Abstract
Recently, it has been proposed that exaggerated top-down processing may generate spontaneous perceptual output, and that this may constitute a cognitive predisposition toward hallucinations. In this experiment, we investigated whether hallucination proneness would be associated with increased auditory-verbal perceptual expectations, and at which processing level this occurs. From 351 undergraduate students screened for hallucination proneness, using the Launay-Slade Hallucination Scale (LSHS), 42 subjects were recruited for participation. Two word recognition tasks were administered, in which top-down influences on perception were manipulated through sentence context (semantic task) or auditory imagery (phonological task). Results revealed that LSHS scores were correlated with the number of semantically primed errors. Subjects with higher levels of hallucination proneness were more likely to report hearing a word that fits the sentence context, when it was not actually presented. This effect remained significant after controlling for general performance on the task. In contrast, hallucination proneness was not associated with phonologically primed errors. We conclude that aberrant top-down processing, particularly in the form of strong semantic expectations, may contribute to the experience of auditory-verbal hallucinations.
Keywords: individual differences, cognitive processes, auditory perception, auditory-verbal hallucinations
Introduction
Auditory-verbal hallucinations (AVH) constitute a characteristic symptom in the schizophrenia spectrum. Up to 70% of patients report this experience at one point during the course of their illness.1 Although generally linked with psychiatric and neurological disorders, it is now acknowledged that during their lifetime, 5%–15% of the general population may have the experience of hearing voices without an objective basis.2,3 These experiences may, to an important degree, resemble the hallucinations observed in schizophrenia.4 Indeed, a growing number of studies consider psychosis to be on a continuum with normal functioning.5 A frequently applied questionnaire to measure these subclinical schizotypal characteristics is the Launay-Slade Hallucination Scale (LSHS).6 Scores on the LSHS can be employed to identify hallucination proneness in subjects from a nonpsychiatric population. The study of such subjects has the advantage that results are not confounded by the contribution of variables such as hospitalization, medication effects, illness duration, and cognitive deficits. Within the theoretical framework of the continuum hypothesis, the study of a subclinical sample can lead the way toward a putative cognitive mechanism underlying hallucination genesis in schizophrenia.
Despite decades of psychological investigation, the cognitive mechanism responsible for the transformation of self-generated mental events into speech perceptions or hallucinations remains unclear.7 Recent theoretical accounts propose the possibility that AVH are due, not to pathologically enhanced mental imagery but to an increased impact of such top-down influences on perception.8–10 Perception is not a passive process, but a reconstructive effort.11 In bottom-up processing, information flows from the senses upward into the perceptual system in the brain. Top-down processing occurs concurrently and has the capacity to reshape this incoming information. In top-down processing, internal models of the (acoustic) environment, ie prior knowledge of the properties and dependencies of the objects in this environment, are employed to interpret sensory information and generate expectations. In the case of hallucinations, there may be a distorted balance between these bottom-up and top-down processing pathways, in such a way that a relatively higher priority is assigned to top-down factors in determining the final percept. 9,10 Indeed, it has been proposed that an excessive top-down processing, eg in the form of serial linguistic expectations, may lead to the generation of spontaneous perceptual experiences.12
A form of top-down processing has been studied using the verbal transformation effect, which refers to the tendency to perceive illusory transformations of repeatedly presented words. For instance, subjects may report hearing “dress” or “stress” upon looped presentation of the word “tress.” The number of such reported transformations was found to be positively correlated with the disoposition toward hallucinations in healthy subjects.13 Another study in schizophrenia patients with and without hallucinations indicated that explicit suggestion may play a crucial role in the verbal transformation effect observed in hallucinating subjects.14
We designed 2 tasks in order to investigate, at a more “automatic” level of processing, whether increased influence from top-down factors in auditory-verbal perception would be related to hallucinatory predisposition in healthy individuals. Second, we sought to test at which level of processing this occurs, namely at the level of semantic processing or the level of phonological processing. This question is important, as patients with schizophrenia (and healthy hallucinators alike) tend to hear meaningful messages and not random auditory stimuli. Thus, it could be hypothesized that semantic expectations play a role in priming hallucinatory perceptual experiences. The stimuli employed in both tasks are auditory verbal and thus bear upon the phenomenological experience of hallucinations, in contrast to previous research where preliminary evidence for increased top-down influences was observed using tone stimuli.8
Methods
Subjects
An abbreviated version of the LSHS,15 using only those items tapping into hallucinatory experiences (items 4, 8, 9, 16), was used to screen 351 undergraduate students from the faculties of Behavioral and Social Sciences, Mathematics and Natural Sciences, and Spatial Sciences at the University of Groningen. The items were selected based on a published factor analysis.16 A total of 42 subjects (17 females), of which half scored in the upper and half in the lower quartile of the abbreviated LSHS, were invited for participation in the actual experiment. This sampling procedure reasonably ensured sufficient variability in hallucination scores in our final sample. Subjects were included only if they reported no current or past psychiatric disorder, and if their native language was Dutch. Ultimately, 3 subjects did not complete the semantic task and 2 failed to complete the phonological task. Furthermore, Cook's D test was used to identify any observations that were exerting a disproportionate effect, which would posit a threat to a valid and reliable analysis. Based on this test, 2 subjects were disqualified as outliers. A final subject was excluded due to an abnormal audiogram (see “Materials and Procedure”).
Materials and Procedure
All participants received oral and written information on the procedures of the experiment, and informed consent was obtained. Participants then filled out the full LSHS-R questionnaire.15 Next, standard audiograms were obtained for each participant, using a descending method with 5-dB steps, within the speech frequency range, in order to ensure adequate auditory perception. Subjects were seated in front of a computer screen and given headphones for sound presentation. Each participant completed both tasks, and task order was counterbalanced across participants.
Semantic Task
Stimuli consisted of short sentences of 5–7 words (eg, see table 1). A pilot study was conducted to test the stimulus materials in an unrelated sample of 28 individuals. Respondents were presented with a number of sentences up to the penultimate word and were asked to fill in the first thing that came to mind. Sentences on which at least 75% of respondents filled in the same word were regarded as highly predictable. Fifty such predictable sentences were selected for the experiment. Subsequently, 50 unpredictable sentences were constructed from the same sentences, by filling in a final word that none of the respondents had reported, within grammatical and semantic constraints. For each of these 100 sentences, the final word was masked by white noise, at a sound level where the stimulus was difficult, but not impossible to detect. [The sound-to-noise level of the embedded words was determined with a small pilot experiment in an unrelated sample of subjects. The same sentences (for the semantic task) and words (for the phonological task) used for the actual experiment were presented with the target word embedded in noise. Subjects were asked to try and identify the target word. Four different sound-to-noise levels were tested. Because we were primarily interested in error rates, we wanted to make sure subjects would make enough errors. Therefore, we selected the sound-to-noise level on which approximately 70% of trials were correctly identified. In addition, all sound stimuli were normalized to the same decibel level, such that all “noise+word” and “noise-alone” stimuli were matched on sound level.] Finally, 50 sentences were created from the same stimuli, by omitting the last word and only presenting a burst of white noise. This was done at the same sound level as the stimuli masked by noise. In this fashion, a total of 150 stimuli were used in the experiment, whereby in a third of cases the predictable word was presented, in a third the unpredictable word was presented, and in a third only white noise appeared. The sequence of stimuli was randomized. Subjects were asked to press the appropriate response button to indicate whether or not they heard a word and subsequently to identify this word out loud. Subjects were encouraged to identify the word only if they were positively convinced, and otherwise to state that they were uncertain of its identity. A total number of 150 trials were presented.
Table 1.
Examples of Stimuli Used in the Semantic Task
| Sentence | Predictable | Unpredictable |
| The thief reported to the | Police | Owner |
| The sailor sells his | Boat | Chair |
| She sunbathes for hours on the | Beach | Roof |
| The accused listened to the plea of his | Attorney | Mother |
| Before bedtime she always tells a | Story | Lie |
| He takes the elevator up to the second | Floor | Meeting |
| She tossed her dirty clothes in the | Hamper | Bushes |
| The unfortunate carpenter hit his | Thumb | Toe |
| The magician pulled a rabbit out of his | Hat | Coat |
| … | … | … |
Note: Sentences were presented up to the penultimate word, after which a burst of white noise appeared. In a third of the trials, only noise was presented. In the rest of the trials, a word was embedded in the noise, at a sound level such that it was difficult to identify. In half of the trials, the word was very predictable given the sentence context. In the other half of the trials, the word was unpredictable, but fit within grammatical and semantic constraints.
Phonological Task
A trial began with the presentation of a spoken prime word. The words were selected from a published list,17 and consisted of adjectives, with similar levels of frequency and familiarity in the Dutch language. After hearing the prime word, a delay period of 2 s followed, wherein subjects were asked to form an auditory mental image of the word. The last phase of the trial consisted of a burst of white noise. On half of the trials, only white noise was presented. In the other trials, a target word was embedded in the noise. On half of these trials, the target word was identical to the “imaged” prime. On the other trials, the word was different. Subjects responded in the same fashion as in the semantic task. A total of 216 trials were presented, divided into 2 blocks.
Statistical Analyses
First, in order to verify that the experimental manipulation was succesful, repeated measures analyses with LSHS score as the between-subjects variable were conducted on the reaction time (RT) and accuracy data acquired from the button press responses. Within-subject variables were “Stimulus Type”: “same,” “different,” or “noise only” in the phonological task and “predictable,” “unpredictable,” or “noise only” in the semantic task.
Our primary analysis concerned the question whether hallucination proneness would be associated with a greater number of top-down erros. To this end, we performed a correlation analysis between LSHS score and the rates of each error type. Subjects could make 2 kinds of “positive” errors, or instances on which a stimulus was erroneously perceived, namely, “top-down errors” and “confabulations.” “Top-down errors” were scored in the phonological task, when the subject's response was the prime word, but the target word was different, or only noise was presented. In the semantic task, a top-down error was scored when the subject's response was the predictable word, but the target was unpredictable or only noise was presented. “Confabulations” were scored when the subject's response was erroneous but unrelated to the prime in the phonological task or to the sentence context in the semantic task. We calculated partial correlations between LSHS score and the error rates while controlling for general task performance (the controlling variable was the percentage of correct button presses, which indexes the ability to simply detect the presented stimuli in the acoustic noise). Further, “misses” were scored when the subject failed to identify a stimulus, and we calculated the number of “unsure” response. Correlations were calculated between these error rates and the LSHS score.
Results
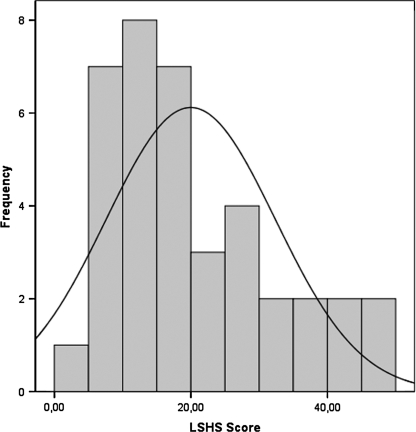
First, we investigated the distribution of LSHS-R scores in the subject sample. A single peak distribution, which was positively skewed (skewness = .848 (.393), kurtosis = −.088 (.768)), was observed in the full LSHS-R scores (see figure 1). Therefore, in the subsequent analyses, we treated LSHS score as a continuous variable, which matches well with the conception of hallucination proneness as a continuum within the population.
Fig. 1.
The Histogram Representing the Frequencies of Full Launay-Slade Hallucination Scale-Revised Scores in Our Subject Sample of First-Year Undergraduate Students, Mean = 19.44 (12.03).
To check if our experimental manipulation was succesful, we conducted a repeated measures analysis which revealed a main effect of Stimulus Type on RT for the “semantic task” (F(2,33) = 8.66, P = .001, Prep = .990, η2 = .294), with the longest RT on unpredictable trials and shortest RT on noise trials, as expected. The analysis for accuracy did not reveal an effect of Stimulus Type (F(2,33) = 1.887, P = .168, Prep = .744, η2 = .066). As we expected, there was neither a significant main effect of LSHS score nor an interaction effect.
The repeated measures analysis on the data from the “phonological task” also revealed a main effect of Stimulus Type on RT (F(2,35) = 7.67, P < .01, Prep = .984, η2 = .271), with the longest RT on the different trials and the shortest RT on the noise trials. In this task, the accuracy analysis did reveal a main effect of Stimulus Type (F(2,35) = 9.476, P = .001, Prep = .998, η2 = .270), with least accurate responses on the different trials, and comparable accuracy on the noise and same trials. There was neither a signifcant main effect of LSHS score nor an interaction effect.
The central question of our investigation was whether hallucination ratings would be associated with an increased number of top-down errors. Consistent with this prediction, we observed a positive and significant correlation between LSHS score and the number of top-down errors in the semantic task (r = .342, P < .05, Prep = .886), indicating an increase in top-down errors with an increase in LSHS score (see figure 2). This correlation was not significant for the phonological task (r = .260, P > .10, Prep = .778). There were no significant correlations between LSHS scores and other error types (confabulations, misses, and unsure responses).
Fig. 2.
Launay-Slade Hallucination Scale Scores on the X-axis and Number of Top-Down Errors in the Semantic Task on the Y-axis. Using partial correlations, we statistically controlled for general ability to detect the stimuli in acoustic noise and observed a positive correlation indicating an increase in number of top-down errors with an increase in hallucination proneness.
Discussion
We designed 2 tasks in order to test whether auditory-verbal perception in hallucination-prone subjects is more liable to be affected by increased top-down attention and whether this takes place at a phonological or semantic level of processing. First, in order to verify that our experimental manipulation of top-down influences was effective, we looked at reaction times and accuracy of stimulus detection in the different task conditions. We observed faster reaction times when the prime word was identical or the sentence context was congruent with the presented target word. This confirms that top-down influences could effectively be manipulated in both tasks. Second, in order to test our specific hypothesis, namely, that hallucination proneness affects the identification of the stimulus, we looked at the type of errors made. We found that in the semantic task, people with a high propensity for experiencing hallucinations were more likely to identify the target word as the word predicted from the sentence context. The instructions urged subjects to make a positive identification only if they were certain and otherwise to state that they were unsure of the stimulus’ identity. This procedure reasonably ensured that an erroneous identification was a true subjective perception, and not a guess or a consequence of task demand characteristics. Furthermore, the fact that higher levels of hallucination proneness were not related to more unsure responses seems to confirm that the effect was not driven by a higher likelihood to guess, despite instructions. Contrary to the findings from the semantic task, higher levels of hallucination proneness were not related to specific error types in the phonological task. These findings thus are consistent with the hypothesis regarding an exaggerated impact of top-down influences on perception, in the case of hallucinatory experiences.9,10 Specifically, in these nonclinical subjects, this seems to take place at the semantic processing level.
The fact that our subject group consisted of nonclinical, high functioning subjects precludes confounding effects from medication, illness-related cognitive deficits, hospitalization, and other variables that complicate research in hallucinating patients. However, the findings may also shed light on the cognitive processes underlying clinical hallucinations. It has been argued frequently that high scorers on questionnaires tapping into schizophrenia-related cognitive or emotional processes show neuropsychological deficits that resemble those found in patients with schizophrenia.18 Previous research has suggested auditory processing in schizophrenia patients may suffer from aberrant top-down processing. A recent study tested individuals with prodromal symptoms,19 who were instructed to repeat any words or phrases that they perceived while listening to “babble” sounds, which consisted of unintelligibly superimposed speech streams. The longest phrase generated by a subject constituted the length of speech illusion (LSI) score. Elevated LSI scores were predictive toward subsequent conversion to schizophrenia. Longer speech illusions were hypothesized to be caused by excessive top-down processing, combined perhaps with distorted perceptual processing or misinterpretation of percepts. When processing a sentence, the perceiver integrates semantic information over several words to form a conceptual model. This internal model then has the ability to bias perception of subsequent incoming stimuli. A relatively large body of research on such semantic priming effects in schizophrenia exists. Several studies have reported increased semantic priming, particulary in fast, automatic processing tasks, which is thought to be due to relatively uninhibited spreading of activation within the semantic network. Sentence context processing is considered to be an automatic process.20 Thus, similar processes to those described in priming experiments in schizophrenia patients may be at work in biasing the perception of hallucination-prone subjects on our semantic task. When stimuli are processed in a more controlled way, as was the case in the phonological task, inhibition of primed content may be easier. In accordance with this notion, it has been suggested that schizophrenia patients are able to inhibit mental representations, but that they need a stronger impetus to do so, compared with controls.21 A similar finding was reported in a study of suppression of cognitive events, using a sentence completion procedure.22 In the high inhibition condition of the task, subjects were required to complete the sentence with a single word that was unrelated to the preceding sentence context. They obtained a positive correlation between hallucination severity in a sample of schizophrenia patients and the number of times a sentence was completed with a contextually appropriate word, against instructions. We observe a similar process in hallucination-prone subjects, but in addition show that the failure to inhibit a “cognitive” event may lead to an actual “perceptual” experience.
Some issues may need further clarification. First, even though we attempted to minimize suggestibility effects, they cannot be completely ruled out. However, had suggestion played a major role in our findings, then we would have expected similar effects in the phonological task. One could even argue that suggestibility effects are more likely there because this task is less automatic and requires controlled processing. Because hallucination proneness was only related to top-down errors in the semantic task, it seems that the effect of suggestion was minimal. Second, because we were particularly interested in AVH, we used auditory-verbal tasks only. In future research, it could be interesting to see if the increased influence of top-down perception extends into other modalities and represents a more general cognitive bias, or whether this processing anomaly is linked to a specific sensory system. Finally, our results are based on correlations between levels of hallucination proneness and number of top-down errors. No causal inference can be drawn from this, and thus it remains unclear whether top-down influences are an antecedent of hallucinations. Another possibility is that those who experience hallucinations become more tuned to internal experiences, which in turn could impact incoming stimuli to a larger extent.
In sum, we observed increased top-down influence on perception of auditory-verbal stimuli in hallucination-prone healthy individuals. Indeed, auditory-verbal “hallucinations” could even be elicited through semantic expectation. Our study sheds new light on a possible cognitive marker for hallucination proneness. Future research may employ neuroimaging methods to investigate how brain systems subserving semantic processes can prime perceptual systems.23 Arguably, an analogous cognitive process could be responsible for auditory hallucinations in schizophrenia. Empirical investigation using similar tasks in patients with schizophrenia could clarify whether hallucinatory experiences and their psychological underpinnings are equivalent in clinical and nonclinical populations.
Funding
Ubbo Emmius grant (180/800 514) from the University of Groningen, The Netherlands, to A.V. Funding to pay the Open Access publication charges for this article was provided by University Medical Center Groningen.
Acknowledgments
The authors thank master students Evelien Platje and Anke Kornfeld for their assistance with subject recruitment and testing.
References
- 1.Slade P, Bentall RP. Sensory Deception: A Scientific Analysis of Hallucination. London: Croon Helm; 1988. [Google Scholar]
- 2.Tien AY. Distributions of hallucinations in the population. Soc Psychiatry Psychiatr Epidemiol. 1991;26(6):287–292. doi: 10.1007/BF00789221. [DOI] [PubMed] [Google Scholar]
- 3.Johns LC, Cannon M, Singleton N, et al. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- 4.Barrett TR, Caylor MR. Verbal hallucinations in normals, V: perceived reality characteristics. Pers Individ Dif. 1998;25(2):209–221. [Google Scholar]
- 5.Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21(8):1125–1141. doi: 10.1016/s0272-7358(01)00103-9. [DOI] [PubMed] [Google Scholar]
- 6.Launay G, Slade P. The measurement of hallucinatory predisposition in male and female prisoners. Pers Individ Dif. 1981;2(3):221–234. [Google Scholar]
- 7.Aleman A, Larøi F. Hallucinations: The Science of Idiosyncratic Perception. Washington, DC: American Psychological Association; 2008. [Google Scholar]
- 8.Aleman A, Bocker KB, Hijman R, de Haan EH, Kahn RS. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr Res. 2003;64(2–3):175–185. doi: 10.1016/s0920-9964(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 9.Behrendt RP. Underconstrained perception: a theoretical approach to the nature and function of verbal hallucinations. Compr Psychiatry. 1998;39(4):236–248. doi: 10.1016/s0010-440x(98)90067-0. [DOI] [PubMed] [Google Scholar]
- 10.Grossberg S. How hallucinations may arise from brain mechanisms of learning, attention, and volition. J Int Neuropsychol Soc. 2000;6(5):583–592. doi: 10.1017/s135561770065508x. [DOI] [PubMed] [Google Scholar]
- 11.Kveraga K, Ghuman AS, Bar M. Top-down predictions in the cognitive brain. Brain Cogn. 2007;65(2):145–168. doi: 10.1016/j.bandc.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman RE, Rapaport J, Mazure CM, Quinlan DM. Selective speech perception alterations in schizophrenic patients reporting hallucinated “voices”. Am J Psychiatry. 1999;156(3):393–399. doi: 10.1176/ajp.156.3.393. [DOI] [PubMed] [Google Scholar]
- 13.Bullen JG, Hemsley DR, Dixon NF. Inhibition, unusual perceptual experiences and psychoticism. Pers Individ Dif. 1987;8(5):687–691. [Google Scholar]
- 14.Haddock G, Slade PD, Bentall RP. Auditory hallucinations and the verbal transformation effect—the role of suggestions. Pers Individ Dif. 1995;19(3):301–306. [Google Scholar]
- 15.Larøi F, Van der Linden M. Nonclinical participants’ reports of hallucinatory experiences. Can J Behav Sci. 2005;37(1):33–43. [Google Scholar]
- 16.Larøi F, Marczewski P, Van der LM. Further evidence of the multi-dimensionality of hallucinatory predisposition: factor structure of a modified version of the Launay-Slade Hallucinations Scale in a normal sample. Eur Psychiatry. 2004;19(1):15–20. doi: 10.1016/S0924-9338(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 17.Hermans D, de Houwer J. Affective and subjective familiarity ratings of 740 Dutch words. Psychol Bel. 1994;34:115–139. [Google Scholar]
- 18.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19(2):233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman RE, Woods SW, Hawkins KA, et al. Extracting spurious messages from noise and risk of schizophrenia-spectrum disorders in a prodromal population. Br J Psychiatry. 2007;191:355–356. doi: 10.1192/bjp.bp.106.031195. [DOI] [PubMed] [Google Scholar]
- 20.Barch DM, Cohen JD, Servan-Schreiber D, et al. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol. 1996;105(4):592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- 21.Titone D, Levy DL, Holzman PS. Contextual insensitivity in schizophrenic language processing: evidence from lexical ambiguity. J Abnorm Psychol. 2000;109(4):761–767. doi: 10.1037//0021-843x.109.4.761. [DOI] [PubMed] [Google Scholar]
- 22.Waters FA, Badcock JC, Michie PT, Maybery MT. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cognit Neuropsychiatry. 2006;11(1):65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 23.Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32(1):175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]