Abstract
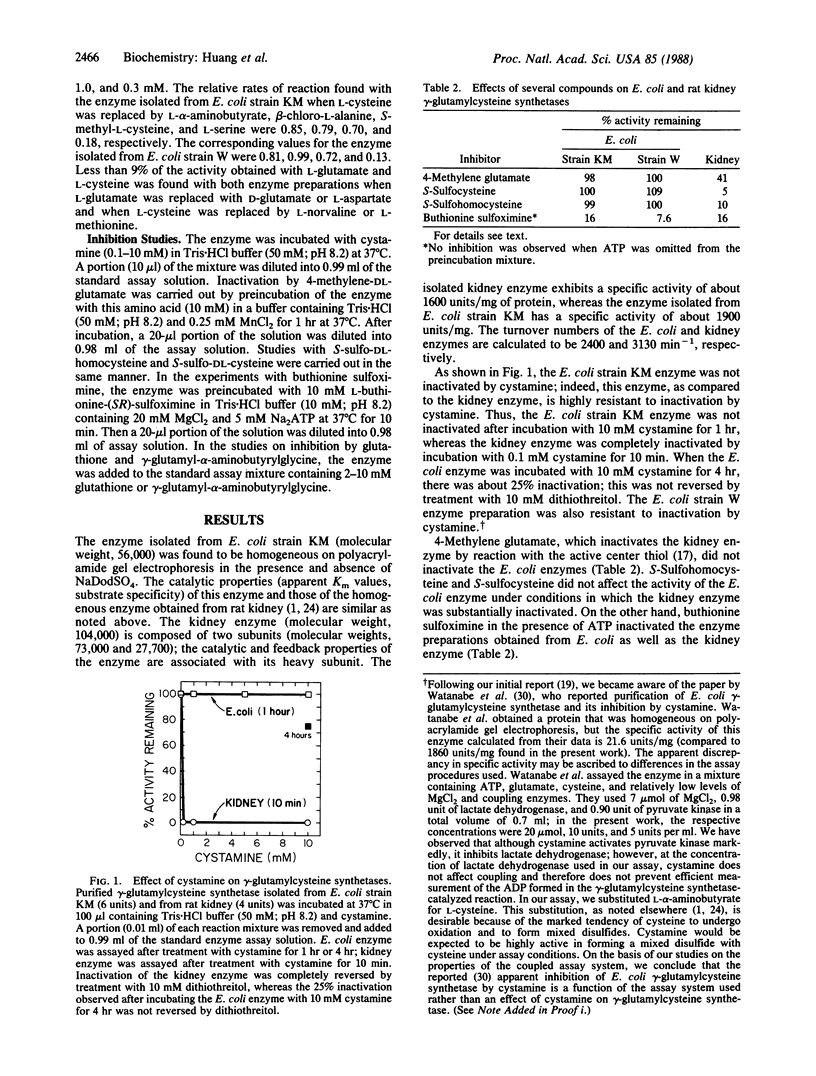
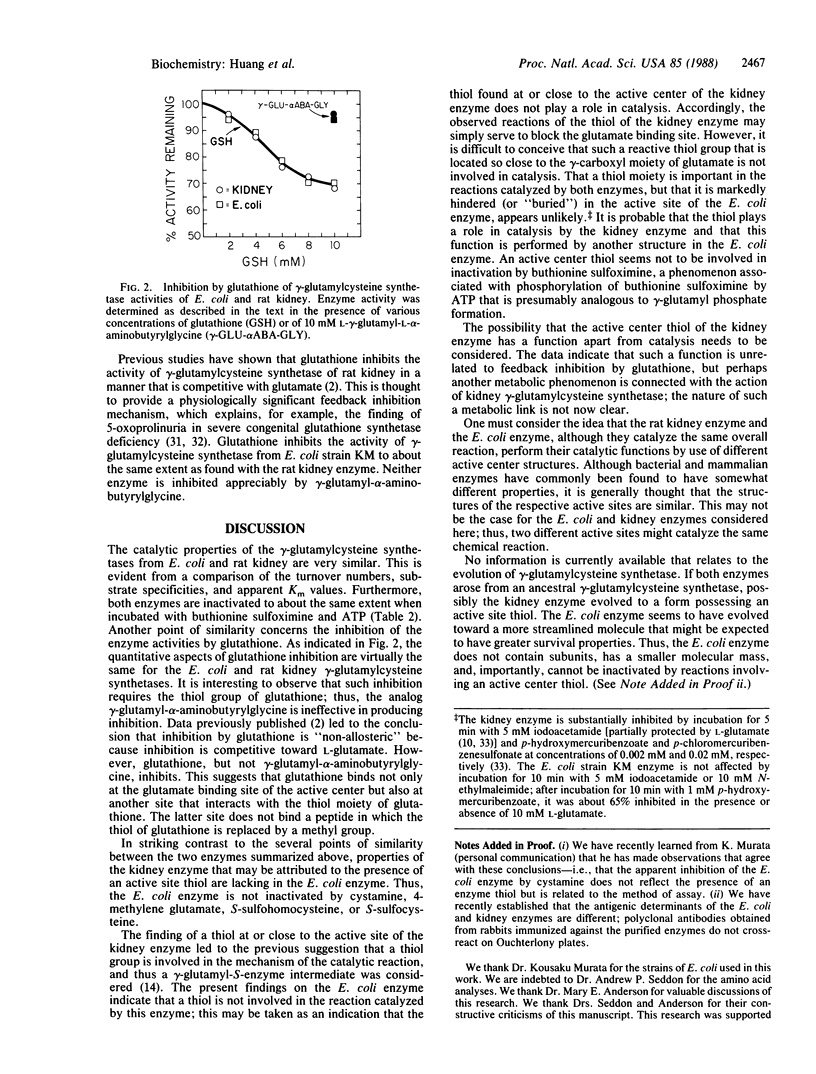
gamma-Glutamylcysteine synthetase (glutamate-cysteine ligase; EC 6.3.2.2) was isolated from an Escherichia coli strain enriched in the gene for this enzyme by recombinant DNA techniques. The purified enzyme has a specific activity of 1860 units/mg and a molecular weight of 56,000. Comparison of the E. coli enzyme with the well-characterized rat kidney enzyme showed that these enzymes have similar catalytic properties (apparent Km values, substrate specificities, turnover numbers). Both enzymes are feedback-inhibited by glutathione but not by gamma-glutamyl-alpha-aminobutyrylglycine; the data indicate that glutathione binds not only at the glutamate binding site but also at a second site on the enzyme that interacts with the thiol moiety of glutathione but not with a methyl group. Both enzymes are inactivated by buthionine sulfoximine in the presence of ATP, suggesting a common gamma-glutamyl phosphate intermediate. However, unlike the rat kidney enzyme that has an active center thiol, the bacterial enzyme is insensitive to cystamine, gamma-methylene glutamate, and S-sulfo amino acids, indicating that it does not have an active site thiol. Thus, the rat kidney and E. coli enzymes share several catalytic features but differ in active site structure. If the active site thiol of the rat kidney enzyme is involved in catalysis, which seems likely, there would appear to be differences in the mechanisms of action of the two gamma-glutamylcysteine synthetases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba S., Tsunekawa H., Imanaka T. New approach to tryptophan production by Escherichia coli: genetic manipulation of composite plasmids in vitro. Appl Environ Microbiol. 1982 Feb;43(2):289–297. doi: 10.1128/aem.43.2.289-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W., Larsson A., Meister A. Inhibition of gamma-glutamylcysteine synthetase by cystamine: an approach to a therapy of 5-oxoprolinuria (pyroglutamic aciduria). Biochem Biophys Res Commun. 1977 Dec 7;79(3):919–925. doi: 10.1016/0006-291x(77)91198-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Gushima H., Miya T., Murata K., Kimura A. Construction of glutathione-producing strains of Escherichia coli B by recombinant DNA techniques. J Appl Biochem. 1983 Feb-Apr;5(1-2):43–52. [PubMed] [Google Scholar]
- Khedouri E., Anderson P. M., Meister A. Selective inactivation of the glutamine binding site of Escherichia coli carbamyl phosphate synthetase by 2-amino-4-oxo-5-chloropentanoic acid. Biochemistry. 1966 Nov;5(11):3552–3557. doi: 10.1021/bi00875a024. [DOI] [PubMed] [Google Scholar]
- Lebo R. V., Kredich N. M. Inactivation of human gamma-glutamylcysteine synthetase by cystamine. Demonstration and quantification of enzyme-ligand complexes. J Biol Chem. 1978 Apr 25;253(8):2615–2623. [PubMed] [Google Scholar]
- Manning J. M., Moore S., Rowe W. B., Meister A. Identification of L-methionine S-sulfoximine as the diastereoisomer of L-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969 Jun;8(6):2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- Moore W. R., Meister A. Enzymatic synthesis of novel glutathione analogs. Anal Biochem. 1987 Mar;161(2):487–493. doi: 10.1016/0003-2697(87)90478-7. [DOI] [PubMed] [Google Scholar]
- Moore W., Wiener H. L., Meister A. Inactivation of gamma-glutamylcysteine synthetase, but not of glutamine synthetase, by S-sulfocysteine and S-sulfohomocysteine. J Biol Chem. 1987 Dec 15;262(35):16771–16777. [PubMed] [Google Scholar]
- Murata K., Abbott W. A., Bridges R. J., Meister A. Glutathione specifically labeled with isotopes. Anal Biochem. 1985 Oct;150(1):235–237. doi: 10.1016/0003-2697(85)90464-6. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. Isolation of highly purified gamma-glutamylcysteine synthetase from rat kidney. Biochemistry. 1971 Feb 2;10(3):372–380. doi: 10.1021/bi00779a003. [DOI] [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Richman P. G., Orlowski M., Meister A. Inhibition of gamma-glutamylcysteine synthetase by L-methionine-S-sulfoximine. J Biol Chem. 1973 Oct 10;248(19):6684–6690. [PubMed] [Google Scholar]
- Ronzio R. A., Meister A. Phosphorylation of methionine sulfoximine by glutamine synthetase. Proc Natl Acad Sci U S A. 1968 Jan;59(1):164–170. doi: 10.1073/pnas.59.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Cystamine-Sepharose. A probe for the active site of gamma-glutamylcysteine synthetase. J Biol Chem. 1982 May 10;257(9):5092–5096. [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Gamma-glutamylcysteine synthetase. Interactions of an essential sulfhydryl group. J Biol Chem. 1984 Mar 25;259(6):3534–3538. [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol. 1985;113:379–390. doi: 10.1016/s0076-6879(85)13050-8. [DOI] [PubMed] [Google Scholar]
- Sekura R., Meister A. Covalent interaction of L-2-amino-4-oxo-5-chloropentanoate at glutamate binding site of gamma-glutamylcysteine synthetase. J Biol Chem. 1977 Apr 25;252(8):2606–2610. [PubMed] [Google Scholar]
- Sekura R., Meister A. gamma-Glutamylcysteine synthetase. Further purification, "half of the sites" reactivity, subunits, and specificity. J Biol Chem. 1977 Apr 25;252(8):2599–2605. [PubMed] [Google Scholar]
- Watanabe K., Yamano Y., Murata K., Kimura A. The nucleotide sequence of the gene for gamma-glutamylcysteine synthetase of Escherichia coli. Nucleic Acids Res. 1986 Jun 11;14(11):4393–4400. doi: 10.1093/nar/14.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wellner V. P., Sekura R., Meister A., Larsson A. Glutathione synthetase deficiency, an inborn error of metabolism involving the gamma-glutamyl cycle in patients with 5-oxoprolinuria (pyroglutamic aciduria). Proc Natl Acad Sci U S A. 1974 Jun;71(6):2505–2509. doi: 10.1073/pnas.71.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]


