Abstract
Monoclonal antibodies against the porcine 1,25-dihydroxyvitamin D3 receptor were immobilized on Sepharose CL-4B and used to obtain a highly purified 1,25-dihydroxyvitamin D3 receptor fraction with a 45% recovery of the 1,25-dihydroxyvitamin D3 binding capacity. The porcine receptor was purified to homogeneity by preparative electrophoresis and digested in sodium dodecyl sulfate/polyacrylamide gels with Staphylococcus aureus strain V8 protease. The resulting peptides were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis, electrophoretically transferred to polyvinylidene difluoride membranes, and directly sequenced. The generation and isolation of peptides by this method allows sequencing of proteins present in trace amounts as well as those whose amino termini have been modified. The 1,25-dihydroxyvitamin D3 receptor amino acid sequence corresponded to the sequence predicted from a recently cloned receptor cDNA obtained from rat kidney mRNAs.
Full text
PDF

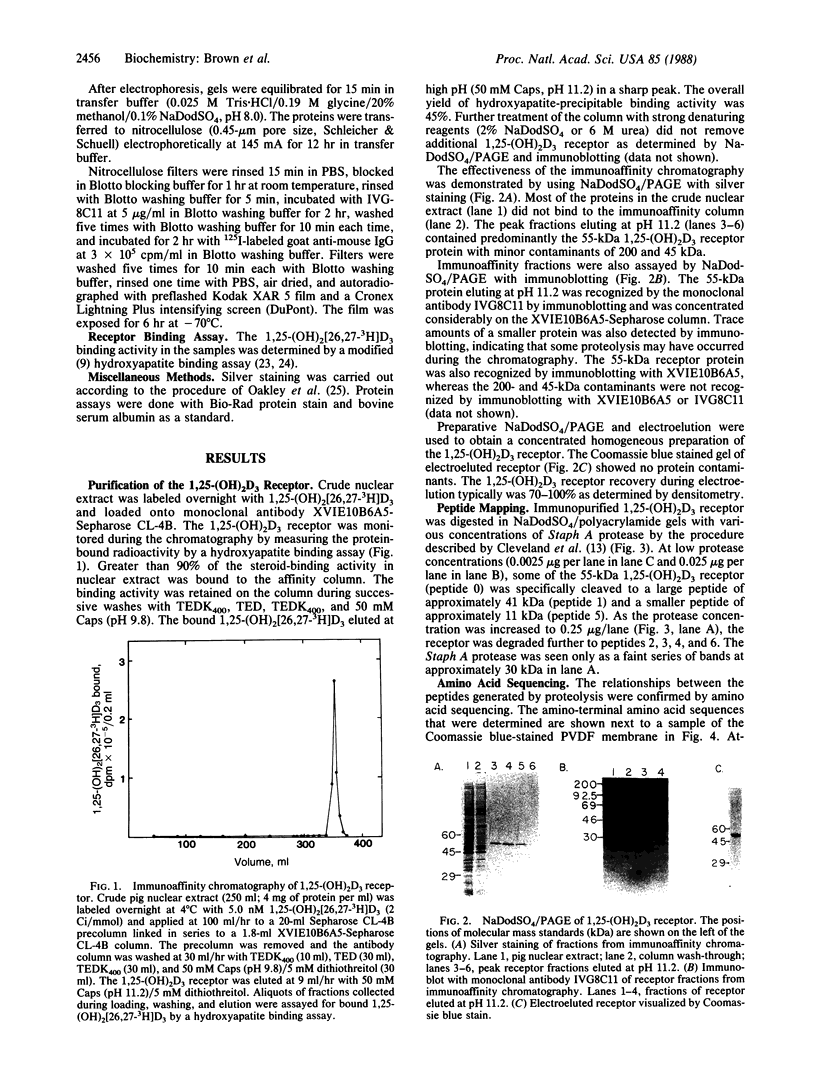
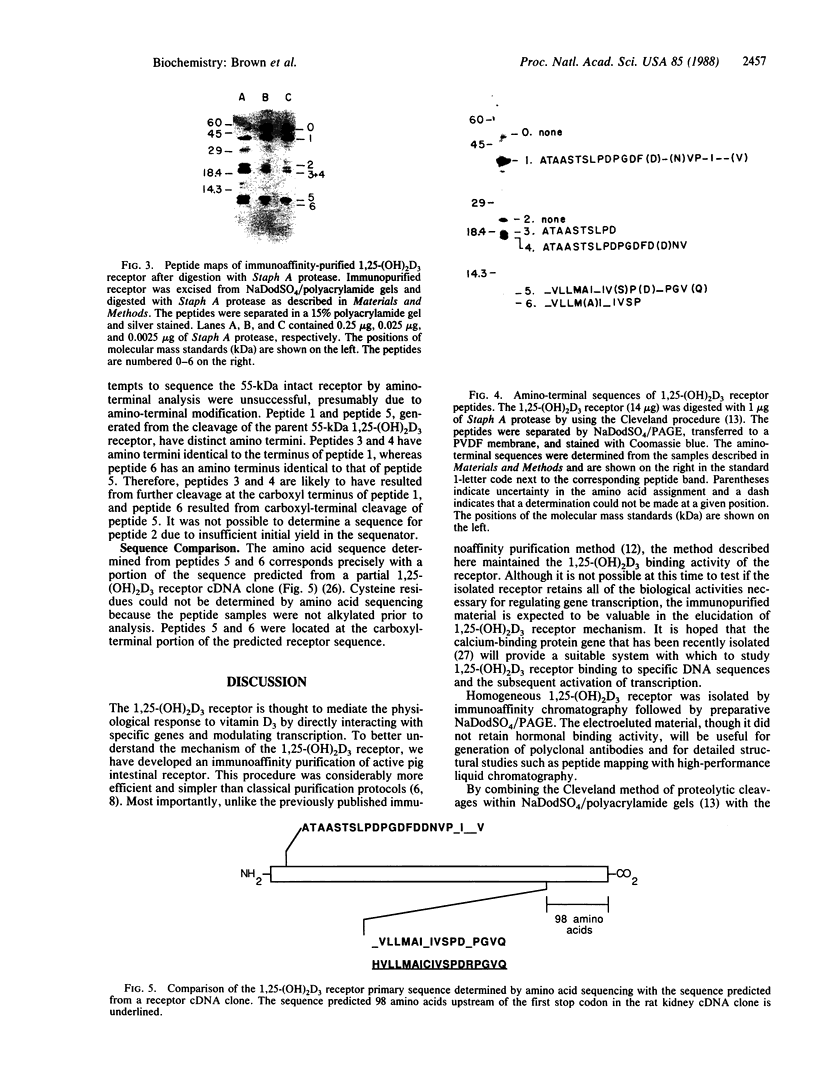

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretto E. A., Pike J. W., Haussler M. R. Immunochemical detection of unique proteolytic fragments of the chick 1,25-dihydroxyvitamin D3 receptor. Distinct 20-kDa DNA-binding and 45-kDa hormone-binding species. J Biol Chem. 1987 Jan 25;262(3):1312–1319. [PubMed] [Google Scholar]
- Burmester J. K., Maeda N., DeLuca H. F. Isolation and expression of rat 1,25-dihydroxyvitamin D3 receptor cDNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1005–1009. doi: 10.1073/pnas.85.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., DeLuca H. F. Identification of the porcine intestinal 1,25-dihydroxyvitamin D3 receptor on sodium dodecyl sulfate/polyacrylamide gels by renaturation and immunoblotting. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7825–7829. doi: 10.1073/pnas.82.23.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., Prahl J. M., Hayes C. E., DeLuca H. F. Monoclonal antibodies to the porcine intestinal receptor for 1,25-dihydroxyvitamin D3: interaction with distinct receptor domains. Biochemistry. 1986 Aug 12;25(16):4523–4534. doi: 10.1021/bi00364a011. [DOI] [PubMed] [Google Scholar]
- Darwish H. M., Krisinger J., Strom M., DeLuca H. F. Molecular cloning of the cDNA and chromosomal gene for vitamin D-dependent calcium-binding protein of rat intestine. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6108–6111. doi: 10.1073/pnas.84.17.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kohn J., Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun. 1982 Aug;107(3):878–884. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Clements P., Carrella M., Strasberg P. M. A high recovery method for concentrating microgram quantities of protein from large volumes of solution. Anal Biochem. 1983 Mar;129(2):513–516. doi: 10.1016/0003-2697(83)90585-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Mellon W. S., Fivizzani M. A., Schnoes H. K., DeLuca H. F. Direct chemical synthesis of 1 alpha,25-dihydroxy[26,27-3H]vitamin D3 with high specific activity: its use in receptor studies. Biochemistry. 1980 May 27;19(11):2515–2521. doi: 10.1021/bi00552a033. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pierce E. A., Dame M. C., DeLuca H. F. Size and charge of the functional 1,25-dihydroxyvitamin D receptor in porcine intestine. J Biol Chem. 1987 Dec 15;262(35):17092–17099. [PubMed] [Google Scholar]
- Pike J. W., Marion S. L., Donaldson C. A., Haussler M. R. Serum and monoclonal antibodies against the chick intestinal receptor for 1,25-dihydroxyvitamin D3. Generation by a preparation enriched in a 64,000-dalton protein. J Biol Chem. 1983 Jan 25;258(2):1289–1296. [PubMed] [Google Scholar]
- Pike J. W., Sleator N. M., Haussler M. R. Chicken intestinal receptor for 1,25-dihydroxyvitamin D3. Immunologic characterization and homogeneous isolation of a 60,000-dalton protein. J Biol Chem. 1987 Jan 25;262(3):1305–1311. [PubMed] [Google Scholar]
- Simpson R. U., DeLuca H. F. Purification of chicken intestinal receptor for 1 alpha, 25-dihydroxyvitamin D3 to apparent homogeneity. Proc Natl Acad Sci U S A. 1982 Jan;79(1):16–20. doi: 10.1073/pnas.79.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M. R. Steroid hormone receptors and the nucleus. Endocr Rev. 1985 Fall;6(4):512–543. doi: 10.1210/edrv-6-4-512. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Norman A. W. An hydroxylapatite batch assay for the quantitation of 1alpha,25-dihydroxyvitamin D3-receptor complexes. Anal Biochem. 1979 Jan 15;92(2):314–323. doi: 10.1016/0003-2697(79)90664-x. [DOI] [PubMed] [Google Scholar]
- Williams D., Gorski J. Equilibrium binding of estradiol by uterine cell suspensions and whole uteri in vitro. Biochemistry. 1974 Dec 31;13(27):5537–5542. doi: 10.1021/bi00724a013. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]