary (Saavedra et al. 2007). Onset of hand movement was determined by a change in resultant velocity of five standard deviations above baseline. The end point was determined as the data point just before the finger marker reached the x-coordinate matching that subject’s target calibration trial. Trials were discarded if the hand was not appropriately located at the start position, if the hand was not stationary at the beginning of the trial or if obvious artifacts were present during the reach portion of the data. Hand data were filtered with a zero lag 4th order low-pass Butterworth filter (cut off frequency 12 Hz) prior to calculating peak velocity, and the number of acceleration changes.
After elimination of trials due to blinks, breaks from fixation, artifacts due to large head movements, or other discontinuities a total of 588 trials from 10 children with CP were compared with 2,201 trials from 30 TD children. Table 2 indicates the average number of trials per task for each group of children.
Table 2.
| Group means (SD) | TD 4–6 yr N=10 |
TD 7–9 yr N=12 |
TD 10–15 yr N=8 |
CP 6–9 yr N=4 |
CP 10–16 yr N=6 |
Significance omnibus | |
|---|---|---|---|---|---|---|---|
| Age | 5yr 9mo | 8yr 5mo | 12yr 3mo | 7yr 5mo | 13yr 3mo | ||
|
(2
tasks)
(average#trials/subject) |
N=8
(36.1) |
N=12
(41.7) |
N=8
(50.4) |
N=4
(37) |
N=6
(46.7) |
Eye F(4,35)= |
|
| Eye RT (ms) |
380 ms (207) |
360 ms (170) |
340 ms (123) |
277 ms (131) |
336 ms (171) |
1.14 | p=.3543 |
| Eye MT (ms) |
97 ms (11.8) |
99 ms (13) |
97 ms (10.2) |
95 ms (8) |
96 ms (11) |
1.18 | p= .3376 |
| Eye amplitude (degrees) |
11.06 (2.08) |
9.64 (1.39) |
9.62 (1.46) |
8.76 (1.33) |
8.50 (1.62) |
18.25 | p < .0001 * |
| Eye accuracy (mm) |
−.87 (5.7) |
−1.08 (6.9) |
−1.23 (5.7) |
−.11 (5.4) |
−.39 (7.9) |
.34 | p = .8492 |
| % saccadic intrusions |
60.4 (27.4) |
31.1 (14.5) |
14.2 (14.9) |
85.5 (20.5) |
58.5 (33.6) |
19.456 | p < .0005 * |
| Head (2 tasks) | Head F(4,35)= |
||||||
| Head azimuth (degrees) |
7.0 (6.1) |
4.8 (4.6) |
3.0 (3.1) |
7.7 (6.4) |
5.0 (5.1) |
12.62 | p < .0001 * |
| View distance (cm) |
34.94 (4.68) |
39.79 (3.41) |
39.79 (3.78) |
40.99 (5.03) |
40.86 (2.86) |
17.00 |
p < .0001 * TD 4–6 sig less than all others |
|
Hand parameters
Eye-Hand task |
Hand F(4,35)= |
||||||
|
N=subjects per group
(average # trials/subject) |
N=8
(16.9) |
N=12
(20.4) |
N=8
(25.9) |
N=4
(17.25) |
N=6
(23) |
||
| Hand RT |
667 ms (250) |
492 ms (123) |
412 ms (96) |
645 ms (169) |
584 ms (159) |
12.62 | p < .0001 * |
| Hand MT |
539 ms (178) |
484 ms (134) |
441 ms (96) |
803 ms (204) |
620 ms (153) |
21.03 | p < .0001 * |
| Hand amplitude (resultant x,y,z) |
30.3 cm (4.91) |
37.5 cm (4.23) |
39.7 cm (8.94) |
41.5 cm (6.79) |
42.2 cm (7.31) |
12.18 |
p < .0001 * TD 4–6 sig less than all others |
| Hand peak velocity (m/sec) |
1.49 (.55) |
1.92 (.47) |
1.98 (.45) |
1.67 (.43) |
1.69 (.52) |
1.91 | p=.1306 |
| Hand submovements (zero acceleration crossings) |
4.54 (2.26) |
3.79 (1.75) |
3.57 (1.53) |
8.06 (2.42) |
5.40 (2.67) |
15.13 | p < .0001 * |
Eye movements were characterized by reaction time (time from target appearance to initiation of movement), movement time (time from initiation to end of movement), amplitude (degrees of horizontal displacement during eye movement), accuracy (distance between target screen coordinates and final eye position), and percentage of saccadic intrusions (breaks from fixation during Control and Hand Only tasks when the eyes were required to remain stable). Eye amplitude (degrees) was calculated for each trial based on view distance at the beginning of the trial and horizontal displacement of gaze. Head movements were characterized by the amplitude of head azimuth (maximum-minimum) and view distance (distance between the head and the screen). Hand movements were characterized by reaction time, movement time, peak velocity (max velocity during hand movement time), and submovements (number of zero acceleration crossings occurring during movement time). Submovements are a valid and sensitive index for quantifying motor performance during reaching in children with CP (Chang et al. 2005).
Statistical Analysis
PROC MIXED (SAS/STAT software Version 9.1, SAS Institute Inc. Cary, NC) was used to evaluate the effect of task x support x group. CP groups (11–16 year olds, 6–9 year olds) were compared with TD peers (10–15 year olds, 7–9 year olds) and younger TD (4–6 year olds) using preplanned contrasts after Bonferroni adjustment. In addition, a priori polynomial contrasts were used to determine the effects of support and paired t-tests were used for post-hoc comparison of differences within groups.
Results
Table 2 shows group means for each dependent variable as well as statistical results for group effects.
Eye Interactions
There were no group differences for saccadic reaction time, movement time or accuracy (Table 2). Eye movements were not influenced by level of postural support but were influenced by concurrent hand use. Across all groups, saccadic reaction time was faster during Eye Hand trials than during Eye Only trials (F(1,35)=5.6 p=.0236). The children with CP had smaller eye movement angles than their peers (CP(11–16) vs TD(10–15) t(35)=−3.86, p=.0005; CP(6–9) vs TD(7–9) t(35)=−2.75, p=.009) despite the fact that they had similar view distances and similar eye accuracy (Table 2).
While the ability to make rapid accurate saccades does not appear to be affected by CP, we found that the ability to inhibit saccades is deficient in these children (Fig 1). Children with CP had significantly higher percentages of saccadic intrusions (i.e., breaks from fixation) than their peers (CP(11–16) vs TD(10–15) p=.002; CP(6–9) vs TD(7–9) p<.0001) but these were not significantly different than those of the TD 4–6 year olds, suggesting this improves with age in children with CP. Saccadic intrusions were not influenced by level of support but were influenced by task (F(1,35)=34.54, p<.0005) being substantially higher in the eye-hand task than the control task, especially for the younger TD children and children with cerebral palsy.
Figure 1.
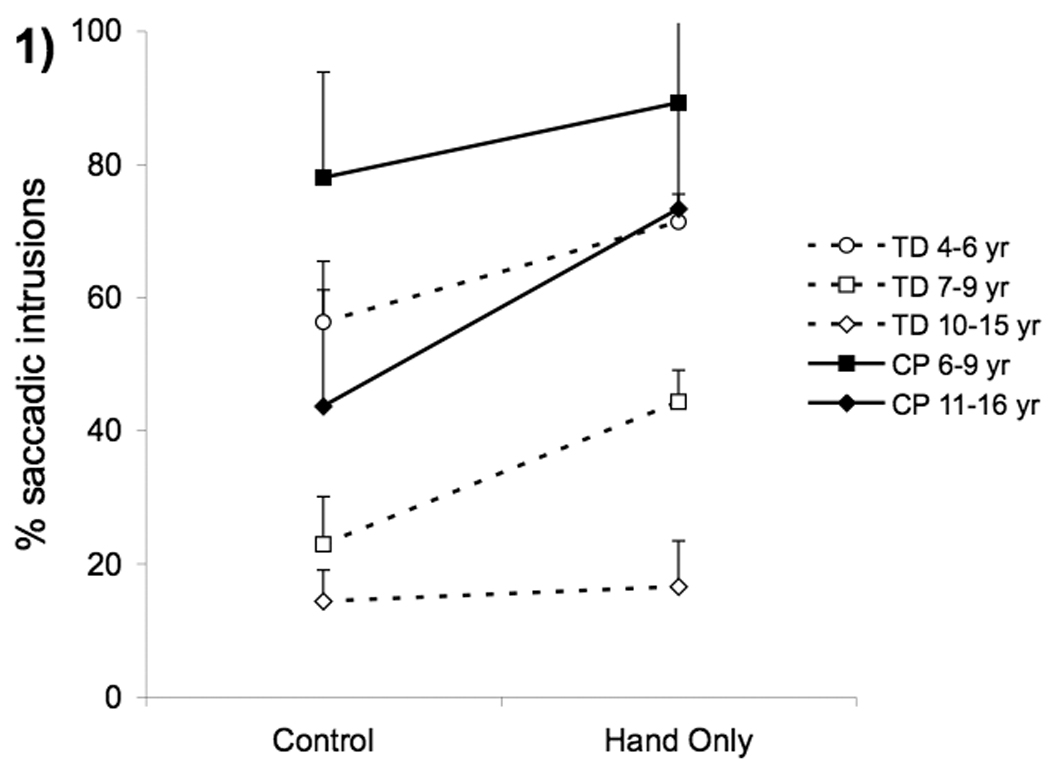
Group means for frequency of saccadic intrusions in typically developing children (unfilled symbols and dashed lines) and children with cerebral palsy (filled symbols and solid lines) during control vs. hand only tasks. Means were collapsed across postural support conditions. Error bars, intersubject SE.
Head movements
Head azimuth could not be statistically compared for Hand Only and Control tasks due to the high number of trials with saccadic intrusions; however, comparisons were possible for the Eye Only vs. Eye-Hand tasks. For these two tasks head azimuth varied significantly by group (Table 2) and task (F(1,35)=97.45, p<.0001) but was not influenced by level of support. All children (CP and TD) used more head movement during eye-hand trials than eye only trials. Across both tasks children with CP used more head azimuth than their peers (CP(6–9) vs TD(7–9) t(35)=3.33 p=.0021 , CP(11–16) vs TD(10–15) t(35)=2.21 p=.0335). Thus, when combined with the results on eye movement excursions, it is apparent that children with CP achieved equal visual accuracy as their TD peers, but did so by combining larger head movements and smaller eye excursions.
Hand interactions
The high level of saccadic intrusions prevented comparison of hand movements made with vs. without eye movements. In the groups with CP, only 3 children (1 child with hemiplegia (7 years) and two children with diplegia (12 & 14 years) were able to inhibit saccades during reaching. We therefore evaluated the effect of postural support during the Eye Hand trials.
Initiation of hand movements
The effect of support on hand reaction time depended on group (group*support interaction, F(8,69)=2.26, p=.0327). The interaction was driven by the youngest groups. The CP(6–9) group, like TD 4–6 year olds (Saavedra et al. 2007), had progressively faster hand reaction time with each additional level of support (linear effect: t(69)=3.02 , p=.0354). Older children (TD & CP) did not have significant differences in hand reaction time across different levels of support. Children with CP were significantly slower than their TD peers (CP(11–16) vs TD(10–15) t(35)=3.73, p=.0054; CP(6–9) vs TD(7–9) t(35)=3.02, p=.0379) but not significantly different than younger TD children.
Execution of hand movements
Hand amplitude and movement time were not influenced by level of postural support. Children with CP were not significantly different than TD peers in hand amplitude; however, they did have significantly longer hand movement times (CP(11–16) vs TD(10–15) t(35)=4.85, p=.0002; CP(6–9) vs TD(7–9) t(35)=7.58, p<.0001) and more submovements. The CP(11–16) group differed significantly from all TD groups except the 4–6 year olds (t(69)=1.99, p=.4354)(Fig. 2). The CP(6–9) group had more submovements than all other groups (p<.0001 all comparisons). Across all groups, children made fewer submovements during trials with hip support (support main effect: F(2,70)=5.20, p=.0078; support quadratic effect: t(69)=−3.20, p=.0021).
Figure 2.
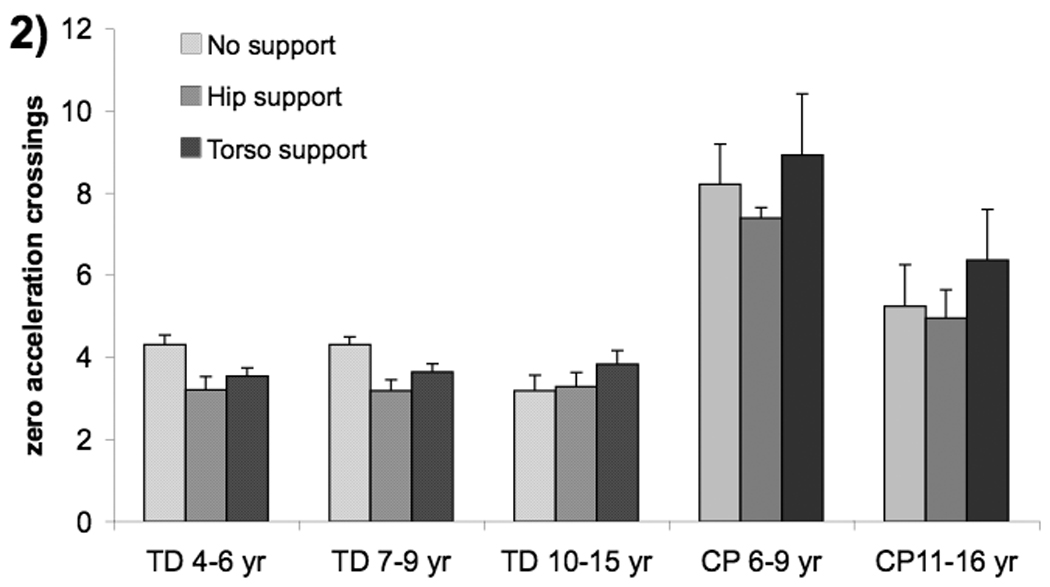
Group means for hand submovements (number of zero acceleration crossings) with three levels of postural support; no support (light shade), hip support (medium shade) and upper torso support (dark shade).Error bars, intersubject SE.
The effect of external support on hand peak velocity depended on group (group * support interaction F(8,69)=2.64, p=.0138) (Fig 3a). The interaction was driven by the children with CP. The CP(11–16) group had increased peak velocity as additional support was added (linear effect of support: t(69)=−2.23, p=.0293), whereas the CP(6–9) group had increased peak velocity with hip support (quadratic effect of support: t(69)=2.76, p=.0073).
Figure 3.
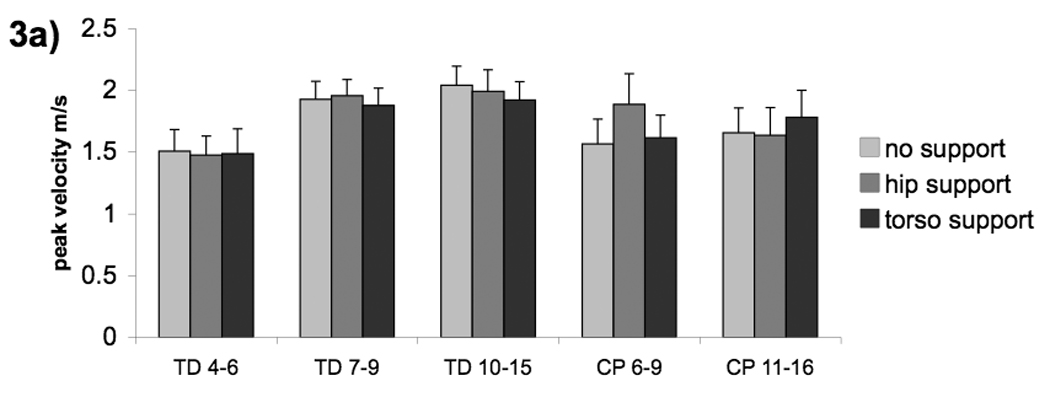
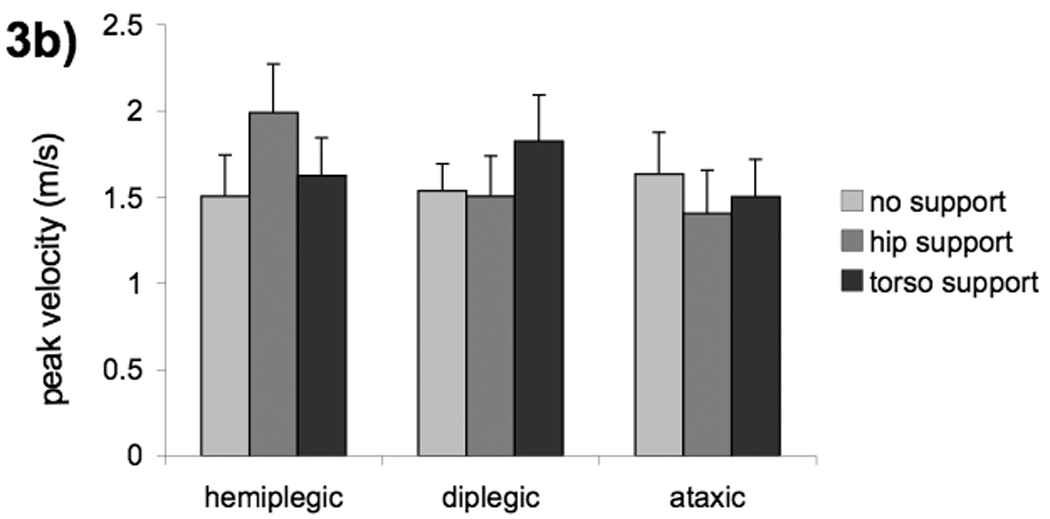
Group means (a) and diagnosis means (b) for hand peak velocity with three levels of postural support; no support (light shade), hip support (medium shade) and upper torso support (dark shade). Error bars, intersubject SE.
The interactions in the data which showed differences in CP groups may be confounded by the fact that diagnostic categories were not evenly distributed between the age groups for CP. All four children with diplegia were in the older group while three out of four children with hemiplegia were in the younger group. Therefore the statistical analysis for peak velocity and hand reaction time was repeated using diagnostic category instead of group. There was no effect of support on hand RT when the subjects were grouped by diagnosis, indicating that this effect is due to age not diagnosis. However, we found a significant support*diagnosis interaction for hand peak velocity (F(6,12)=5.40, p=.0064) (Fig 3b). This was driven by different reactions to support for the children with diplegia vs. hemiplegia (support: hemiplegia vs diplegia F(2,12)=7.46, p=.0078). Children with hemiplegia had increased peak velocity with hip support (hemiplegia: quadratic effect of support: t(12)=3.28, p=.0397) while children with diplegia had increased peak velocity with torso support (diplegia: linear effect of support: t(12)=−3.68, p=.0189). Examination of effect size indicates that there was a larger effect of hip support when comparing peak velocity by diagnostic groups (Cohen’s d=1.0) than when comparing by age groups (d=.5).
Hemiplegia, impaired vs. unimpaired hand
We were able to collect data on both hands for 3 of the children with hemiplegia (1 with mixed ataxia/hemiplegia). Children with hemiplegia had significantly longer movement time (F(1,2)=28.24, p=.0336) and more submovements (F(1,2)=50.84, p=.0191) with their impaired hand across all levels of support. There were no hand by support interactions and there was no main effect of support.
Discussion
In this study of eye hand coordination in children with CP, we examined the functional coupling of the eye and hand across development and determined the extent to which it was constrained by trunk postural control. For this purpose children with CP aged 6–16 years were compared to a control group of TD children aged 4–15 years. The children made eye and hand movements either together or in isolation with different levels of external postural support. Across all diagnostic categories, children with CP had slower, less efficient hand movements and were significantly delayed in their ability to isolate their eye, head and hand movements. Postural support did not affect eye or head movements, whereas it did influence initiation and execution of hand movements.
Our primary motivation in this study was to investigate the functional coupling between various effectors involved in reaching and determine whether children with CP differed in their coordination of these systems when compared with TD children. We predicted that children with CP would have more difficulty when task demands involved coordination of multiple systems than when they used the systems in isolation.
Eye Hand Interactions
Our findings that eye movements in children with CP were as fast and accurate as their peers are in agreement with previous studies (Katayama & Tamas 1987; Lee et al. 1995, Verrel et al 2008). Likewise our results for hand movements agree with previous studies indicating that children with CP have slower hand reaction times (van Thiel et al 2000; Utley & Sugden 1998), as well as slower movement times and increased submovements (van der Heide et al. 2005; Chang et al. 2005; Mutsaarts et al. 2006), when compared to their peers or when comparing affected and unaffected arms in children with hemiplegia (Mackey et al. 2006; Ronnqvist & Rosblad 2007; Hung et al. 2004, Steenbergen et al 1996).
We had predicted that visual responses would reflect compensation for manual motor deficits and would therefore be delayed during the Eye Hand task compared to Eye Only task. However, we found that eye movements were initiated faster when paired with hand movements across all children (TD & CP) even though hand movements were initiated and executed more slowly by children with CP. Moreover, eye movements remained constant across levels of support and group even though hand movements were affected by these variables. This suggests that children with CP do not adjust their eye movements to accommodate their altered hand movements, and have difficulty inhibiting unwanted saccades. Contrary to the present results, Verrel and colleagues (2008) concluded that children with CP adjusted eye movements when using the affected hand compared to the unaffected hand. Task characteristics between the two studies may account for the differences in interpretation of the findings. In the Verrel study, subjects had to reach for and grasp an object, pick it up and transport it to the target location. Delayed initiation of saccades at the beginning of the transport phase was interpreted as increased visual attentiveness during reach with the affected hand. However, this result may have reflected use of vision to accommodate for the effect of sensory motor deficits on prehension rather than coordination of the eyes with the reaching task. Their result showing a greater lag between eye end time and hand end time on the more affected side compared to the less affected side is in agreement with our findings that eye movement time is not altered by differences in hand movement time. In addition, their report of no deficit in anticipatory gaze control in the participants with CP is in agreement with our findings.
The high rate of saccadic intrusions in the children with CP prevented comparison of hand movements made with compared to without eye movements. Therefore, we were not able to evaluate hand movements in isolation. The children with CP had difficulties isolating the eye from the hand that were similar to those in the TD 4–6 year olds. We suggest that this indicates that children with CP are delayed in their ability to inhibit the natural tendency to prepare and execute simultaneous eye and hand movements.
Eye Head Interactions
An advantage of this study is that it allowed head free eye-tracking which was easier for the young TD children and the children with CP to tolerate. This method also allowed us to examine the interaction between the eyes and head. It is important to note that the target eccentricity used in this study required gaze shifts of 11° or less for all subjects. When gaze is shifted more than 15°, eye movements are typically supplemented by head movements (Stoffregen et al 2006).
We found that children with CP used more head movement and less eye movement to accurately direct gaze towards the target and that this occurred regardless of hand movements or postural demands. This is not similar to TD 4–6 year olds who had both larger head movements and larger eye excursions. Increased head movement in children with CP has been noted in previous postural studies (Dan et al. 2000) and in studies examining eye movements (Jacobson & Dutton 2000; Good et al. 2001). There is a possibility that saccade amplitude in children with CP was adapted to smaller levels to compensate for excessive head movement. Alternatively, head movements may have increased to accommodate oculomotor deficits.
Cortical visual impairment (CVI) has been reported in children with CNS injury (Jacobson & Dutton 2000) and might account for increased head movement seen in our study. We believe this is unlikely. The primary deficits in CVI are decreased visual acuity and impaired fixation (Salati et al. 2002; Good et al. 2001). Examination of children with CVI indicates that they exhibit slow, inefficient and highly variable visual performance (Good et al. 2001). The children in this study had fast, accurate saccades with reaction times similar to TD peers; in addition the techniques used required that children fixate when instructed and had parental reports of normal or corrected to normal visual acuity.
Combined with the difficulty isolating eye movement from hand movements, increased head movements in CP may be related to a lack of ability to isolate eye movements from head movements. While the most stringent method of evaluating eye movements is to hold the head rigidly in place and observe the eyes, it may have limitations. Use of the current technique, allows consideration of the possibility that the child may be constrained to move the eyes and head together and this has significant implications for researchers. If eye and head movements are indeed coupled, eye motility may be misjudged in young TD children or children with CP in whom fixed head examinations are used.
Increased saccadic intrusions paired with increased head movement during gaze shifts may contribute to postural instability in children with CP. In healthy adults visual fixation and gaze shifts of less than 15° result in reduction of postural sway (Stoffregen et al. 1999, Stoffregen et al 2006). We are not aware of any studies evaluating the influence of eye gaze or head motion on postural stability in children with CP.
Overall, our data suggest that the eye, head and hand may be constrained to act together in children with CP. A similar pattern of eye hand coordination was seen in TD children who were 4–6 years of age. The hypothesis that children with CP are constrained in their ability to activate all necessary effectors independently contributes to understanding some of the modifications of behavior in children with CP noted in previous reaching studies.
A key strategy noted in reaching studies has been that children with CP alter the overall movement time during a reach (van Thiel et al 2000; van Roon et al 2004, 2005a). Van Roon et al (2005a) measured pen force during a line drawing task that altered level of difficulty by increasing accuracy demands and blocking vision. They noted that while TD subjects increased proprioceptive input by increasing pen force during more difficult tasks, the subjects with CP did not adapt pen force but instead used a strategy of slowing their movement time during more difficult tasks. If independent control of effectors is not available, altering the proprioceptive input by increasing pen force may not be of benefit to the child with CP. Likewise vision of the arm during movement may not be of benefit (van Roon 2005a).
The only adaptation available to children with CP may be the alteration of the preplanned ballistic movement. This would restrict them to the completion of one movement followed by adjustments for the next movement during sequenced activities and might explain the “step by step” planning reported in several studies (Mutsaarts et al 2005, Steenbergen & van der Kamp 2004).
Finally, if ballistic, all or none movements are being performed by children with CP, sensory feedback may not be beneficial. In TD children Hay (1978, 1979) demonstrated that children do not use vision during reaching to make online corrections until they reach the age of 7–8 years. This coincides with the time during TD development in which the ability to isolate various effectors matures (Saavedra et al 2007). Future research should both explicitly examine the ability of children with CP to make online corrections and determine the benefit of various sensory resources to those corrections.
Effects of Postural Support
Our secondary goal in this study was to examine the effect of concurrent postural demands on reaching performance and to determine whether children with CP differed from TD children in their response to postural manipulations during reach. We predicted that all children would have improved reach when the trunk was in neutral vertical alignment during the hip support condition. We predicted that the children with CP would have additional benefits from reduction of postural demands during the torso support condition.
As predicted, all children benefited from improved trunk alignment during the hip support condition. Across all groups (TD & CP) children made less submovements when we aligned their spines vertically with hip support but this improvement did not remain when additional thoracic support was provided (Fig 2). Restricting the degrees of freedom of the trunk with external support affected the children with CP similarly to TD peers in regards to smoothness of reaching trajectory.
There was a risk that performance in children with CP could deteriorate in the torso support condition due to interference with compensatory movements of the trunk used to extend reaching distance (Mackey et al. 2006; Ricken et al. 2005; Steenbergen & Meulenbroek 2006; van Roon et al. 2004). However, we adjusted reaching distance to prevent the need for compensatory trunk motion. The fact that hand amplitude was not influenced by the level of support in any of the groups reinforces the success of this attempt.
The youngest children (CP & TD) initiated hand movements progressively faster with each additional level of support, while reaction times in the older children (TD7-9, TD10-15 and CP 10–16) were not affected by the level of support. Evaluation of hand RT by diagnosis showed no effect of support. This evidence indicates that the benefit of postural support on planning of movements is related to age and is not specific to children with CP.
In contrast, the interaction between hand peak velocity and support was stronger when examined by diagnosis than when examined by age (Fig 3a, b). This finding suggests that children with diplegic CP and those with hemiplegic CP may have different levels of trunk postural control. Children with hemiplegia had marked improvement from alteration of trunk alignment while those with diplegia benefited most from reduction of postural demands with torso support. Hadders-Algra and coworkers (2007) also found improvements for hemiplegia but not diplegia when the trunk was aligned more vertically. Van Roon and colleagues (2005b) demonstrated improvements in subjects with hemiplegic CP but not quadriplegic CP with trunk fixation. It is unknown whether these improvements were the result of reduced postural demands or improved alignment. However it is clear that spinal control differed between those with hemiplegic CP and those with quadriplegic CP. While our results are confounded by small sample size and asymmetrical distribution of diagnostic categories within age groups, they suggest that further studies examining influences of spinal control on reaching in children with spastic cerebral palsy are warranted.
Conclusions
We found that for most variables measured, children with CP had lower performance than their TD peers. Children with CP, like TD 4–6 year olds, have rapid accurate saccadic responses but initiate and complete hand movements more slowly than their peers. They use more concurrent head movement and have significantly greater difficulty isolating eye, head and hand movements. We have previously suggested that coupled eye, head and hand movements may be the default output of the CNS early in development and the ability to isolate the effectors may be necessary in order to gain feedback control of motor actions (Saavedra et al. 2007). Results of this study suggest that a primary deficit across all diagnostic groups in CP may be the inability to isolate the various effectors.
While the benefits of postural support on reaction time and submovements were similar between children with CP and TD children. The effects of external postural support on peak velocity indicate that spinal control may differ depending on diagnostic category of CP. Specifically, it appears that children with hemiplegia are influenced primarily by trunk alignment while those with diplegic CP are affected more by demands for trunk postural control.
Acknowledgements
The authors wish to thank Cooper Boydston for providing computer support, Robin High for providing statistical consultation and Robert Nickel, MD, for neurologic evaluation of subjects.
Abbreviations
- CP
cerebral palsy
- TD
typically developing
References
- Bernstein NA. The co-ordination and regulation of movement. In: Whiting, editor. Human Motor Actions Bernstein Reassessed. The Netherlands: Elsevier Science Publishers BV, Amsterdam; 1984. [Google Scholar]
- Biguer B, Jeannerod m, Prablanc C. The coordination of eye, head and arm movements during reaching at a single visual target. Exp Brain Res. 1982;46(2):301–304. doi: 10.1007/BF00237188. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Wu TI, Wu WL, Su FC. Kinematical measure for spastic reaching in children with cerebral palsy. Clinical Biomech. 2005;20:381–388. doi: 10.1016/j.clinbiomech.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Chen YP, Yang TF. Effect of task goals on the reaching patterns of children with cerebral palsy. J Mot Behav. 2007;39:317–324. doi: 10.3200/JMBR.39.4.317-325. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–382. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Dan B, Bouillot E, Bengoetxea A, Noel P, Kahn A, Cheron G. Head stability during whole body movements in spastic diplegia. Brain and Dev. 2000;22:99–101. doi: 10.1016/s0387-7604(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Favilla M. Reaching movements in children: accuracy and reaction time development. Exp Brain Res. 2006;169:122–125. doi: 10.1007/s00221-005-0291-8. [DOI] [PubMed] [Google Scholar]
- Fedrizzi E, Bova S, Farinotti M, Inverno M, Savoiardo S. Eye-movement disorders and visual-perceptual impairment in diplegic children born preterm: a clinical evaluation. Dev Med Child Neurol. 1998;40:682–688. doi: 10.1111/j.1469-8749.1998.tb12328.x. [DOI] [PubMed] [Google Scholar]
- Gillen G, Boiangiu C, Neuman M, Reinstein R, Schaap Y. Trunk posture affects upper extremity function of adults. Percept Mot Skills. 2007;104:371–380. doi: 10.2466/pms.104.2.371-380. [DOI] [PubMed] [Google Scholar]
- Good WV, Jan JE, Burden SK, Skoczenski A, Candy R. Recent advances in cortical visual impairment. Dev Med Child Neurol. 2001;43:56–60. doi: 10.1017/s0012162201000093. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M, van der Heide JC, Fock JM, Stremmelaar E, van Eykern LA, Otten B. Effect of seat surface inclination on postural control during reaching in preterm children with cerebral palsy. Phys Ther. 2007;87:861–871. doi: 10.2522/ptj.20060330. [DOI] [PubMed] [Google Scholar]
- Hay L. Accuracy of children on an open-loop pointing task. Percept Mot Skills. 1978;47:1079–1082. doi: 10.2466/pms.1978.47.3f.1079. [DOI] [PubMed] [Google Scholar]
- Hay L. Spatial-temporal analysis of movements in children: motor programs versus feedback in the development of reaching. J Mot Behav. 1979;11:189–200. doi: 10.1080/00222895.1979.10735187. [DOI] [PubMed] [Google Scholar]
- Hung YC, Charles J, Gordon AM. Bimanual coordination during a goal-directed task in children with hemiplegic cerebral palsy. Dev Med Child Neuro. 2004;46:746–753. doi: 10.1017/s0012162204001288. [DOI] [PubMed] [Google Scholar]
- Huys R, Daffertshofer A, Beek P. Multiple time scales and multiform dynamics in learning to juggle. Motor Control. 2004;7:188–212. doi: 10.1123/mcj.8.2.188. [DOI] [PubMed] [Google Scholar]
- Huys R, Daffertshofer A, Beek P. Multiple time scales and subsystem embedding in learning to juggle. Hum Mov Sci. 2004:315–336. doi: 10.1016/j.humov.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Survey of Ophalmology. 2000;45:1–13. doi: 10.1016/s0039-6257(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. In The Neural and Behavioural Organization of Goal-Directed Movements. New York: Oxford University Press; 1990. The coordination of eye, head, and arm movements during reaching for a visual target; pp. 41–50. [Google Scholar]
- Katayama M, Tamas LB. Saccadic Eye-Movements of Children with Cerebral-Palsy. Dev Med Child Neurol. 1987;29:36–39. doi: 10.1111/j.1469-8749.1987.tb02105.x. [DOI] [PubMed] [Google Scholar]
- Kozeis N, Anogeianaki A, Mitova DT, Angianakis G, Mitov T, Klisarova A. Visual function and visual perception in cerebral palsied children. Ophthalmic Physiol Opt. 2007;27:44–53. doi: 10.1111/j.1475-1313.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- Lee SK, LeGare M, Zhang HP. Conjugate eye movements: comparison of cerebral palsied and normal adults. Percept Mot Skills. 1995;81:575–591. doi: 10.1177/003151259508100244. [DOI] [PubMed] [Google Scholar]
- Mackey AH, Walt SE, Stott NS. Deficits in upper-limb task performance in children with hemiplegic cerebral palsy as defined by 3-dimensional kinematics. Arch Phys Med Rehabil. 2006;87:207–215. doi: 10.1016/j.apmr.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Mutsaarts M, Steenbergen B, Bekkering H. Anticipatory planning of movement sequences in hemiparetic cerebral palsy. Motor Control. 2005;9:439–458. doi: 10.1123/mcj.9.4.439. [DOI] [PubMed] [Google Scholar]
- Mutsaarts M, Steenbergen B, Bekkering H. Anticipatory planning deficits and task context effects in hemiparetic cerebral palsy. Exp Brain Res. 2006;172:151–162. doi: 10.1007/s00221-005-0327-0. [DOI] [PubMed] [Google Scholar]
- Nwaobi OM, Brubaker CE, Cusick B, Sussman MD. Electromyographic investigation of extensor activity in cerebral-palsied children in different seating positions. Dev Med Child Neurol. 1983;25:175–183. doi: 10.1111/j.1469-8749.1983.tb13741.x. [DOI] [PubMed] [Google Scholar]
- Nwaobi OM. Effects of body orientation in space on tonic muscle activity of patients with cerebral palsy. Dev Med Child Neurol. 1986;28:41–44. doi: 10.1111/j.1469-8749.1986.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Nwaobi OM. Seating orientations and upper extremity function in children with cerebral palsy. Physical Therapy. 1986;67:1209–1212. doi: 10.1093/ptj/67.8.1209. [DOI] [PubMed] [Google Scholar]
- Ricken AXC, Bennett SJ, Savelsbergh GJP. Coordination of reaching in children with spastic hemiparetic cerebral palsy under different task demands. Motor Control. 2005;9:357–371. doi: 10.1123/mcj.9.4.357. [DOI] [PubMed] [Google Scholar]
- Ronnqvist L, Rosblad B. Kinematic analysis of unimanual reaching and grasping movements in children with hemiplegic cerebral palsy. Clinical Biomech. 2007;22:165–175. doi: 10.1016/j.clinbiomech.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Saavedra S, Woollacott M, van Donkelaar P. Effects of postural support on eye hand interactions across development. Exp Brain Res. 2007;180:557–567. doi: 10.1007/s00221-007-0874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salati R, Borgatti R, Giammari G, Jacobson L. Oculomotor dysfunction in cerebral visual impairment following perinatal hypoxia. Dev Med Child Neurol. 2002;44:542–550. doi: 10.1017/s0012162201002535. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Hulstign W, de Vries A, Berger M. Bimanual movement coordination in spastic hemiparesis. Exp Brain Res. 1996;110:91–98. doi: 10.1007/BF00241378. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, van der Kamp J. Control of prehension in hemiparetic cerebral palsy: similarities and differences between the ipsi-and contra-lesional sides of the body. Dev Med Child Neurol. 2004;46:325–332. doi: 10.1017/s0012162204000532. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Meulenbroek RGJ. Deviations in upper-limb function of the less-affected side in congenital hemiparesis. Neuropsychologia. 2006;44:2296–2307. doi: 10.1016/j.neuropsychologia.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Stoffregen TA, Smart LJ, Bardy BG, Pagulayan RJ. Postural stabilization of looking. J Exp Psychol Hum Percept Perform. 1999;25:1641–1658. [Google Scholar]
- Stoffregen TA, Bardy BG, Bonnet CT, Pagulayan RJ. Postural stabilization of visually guided eye movements. Ecol Psych. 2006;18(3):191–222. [Google Scholar]
- Utley A, Sugden D. Interlimb coupling in children with hemiplegic cerebral palsy during reaching and grasping at speed. Dev Med Child Neurol. 1998;40:396–404. [PubMed] [Google Scholar]
- van der Heide JC, Otten B, Stremmelaar E, Hadders-Algra M. Kinematic characteristics of reaching movements in preterm children with cerebral palsy. Pediatr Res. 2005;57:883–889. doi: 10.1203/01.PDR.0000157771.20683.14. [DOI] [PubMed] [Google Scholar]
- van der Heide JC, Otten B, Stremmelaar E, van Eykern LA, Hadders-Algra M. Postural control during reaching in preterm children with cerebral palsy. Dev Med Child Neurol. 2004;46:253–266. doi: 10.1017/s0012162204000416. [DOI] [PubMed] [Google Scholar]
- van Roon D, Steenbergen B, Meulenbroek RGJ. Trunk recruitment during spoon use in tetraparetic cerebral palsy. Exp Brain Res. 2004;155:186–195. doi: 10.1007/s00221-003-1716-x. [DOI] [PubMed] [Google Scholar]
- van Roon D, Steenbergen B, Meulenbroek RGJ. Movement-accuracy control in tetraparetic cerebral palsy: effects of removing visual information of the moving limb. Motor Control. 2005;9:372–394. doi: 10.1123/mcj.9.4.372. [DOI] [PubMed] [Google Scholar]
- van Roon D, Steenbergen B, Meulenbroek RGJ. Trunk use and co-contraction in cerebral palsy as a regulatory mechanism for accuracy control. Neurospychologia. 2005:497–508. doi: 10.1016/j.neuropsychologia.2004.07.014. [DOI] [PubMed] [Google Scholar]
- van Thiel E, Meulenbroek RGJ, Hulstijn W, Steenbergen B. Kinematics of fast hemiparetic aiming movements toward stationary and moving targets. Exp Brain Res. 2000;132:230–242. doi: 10.1007/s002219900331. [DOI] [PubMed] [Google Scholar]
- van Thiel E, Steenbergen B. Shoulder and hand displacements during hitting, reaching, and grasping movements in hemiplegic cerebral palsy. Motor Control. 2001;5:166–182. doi: 10.1123/mcj.5.2.166. [DOI] [PubMed] [Google Scholar]
- von Hofsten C, Ronnqvist L. Preparation for grasping an object: a developmental study. J Exp Psychol Hum Percept Perform. 1988;14:610–621. doi: 10.1037//0096-1523.14.4.610. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. Action in development. Dev Sci. 2007;10:54–60. doi: 10.1111/j.1467-7687.2007.00564.x. [DOI] [PubMed] [Google Scholar]


