Abstract
Symptom development during the prodromal phase of psychosis was explored retrospectively in first-episode psychosis patients with special emphasis on the assumed time-related syndromic sequence of “unspecific symptoms (UN)–predictive basic symptoms (BS)–attenuated psychotic symptoms (APS)–(transient) psychotic symptoms (PS).” Onset of syndromes was defined by first occurrence of any of their respective symptoms. Group means were inspected for time differences between syndromes and influence of sociodemographic and clinical characteristics on the recalled sequence. The sequence of “UN–BS/APS–PS” was clearly supported, and both BS and, though slightly less, APS were highly sensitive. However, onset of BS and APS did not show significant time difference in the whole sample (N = 126; 90% schizophrenia), although when each symptom is considered independently, APS tended to occur later than first predictive BS. On descriptive level, about one-third each recalled an earlier, equal and later onset of BS compared with APS. Level of education showed the greatest impact on the recall of the hypothesized sequence. Thereby, those with a higher school–leaving certificate supported the assumed sequence, whereas those of low educational background retrospectively dated APS before BS. These findings rather point out recognition and recall bias inherent to the retrospective design than true group characteristics. Future long-term prospective studies will have to explore this conclusively. However, as regards the criteria, the results support the notion of BS as at least a complementary approach to the ultrahigh risk criteria, which may also allow for an earlier detection of psychosis.
Keywords: psychosis, early course, phenomenology, early initial prodromal state, late initial prodromal state
Introduction
In early detection of psychoses, the widely applied “ultrahigh risk” (UHR) criteria of the prodrome of first-episode psychosis aim at defining an imminent risk of psychosis with conversion within 1 year not only by attenuated psychotic symptoms (APS) but also by brief limited intermittent psychotic symptoms (BLIPS) and a combination of a risk factor for psychosis and recent functional decline.1,2 A complementary approach employs a subgroup of basic symptoms (BS; ie, subtle, subclinical self-experienced disturbances in thought, speech, and perception processes that are rarely perceivable from outside).3–6 In the Cologne Early Recognition (CER) study,4,6 presence of these cognitive-perceptive BS at baseline examination predicted development of schizophrenia within a mean follow-up period of 9.6 years with good predictive accuracy, thereby preceding onset of psychosis by less than 1 year in less than 1% of converted cases and by more than 4 years in 48%.6
Within the early detection and intervention projects of the German Research Network on Schizophrenia (GRNS),7 the BS and UHR approach were used to define an early initial prodromal state (EIPS) and a late initial prodromal state (LIPS).5,7,8 The EIPS was defined by the presence of any one of the 10 predictive BS4,6 in the absence of APS and BLIPS (ie, thought interference, perseveration, pressure or blockages, disturbance of receptive language, decreased ability to discriminate between ideas and perception, unstable ideas of reference, derealization, and visual or acoustic perception disturbances) and, alternatively, by the combination of a genetic or obstetric risk factor for psychosis and recent functional decline.5,7,8 In line with the UHR criteria, the LIPS was characterized by APS (ie, ideas of reference, unusual perceptual experiences including body-related illusions, paranoid ideation/mistrust, magical thinking, and odd speech) and, alternatively, by BLIPS.5,7,8
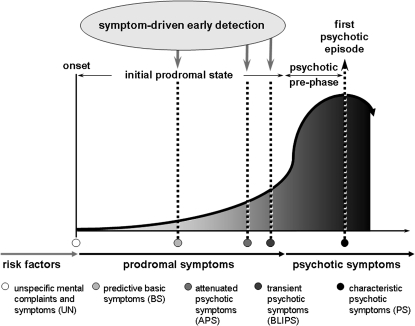
Based on data of different prospective studies, a sequence of symptom development relating to the symptomatic definitions of EIPS and LIPS was proposed.8 It assumes that the psychotic prodrome typically starts off with unspecific mental problems (UN) that, in combination with a risk factor and functional decline, can well be part of the EIPS. UN are then followed by predictive BS and, subsequently, by APS, before (transient) psychotic symptoms (PS) finally develop8 (see figure 1).
Fig. 1.
Model of Psychopathological Development of Psychosis Related to the Definition of the Early and Late Initial Prodromal State, Modified According to Klosterkötter et al.8
This sequence has not yet been prospectively studied in samples defined by presence of UN only. Such studies would require immensely large sample sizes and follow-ups of 10 years or more to avoid false classification of outcomes, especially so-called “false false positives,”9 due to an insufficiently long observation period. Therefore, a prospective evaluation is not conceivable within the nearer future. Furthermore, truly prospective studies will suffer from a selection bias in favor of persons already seeking help very early in the prodromal phase, at the first onset of mental problems. For these reasons, the present study aims at a first retrospective evaluation of the proposed sequence “UN–BS–APS–PS” in a less selected sample of first-episode psychosis patients.
There is, however, some indication that certain clinical and/or sociodemographic factors are associated with differences in the course of symptoms in the prodromal phase.3–6,10–16 Therefore, 6 factors were analyzed for their influence on the proposed sequence for the following reasons: the CER study3–6 had its focus on the development of schizophrenia; thus, it is not clear whether its results can be assigned to other psychoses. Consequently, type of first-episode psychosis at discharge was studied as a moderating variable.
Furthermore, analyses of the CER data10 as well as a computer simulation model of the occurrence of speech disturbances and acoustic hallucinations11 suggested that the duration of the prodromal phase as well as age at onset of illness were associated with differences in psychopathology. For these reasons, prodrome duration and age at illness onset were also considered.
Prospective early detection studies rely on help-seeking samples, yet, only a fraction of first-episode patients appear to seek help for mental problems in this early phase12 and, thus, might introduce a selection bias in models relying on these studies. One major variable associated with help-seeking behavior not only for mental but also for any health-related problems is the sociodemographic background including level of education on leaving high school13; thus level of education was studied as yet another moderating variable.
A positive family history of psychosis is the strongest risk factors for psychoses14 and, in combination with recent functional deterioration, an important predictor of psychosis.15 Furthermore, it was found to be associated with a longer prodromal phase.16 Family history of psychiatric disorders, therefore, was also studied for its influence on the supposed symptom sequence.
Because gender differences are considered to be an important variable in understanding schizophrenia,17 it was included as the sixth and final moderating variable.
Method
Sample
One hundred twenty-eight inpatients with first-episode psychosis were assessed as part of the multicenter awareness project of the GRNS7 after informed written consent had been obtained. Participation in the study was voluntary; the study was approved by the local ethic committee. Two patients did not report on any symptoms prior to the admission for psychosis and, consequently, were not included in the analyses that were based on the remaining 126 patients (table 1).
Table 1.
Sociodemographic and Clinical Characteristics of the First-Episode Sample
| Total (N = 126; 100%) | |
| Age at admission in y: mean ± SD; median (range) | 30.10 ± 8.64; 29 (18–55) |
| Age at first positive symptom in y: mean ± SD; median (range) | 27.94 ± 10.42; 25 (4–51) |
| Age at first symptom in y: mean ± SD; median (range) | 22.38 ± 9.78; 20 (8–50) |
| DUI in y: mean ± SD; median (range) | 8.17 ± 7.53; 6 (0–43) |
| DUP in y: mean ± SD; median (range) | 2.27 ± 3.91; 1 (0–20) |
| Duration of prodrome in y: mean ± SD; median (range) | 5.90 ± 7.09; 4 (0–38) |
| Diagnosis at dischargea (%) | |
| Schizophrenia | 88.1 |
| Schizophreniform disorder | 5.6 |
| Schizoaffective disorder | 2.4 |
| Delusional disorder | 0.8 |
| Brief psychotic episode | 3.2 |
| Marital status (%) | |
| Single | 77.8 |
| Married/living with steady partner | 13.5 |
| Separated/divorced | 7.1 |
| Widowed/living apart, not separated | 1.6 |
| Current steady partner (%): | |
| No | 72.2 |
| Yes | 27.8 |
| Level of educationb (%): | |
| No certificate/CSE | 35.7 |
| O level/VBD | 29.4 |
| A level/still in high school | 34.9 |
| Current occupation (%): | |
| None | 47.2 |
| Protected/therapeutic place | 8.1 |
| Normal occupation | 44.7 |
| Family historyc (%): | |
| No mental disorder known | 65.1 |
| Psychosis | 11.1 |
| Other disorder (affective disorders) | 23.8 (12.7) |
Clinical diagnosis, not assessed in a standardized manner.
Level of education on leaving school was translated into British school–leaving certificates. CSE: Certificate of Secondary Education, VBD: Vocational baccalaureate diploma. CSE and O levels require 10, VBD 12, and A levels 13 years of schooling, provided that no class had been repeated.
With regard to biological first-degree relatives.
Instruments
Symptom presence and onset were assessed with the “Early Recognition Instrument based on the Instrument for the Retrospective Assessment of the Onset of Schizophrenia” (ERIraos)18 according to the methods used in the Age-Beginning-Course (ABC) study of schizophrenia.9 The ERIraos was developed within the GRNS6,18 and is the result of a review of literature on the early detection of psychoses and early detection instruments such as the “Structured Interview for Prodromal Syndromes” (SIPS),19 the “Comprehensive Assessment of At-Risk Mental States” (CAARMS),20 and the “Bonn Scale for the Assessment of Basic Symptoms” (BSABS).21 Its main source, however, was part IV of the “Instrument for the Retrospective Assessment of the Onset of Schizophrenia” (IRAOS),22 the core instrument in the ABC study of the early course of schizophrenia on a representative sample of 232 first-episode schizophrenia patients, 130 patients first hospitalized for depression, and 130 healthy controls.23 Thus, the ERIraos was created as an extended substitute for the IRAOS part IV, ie, the symptom list, which includes BS according to the BSABS and, contrary to their syndromic definitions in the SIPS and CAARMS, APS according to the corresponding symptoms of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria of schizotypal personality disorder. Thereby, the ERIraos has kept its advantages as an instrument for a valid and reliable retrospective dating of symptom onset.24
In line with the ABC study,9 patients were interviewed in remission, around the time of discharge; clinical records were used as a complimentary information source. Assessed for the month of first occurrence and with the day of admission as anchor point, onset of UN was defined by the time (in years) between admission and the earliest onset of any one reported of 88 nonpsychotic ERIraos items not included in BS or UHR criteria (eg, increased worrying, depressive mood, or sleeping problems). Correspondingly, onset of BS and UHR, respectively, were operationalized by the earliest occurrence of any one of the 10 predictive BS and any one of the 5 APS; onset of psychosis by that of any PS, irrespective of its duration. Although the ERIraos allows the rating of BLIPS, patients were mainly unable to recall the exact duration of a symptom at certain times in the past and sometimes years back. As a result, in this retrospective study, no distinction between BLIPS, which are part of the UHR and LIPS criteria, and PS was made. Yet, to allow for possible BLIPS, other symptoms (UN, BS, and APS) were included as prodromal, even if their onset was reported later in time than that of the earliest PS.
Data Analysis
To account for large variations in the duration of untreated illness (DUI) across patients (see table 1) and to avoid distortions by extremely short or long DUIs in the calculation of group means, z transformations of time data of symptoms (difference between date of admission and month of onset of the respective earliest symptom) were carried out for each patient across his/her symptom onset data. By these 126 individual z transformations, onset data of symptoms given as the individual SDs (ranging from “0.01” to “11.45” across patients) from the individual mean (ranging from “−0.63” to “11.08”) is standardized as number of SD from the mean, which is fixed at “0.” By means of this transformation, the onset of syndromes or phases is comparable across patients irrespective of the length of the individual DUI.
Differences between the time intervals of phases within groups were calculated by 2-tailed paired, single-sample t tests of z-transformed data for patients showing both respective phases; thus, subsample sizes for onset comparisons differed even within the same subgroups. At the given subsample sizes of 101 to 2 patients and a power of 95%, only medium to very large effects of d ≥ 0.5 could be expected to become significant. Thus, these subgroup comparisons served mainly explorative purposes, and no further adjustment for multiple testing was made. At n = 1, no analyses could be carried out for the diagnostic subgroup of delusional disorders.
Spearman correlation coefficients of the 6 sociodemographic and clinical variables were calculated to reveal significant interactions that should be considered as additional covariates in the stepwise logistic regression analyses. These were calculated according to the forward Wald method (threshold probability of .5, probability of inclusion of .05 and of exclusion of .10) on 2 different subgroups: (I) patients who had reported both BS and APS (n = 81) with BS occurring earlier than or within the same months as APS (“BS ≥ APS”) as positive event and (II) patients who had reported different onset of BS and APS (n = 52) with “BS > APS” as positive event.
To limit redundancies, the resulting logistic models were tested for their threshold-independent classification accuracy in a slightly extended subsample of 91 patients with at least BS preceding PS (“BS ≥ APS” or, in absence of APS, “BS > PS” as positive event), by analyses of the area under the receiver operating characteristic (ROC) curve, AUC.
Results
Occurrence and Onset of Symptoms
All 126 patients reported UN as well as PS, 101 (80.2%) BS according to the adapted EIPS criterion and 91 (74.2%) APS according to the adapted LIPS criterion. Eighty-one (64.3%) reported both APS and BS; of these, 27 (33.3%) reported onset of BS before onset of APS, 29 (35.8%) within the same months as APS, and 25 (30.9%) after onset of APS.
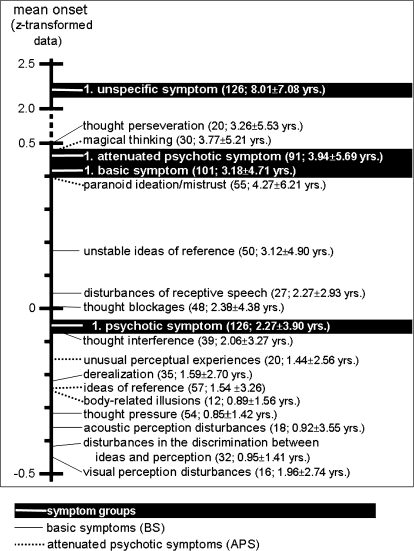
Within the sample, the onset of UN occurred on average 8.2 years before admission (z = 2.24), followed by the onset of APS 3.9 years (z = 0.46), and by that of BS 3.3 years (z = 0.42) before admission; PS preceded admission on average by 2.3 years (z = −0.05; figure 2). Time differences between UN and BS (t = 10.86, df = 100, P = .000), APS (t = 8.10, df = 90, P = .000) and PS (t = 15.10, df = 125, P = .000), respectively, and between PS and BS (t = 2.56, df = 100, P = .012) and APS (t = 2.55, df = 90, P = .012), respectively, became significant but not between BS and APS (t = −0.89, df = 80, P = .376). The early occurrence of APS was mainly due to “mistrust” and “magical ideation” (see figure 2), though, when regarded separately in persons showing the respective symptom pairs, each single APS had its mean onset following that of BS. Thereby, mistrust (t = 1.42, df = 52, P = .161) and magical ideation (t = .255, df = 26, P = .801) showed no significant time difference between their respective onset and BS, whereas ideas of reference (t = 2.95, df = 51, P = .005), body-related illusions (t = 2.11, df = 11, P = .059), and unusual perceptual experiences (t = 2.37, df = 18, P = .029) did at least at trend level.
Fig. 2.
Mean Onset of Phases, Basic Symptoms, and Attenuated Psychotic Symptoms. Number of persons reporting the respective symptom/symptom group is given in brackets along with mean ± SD of raw time data.
Impact of Sociodemographic and Clinical Characteristics
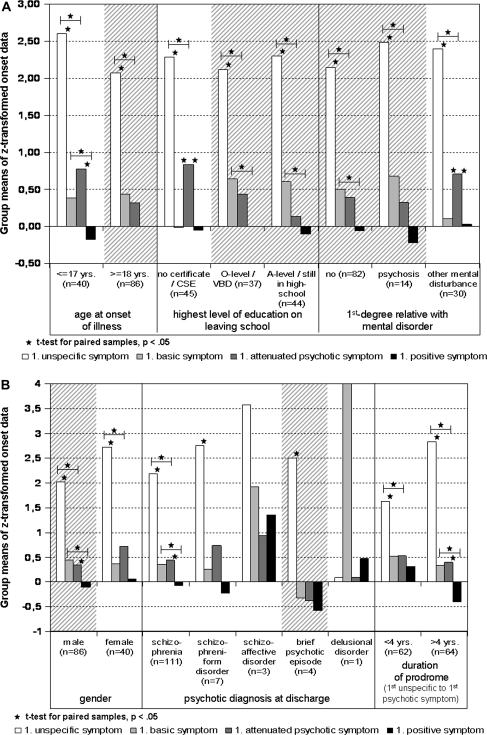
Considering gender, age at onset of illness (dichotomized at 18 years, ie, the threshold age for adult psychiatry), duration of prodrome (dichotomized at the median of 4 years), highest level of education, psychotic diagnosis at discharge, and history of a first-degree relative with mental disorder, in all but those subgroups with n ≤ 3, UN significantly preceded BS and PS, respectively, as well as APS, in all but those subgroups with n ≤ 7, (see figure 3a and 3b). Except for gender and a short duration of prodrome, the time difference between APS and PS became significant only in subgroups with a converse onset of prodromal symptoms, ie, in those reporting a mean onset of APS before BS, and n ≥ 30 (see figure 3a and 3b). Furthermore, BS significantly preceded PS in patients with an onset of illness before the age of 18 years, with A levels or aiming for it, with no family history of psychiatric disorders, of male gender, and with a long prodrome. In all but 2 subgroups, the time difference between onset of BS and APS did not differ significantly.
Fig. 3.
(a) Sequences of Phases According to Age at Onset of the Illness, Highest Level of Education on Leaving School, and Family History of Mental Disorders. Subgroups following the proposed sequence are highlighted with gray lines. (b) Sequences of phases according to gender, clinical diagnosis of psychosis at discharge, and duration of the prodromal phase. Subgroups following the proposed sequence are highlighted with gray lines.
At a purely descriptive level, the expected sequence “UN–BS–APS–PS” occurred for men, patients of at least 18 years of age at onset of illness, patients with a higher education, patients with no family history of mental disorders or one of psychosis and the few patients with a diagnosis of a brief psychotic episode (see figure 3a and 3b). In none of these subsamples, the time difference between BS and APS became significant.
Female patients, patients reporting an adolescent onset of illness, patients with no school-leaving certificate or only CSE, patients with first-degree relatives with a nonpsychotic, mainly depressive mental disorder, and patients with a schizophrenic or schizophreniform diagnosis at discharge reported the expected pattern of UN occurring first and PS last but an onset of APS before BS (see figure 3a and 3b). The latter became significant for the low educated subsample (n = 45) and those with a nonpsychotic family history of mental disorders (n = 30; see figure 3a). The duration of the prodrome—at least at the chosen median cutoff—was mainly unrelated to the reported sequence of phases.
The correlation analyses of the sociodemographic and clinical variables showed that, with 2 exceptions, these were mainly independent of each other: prodrome duration was inversely correlated with age at onset of illness (rs = −0.406, P < .01), and diagnosis at discharge was positively correlated with gender (rs = 0.237, P < .01), ie, men were more likely to receive a diagnosis of schizophrenia (see table 2).
Table 2.
Correlation Between Variables Examined for Their Influence on the Hypothesised Sequence of Symptom Types (Nonparametric Spearman Correlation Coefficient)
| Level of Education on Leaving School | First-Degree Relative With Mental Disorder | Gender | Diagnosis at Discharge | Duration of Prodrome | |
| Age at onset of illness, y | −0.143 | −0.059 | 0.152 | −0.118 | −0.406** |
| Level of education | 0.108 | −0.136 | 0.006 | −0.017 | |
| First-degree relative with mental disorder | 0.087 | 0.103 | 0.026 | ||
| Gender | 0.237** | 0.157 | |||
| Diagnosis at discharge | 0.119 |
**P < 0.01.
Prediction of Hypothesized Sequence
Testing the impact of the 6 sociodemographic and clinical variables as well as the 2 significant correlative interactions on the recall of the hypothesized sequence in 2 subsamples, at odds ratios around 2.7, level of education showed the greatest impact in both logistic models (see table 3). In addition to level of education, age at onset of illness was selected in the first (M1) and family history in the second model (M2; see table 3). Both logistic models became significant and correctly classified 72% of patients. And with areas under the ROC curve (AUC) between 0.717 for M2 (95% confidence limit [CL]: 0.602/0.833; P = .001) and 0.737 for M1 (95% CL: 0.629/0.854; P = .000), they were also able to correctly classify patients above chance level for their probability to report the assumed sequence in the extended subsample (n = 91; with positive events equal “BS ≥ APS” or “BS > PS” in absence of APS).
Table 3.
Stepwise Logistic Regression Analysis (Wald Method, Forward) to Predict Onset of BS Prior to APS in First-Episode Patients Showing Both Types of Symptoms
| 95% CI for Exp(β) |
||||||||
| Model (Mx) | Selected Variable | β | SE | Wald (df = 1) | P | Exp(β) | Lower | Upper |
| M1: BS ≥ APSa (n = 81); BS = APS equals positive event | Age at onset of illness (in y) | 0.055 | 0.028 | 4.013 | .045 | 1.057 | 1.001 | 1.116 |
| Level of educationb | 0.960 | 0.323 | 8.821 | .003 | 2.611 | 1.386 | 4.920 | |
| Constant term | −2.224 | 0.915 | 5.906 | .015 | 0.108 | |||
| M2: BS > APSc (n = 52); BS = APS excluded | Level of education | 0.999 | 0.385 | 6.747 | .009 | 2.715 | 1.278 | 5.768 |
| First-degree relative with mental disorderd | −0.736 | 0.368 | 4.006 | .045 | 0.479 | 0.233 | 0.985 | |
| Constant term | −1.432 | 0.783 | 3.341 | .068 | 0.239 | |||
Note: Entered variables—“age at onset of illness”; “level of education,” “first-degree relative with mental disorder,” “gender,” “diagnosis at discharge,” “duration of prodrome,” and the significant interactions “age at onset of illness × duration of prodrome,” “diagnosis at discharge × gender.” BS, basic symptoms; APS, attenuated psychotic symptoms; CI, confidence interval.
Model fit: χ2 = 13 680, df = 2, P = .001 (40.0% right negative [10/25], 87.5% right positive [49/56] and altogether 72.8% right classifications [59/81]).
Low value equals low education.
Model fit: χ2 = 11 438, df = 2, P = .003 (68.0% right negative [17/25], 74.1% right positive [−20/27], and altogether 71.2% right classifications [37/52]).
Values distributed as follows: 0 = none; 1 = psychosis; 2 = other mental disturbance.
Discussion
We aimed at exploring retrospectively a sequence of symptom development relating to the definitions of the EIPS and LIPS in a first-episode psychosis sample. These 2 prodromal states had been suggested as part of the early detection and intervention studies of the GRNS.7 Based on annual transition rates reported for UHR subjects not specifically treated for beginning psychosis1,2,19,25–28 and for subjects with predictive BS,3,4,6 it was assumed that the psychotic prodrome starts off with UN, which are followed by predictive BS first, attenuated (APS) next and–transient–psychotic symptoms (BLIPS/PS) last.8
All but 2 patients (98.4%; n = 126) reported a prodromal phase of at least 1-month duration. This rate clearly exceeds the 73% of first-episode schizophrenia patients with an initial prodrome of equal minimum 1-month duration of the ABC study.29 This difference might result from the inclusion of any first-episode psychosis and not only schizophrenia in our sample. However, the higher frequency of prodromes in our sample more likely reflects the effect of the extended ERIraos symptom list that, compared with the original IRAOS symptom list, catches a greater number of prodromal complaints. By picking up earlier UN, this would have also affected the duration of the prodrome that, at a mean duration of 5.9 years, was slightly longer than the 5.0-year mean prodromal period of the ABC study.29
Of the 126 patients who recalled a prodromal phase, 111 (88.1%) reported either BS or APS following UN and preceding PS, 81 (64.3%) both. A total of 15.9% recalled BS preceding (BLI)PS in absence of APS, 7.9% APS preceding (BLI)PS in absence of BS. Thus, BS as well as APS showed a high sensitivity of .80 and .72, respectively, in the total sample that confirms their role as important and partly complementary predictors of psychosis.1–6,25–30
Each of roughly one-third of patients recalled an earlier onset of BS, a rather simultaneous onset and an earlier onset of APS, respectively. When the mean time of onset was considered in the whole sample irrespective of any moderating variables, the model was supported for the general sequence of UN—more specific prodromal symptoms (BS or APS)—(BLI)PS, but the hypothesized sequence of BS and APS did not occur due to a statistically insignificant difference between their respective onset. However, if we compared individual APS with the syndromic BS criterion, we did observe that APS occurred later than BS; at times these were significant differences. The comparison to the BS criterion as a whole was chosen over that to single BS in order to keep subsample sizes at a statistically reasonable level.
One limitation of the study that might account for the partial lack of support of the assumed sequence was that the definitions used in the present work do not fully reflect GRNS prodromal criteria. Within the GRNS, an EIPS was defined alternatively by at least any one predictive BS or by presence of a risk factor in combination with a recent significant functional decline (trait-state criterion).5 Yet, the present definition of an early prodromal state included the BS-criterion alone; the trait-state criterion was not considered. Thus, it could be argued that EIPS criteria might have been met earlier by the 11% of patients with a positive family history for psychosis: still, the hypothesized sequence showed in the mean time data of this genetic high-risk subsample already. Consequently, an additional consideration of this trait-state criterion is unlikely to have affected the overall findings. Consideration of the second risk factor employed in the GRNS, obstetric complications, however, might have affected the present findings in 2 ways: (1) by dating back the onset of EIPS in some cases or (2) by identifying more EIPS cases. Both would have been in favor of the hypothesized sequence.
A similar limitation applies to the LIPS definition. Within the GRNS, a LIPS was defined by either APS or BLIPS.5 Yet, the present definition of a late prodromal state by APS alone considered only one of the 2 alternative criteria. Because patients reported great difficulties in recalling the exact duration of certain symptoms in the past and often years back in addition to their onset, no distinction was made between BLIPS and PS. However, only 1.5% of all putatively prodromal clients who consulted the Cologne Early Recognition and Intervention Centre (FETZ) between 1998 and 200313 reported BLIPS as the sole prodromal criterion, whereas 0.5% reported APS, 15.2% APS and BS, and 3.0% only BS in addition to BLIPS. This is in line with other UHR-based studies reporting not BLIPS but APS for the vast majority of cases.1,2,19,20,25–28,30,31 Thus, assuming a 1.5% rate of cases with only BLIPS, in the present study, the presence and, consequently, the onset of LIPS might have been missed by its definition by APS alone in mere 1 or 2 cases.
When examining the influence of sociodemographic and clinical variables on the early course, only duration of the prodrome seems to have no apparent effect on the sequence of symptom groups. It is unclear if this finding results from the chosen cutoff, or, considering the varied onset of the single symptoms constituting the prodromal criteria, if differences in psychopathology that are related to the duration of the prodrome are more likely restricted to single symptoms as reported from the CER study10 as well as from a computer simulation of the elimination of synaptic connections in normal development and psychotic symptom formation.11
The other 5 examined variables, however, seem to have an impact on the mean retrospective dating of time of onset of criteria, especially of BS and APS. At a descriptive level, the assumed sequence occurred for male patients, for patients who were older at illness onset or had higher level of education and for patients who had no family history of psychiatric disorders or, alternatively, one of psychosis as well as for patients with a diagnosis of a brief psychotic episode. Of these, level of education had by far the greatest influence on the presentation of the sequence, nearly tripling the odds of its occurrence. Furthermore, age at onset of illness and family history partly impacted on the sequence, though hardly raising the model's odds, whereas impact of gender and, even more so, of diagnosis at discharge were insignificant.
The impact of level of education on the presentation of the hypothesized syndrome sequence can be explained in 2 ways. First, the sequential approach was generated on data of potentially prodromal samples seeking help for mental problems in specialized centers. Health, and especially mental health service utilization, however, is associated with socioeconomic variables including level of education.13,32–36 Accordingly, as regards percentages of patients with higher education, especially A levels, both truly prodromal patients included in the CER study10 (45%) as well as potentially prodromal persons seeking help for their mental problems in the FETZ (60%) were overrepresented in comparison to the general population (34%)13 as well as to the less selected present sample of first-episode psychosis inpatients (35%). Consequently, with the model being based on results of samples of higher than average education, ie, with the model generation being influenced by a selection bias in favor of the better educated, it is quite perceivable that it could only be replicated in and might even only be valid for this subgroup.
A second explanation of the impact of level of education may be that it can be regarded as a rough approximation of intelligence. While the hypothesized sequence showed most clearly in those with at least O levels, the most prominent deviation from the expected pattern showed for patients with no or low school–leaving certificate. Within this lowly educated subsample, cognitive-perceptive BS were not only recalled having occurred significantly later than APS but almost at the same time as PS. Because it was shown that present BS can be assessed even in psychotic patients with mild mental retardation, in whom they occur in the same frequency as in nonmentally retarded chronic schizophrenia patients,36 it is unlikely that they are less frequent or less assessable in patients of lower intelligence or lower level of education. Unlike the assessment of current symptoms, in that the awareness can be risen to symptoms not spontaneously recognized, the retrospective assessment and dating of symptoms is far more challenging as it requires (1) spontaneous and highly differentiated symptom recognition by the patient him-/herself at the time of its first occurrence, (2) the attribution of the recognized symptom as meaningful or important—a prerequisite for storage in long-term memory, and (3) the correct retrieval of the exact symptom and its date of onset from memory. Thus, the late dating of onset of very subtle BS in the lower educated subsample might be partially caused by lack of their spontaneous recognition or lack of their attribution as important during earlier times of the illness and/or by incorrect retrospective timing toward a time of great changes, ie, the time of onset of PS. This, consequently, challenges the broad applicability of a retrospective study design in prodromal psychosis. Because these limiting factors, however, do not impact much on the assessment of present symptoms, predictive BS should be picked up earlier and the proposed symptom sequence may also show in samples of low education when patients are interviewed for present symptoms in prospective studies.
Contrary to the more stable, highly significant impact of level of education, regression analyses demonstrated no or only marginal impact of gender, diagnosis at discharge, age at illness onset, and family history of mental disorder on the reported sequence. Thus, it is unlikely that these represent strong moderator variables on the expression of potential symptom patterns in the prodrome.
In summary, the retrospective data supported the proposed sequence in that UN were regularly found first, followed by more specific prodromal symptoms, ie, cognitive-perceptive BS and/or APS, before the onset of first PS. Furthermore, with both BS and APS criterion being highly sensitive already on their own and not overlapping in a considerable portion of patients, their combination should be able to detect the vast majority of first-episode psychosis cases. As regards the proposed regular pattern of BS preceding APS, however, the results are inconclusive. Future large-scale prospective long-term follow-up studies assessing BS and UHR criteria will have to show whether this is due to (1) a selection bias in studies underlying the proposed sequence, (2) the retrospective study design and, consequently, a recognition and related recall bias in the present sample, (3) the definition of EIPS and LIPS in the present study, and/or (4) the fact that the proposed sequence is not valid or only in certain subgroups.
Because all 3 possible sequential onset patterns of predictive BS and APS occurred with equal frequency, the results caution against solely global examinations of criteria as a whole—as often done with the UHR criteria,9,20 as well as against an untimely definition of a regular sequence—either in terms of EIPS and LIPS5,8 or in terms of self-disturbances or BS as a second step in a “close-in” UHR-based approach to an early detection.37 Future long-term prospective follow-up studies should attempt to determine the different patterns in which symptoms occur and the variation that may occur in different subgroups. It is possible that the identification of such symptom patterns will be an important step forward in attempts at a better timing of a patient's current risk status and, thus, help determine appropriate phase- or risk-adapted treatment.
Funding
The awareness study was funded by the German Federal Ministry of Education and Research (grant 01 GI 0235 to J.K., Cologne, and W.M., Bonn). Data assessments were supported by Daniel Köhn, formerly Cologne; Verena Veith, Cologne, Heinz Picker, Cologne; Sarah von der Laage, Cologne; Annett Nüchter, Cologne; Julia Berning, Bonn, Julia Bludau, Bonn, and Antje Niedersteberg, Duisburg.
Acknowledgments
Readers interested in an English manual for the assessment of BS and their differentiation to attenuated positive and negative symptoms are kindly referred to the Schizophrenia Proneness Instrument, Adult version by F. Schultze-Lutter, J. Addington, S. Ruhrmann, and J. Klosterkötter available at www.fioriti.it.
References
- 1.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. Br J Psychiatry. 1998;173(suppl 33):14–20. [PubMed] [Google Scholar]
- 2.Phillips LJ, Yung AR, McGorry PD. Identification of young people at risk of psychosis: validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Aust N Z J Psychiatry. 2000;34(suppl):S164–S169. doi: 10.1080/000486700239. [DOI] [PubMed] [Google Scholar]
- 3.Schultze-Lutter F. Prediction of psychosis is necessary and possible. In: McDonald C, Schultz K, Murray R, Wright P, editors. Schizophrenia: Challenging the Orthodox. London, UK: Taylor & Francis; 2004. pp. 81–90. [Google Scholar]
- 4.Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 5.Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003;36(suppl 3):162–167. doi: 10.1055/s-2003-45125. [DOI] [PubMed] [Google Scholar]
- 6.Schultze-Lutter F, Ruhrmann S, Klosterkötter J. Can schizophrenia be predicted phenomenologically? In: Johannessen JO, Martindale B, Cullberg J, editors. Evolving Psychosis. Different Stages, Different Treatments. London, UK: Routledge; 2006. pp. 104–123. [Google Scholar]
- 7.Häfner H, Maurer K, Ruhrmann S, et al. Early detection and secondary prevention of psychosis: facts and visions. Eur Arch Psychiatry Clin Neurosci. 2004;254:117–128. doi: 10.1007/s00406-004-0508-z. [DOI] [PubMed] [Google Scholar]
- 8.Klosterkötter J, Schultze-Lutter F, Ruhrmann S. Früherkennungssysteme der schizophrenen Erkrankung. In: Falkai P, Pajonk FG, editors. Psychotische Störungen. Systematische Therapie mit modernen Neuroleptika. Stuttgart, Germany: Georg Thieme Verlag; 2003. pp. 23–35. [Google Scholar]
- 9.Yung AR, Yuen HP, Berger G, et al. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr Bull. 2007;33:673–681. doi: 10.1093/schbul/sbm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultze-Lutter F, Ruhrmann S, Hoyer C, Klosterkötter J, Leweke FM. The initial prodrome of schizophrenia: different duration, different underlying deficits? Compr Psychiatry. 2007;48:479–488. doi: 10.1016/j.comppsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 11.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 12.Köhn D, Pukrop R, Niedersteberg A, et al. Wege in die Behandlung: Hilfesuchverhalten schizophrener Ersterkrankter. Fortschr Neurol Psychiatr. 2004;72:635–642. doi: 10.1055/s-2004-818418. [DOI] [PubMed] [Google Scholar]
- 13.Schultze-Lutter F, Picker H, Ruhrmann S, Klosterkötter J. Das Kölner Früh-Erkennungs- & Therapie-Zentrum für psychische Krisen (FETZ): Evaluation der Inanspruchnahme. Med Klin. 2008;103:81–89. doi: 10.1007/s00063-008-1012-4. [DOI] [PubMed] [Google Scholar]
- 14.Mäki P, Veijola J, Jones PB, et al. Predictors of schizophrenia—a review. Br Med Bull. 2005;73–74:1–15. doi: 10.1093/bmb/ldh046. [DOI] [PubMed] [Google Scholar]
- 15.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman RM, Malla AK, Manchanda R. Delay in treatment for psychosis: its relation to family history. Soc Psychiatry Psychiatr Epidemiol. 2007;42:507–512. doi: 10.1007/s00127-007-0174-3. [DOI] [PubMed] [Google Scholar]
- 17.Addington J, Schultze-Lutter F. Prodromal phase of psychosis in adolescent women. In: Romans S, Seeman MV, editors. Women's Mental Health: A Life Cycle Approach. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 123–132. [Google Scholar]
- 18.Maurer K, Hörrmann F, Trendler G, Schmidt M, Häfner H. Früherkennung des Psychoserisikos mit dem Early Recognition Inventory (ERIraos). Beschreibung des Verfahrens und erste Ergebnisse zur Reliabilität und Validität der Checkliste. Nervenheilkunde. 2006;1–2:11–16. [Google Scholar]
- 19.Miller TJ, McGlashan TH, Lifshey Rosen J, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 20.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 21.Gross G, Huber G, Klosterkötter J, Linz M. Bonner Skala für die Beurteilung von Basissymptomen (BSABS; Bonn Scale for the Assessment of Basic Symptoms) Berlin, Germany: Springer; 1987. [Google Scholar]
- 22.Häfner H, Riecher A, Maurer K, et al. Ein Instrument zur retrospektiven Einschätzung des Erkrankungsbeginns bei Schizophrenie (Instrument for the retrospective assessment of the onset of schizophrenia—“IRAOS”)—Entwicklung und erste Ergebnisse. Z Klin Psychol. 1990;19:230–255. [Google Scholar]
- 23.Häfner H, Maurer K, Trendler G, et al. Schizophrenia and depression: challenging the paradigm of two separate diseases—a controlled study of schizophrenia, depression and healthy controls. Schizophr Res. 2005;77:11–24. doi: 10.1016/j.schres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Maurer K, Häfner H. Methodological aspects of the onset assessment in schizophrenia. Schizophr Res. 1995;15:265–276. doi: 10.1016/0920-9964(94)00051-9. [DOI] [PubMed] [Google Scholar]
- 25.McGorry PD, Yung AR, Phillips LJ, et al. A randomized controlled trial of interventions designed to reduce the risk of progression to first episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 26.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 27.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk. Br J Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 28.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 29.Häfner H, Nowotny B, Löffler W, an der Heiden W, Maurer K. When and how does schizophrenia produce social deficits? Eur Arch Psychiatr Clin Neurosci. 1995;246:17–28. doi: 10.1007/BF02191811. [DOI] [PubMed] [Google Scholar]
- 30.Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Broome RM, Woolley JB, Johns LC, et al. Outreach and support in south London (OASIS): implementation of a clinical service for prodromal psychosis and the at risk mental state. Eur Psychiatry. 2005;20:372–378. doi: 10.1016/j.eurpsy.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Andrade LH, Viana MC, Tófoli LFF, Wang YP. Influence of psychiatric morbidity and sociodemographic determinants on use of service in a catchment area in the city of São Paulo, Brazil. Soc Psychiatry Psychiatr Epidemiol. 2008;43:45–53. doi: 10.1007/s00127-007-0263-3. [DOI] [PubMed] [Google Scholar]
- 33.Steele L, Dewa C, Lee K. Socioeconomic status and self-reported barriers to mental health service use. Can J Psychiatry. 2007;52:201–206. doi: 10.1177/070674370705200312. [DOI] [PubMed] [Google Scholar]
- 34.Pevalin DJ. Socio-economic inequalities in health and service utilization in the London Borough of Newham. Public Health. 2007;121:596–602. doi: 10.1016/j.puhe.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Lampert T. Schichtspezifische Unterschiede im Gesundheitszustand und Gesundheitsverhalten. Berlin, Germany: Berliner Zentrum Public Health, Blaue Reihe; 2005. [Google Scholar]
- 36.Schultze-Lutter F, Klosterkötter J. Do basic symptoms provide a possible explanation for the elevated risk for schizophrenia among mentally retarded? Neurol Psychiatry Brain Res. 1995;3:29–34. [Google Scholar]
- 37.Nelson B, Yung AR, Bechdolf A, McGorry PD. The Phenomenological Critique and Self-disturbances: Implications for Ultra-High Risk (“Prodrome”) Research. Schizophr Bull. 2008;34:381–392. doi: 10.1093/schbul/sbm094. [DOI] [PMC free article] [PubMed] [Google Scholar]