Abstract
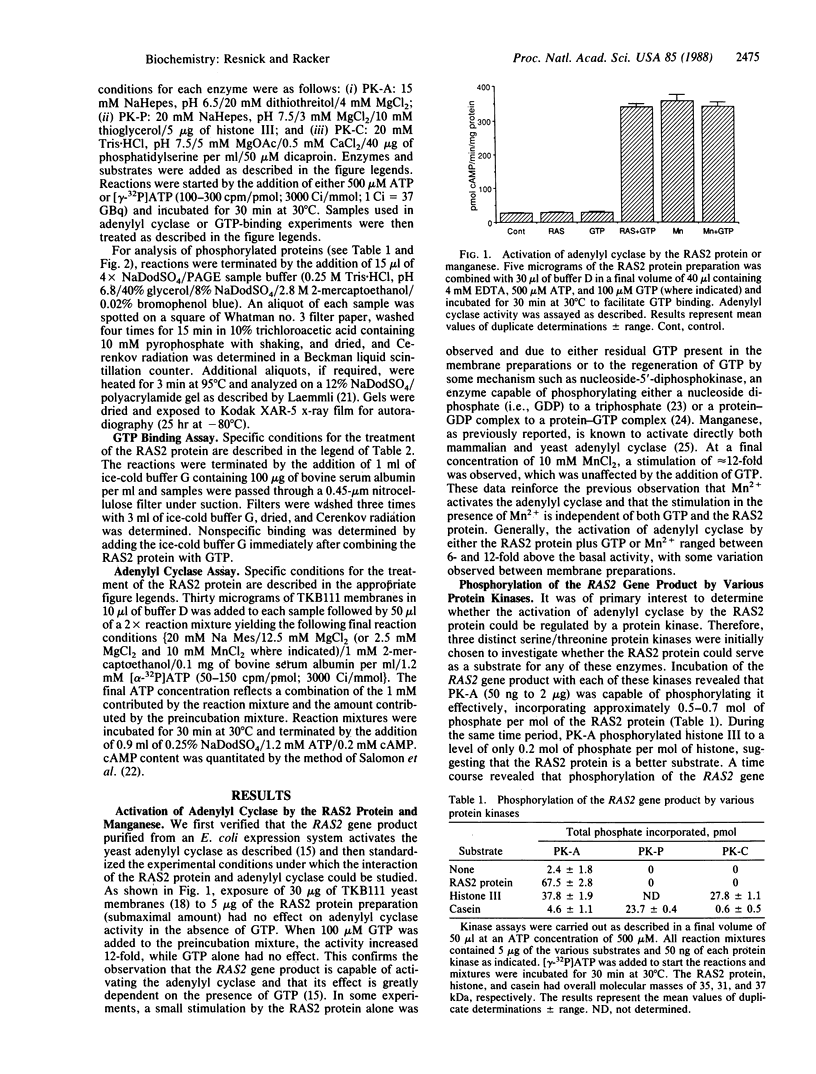
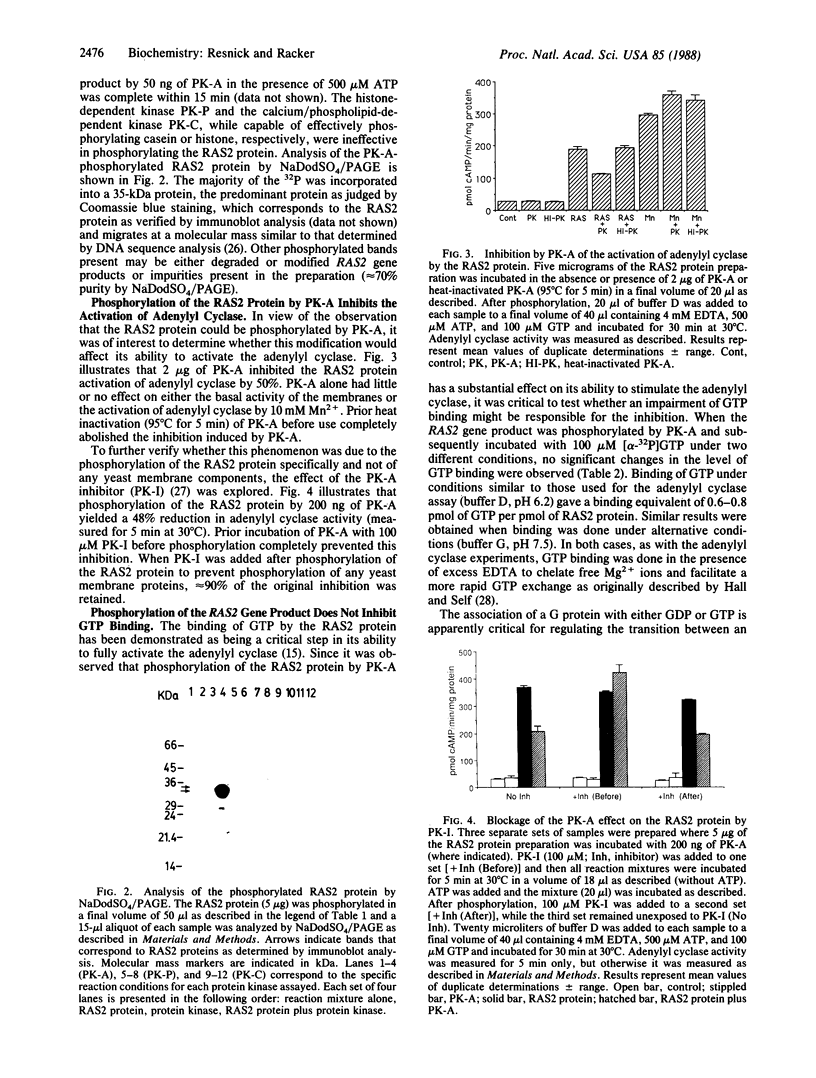
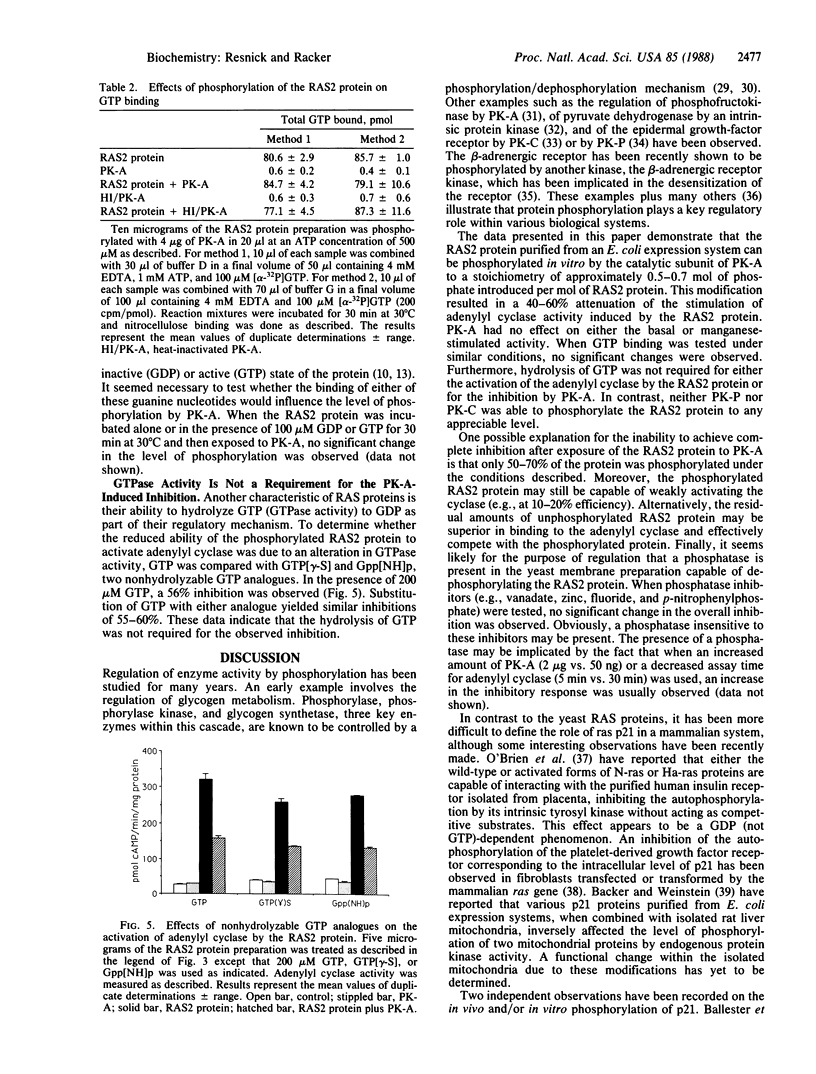
The RAS2 gene product of Saccharomyces cerevisiae expressed in Escherichia coli was phosphorylated by protein kinase A in vitro to approximately 0.5-0.7 mol of phosphate per mol of protein. Neither protein kinase C nor protein kinase P phosphorylated the RAS2 protein significantly. The RAS2 protein is known to activate, in the presence of either Mg2+ and GTP or Mn2+, a yeast membrane preparation with an overexpressed adenylyl cyclase and a deficiency in endogenous RAS1 and RAS2 proteins. When the RAS2 protein was phosphorylated by protein kinase A prior to exposure to the yeast membranes, its capacity to activate the adenylyl cyclase was diminished by 40-60%, while activation by Mn2+ remained unaffected. The phosphorylated protein retained, however, its ability to bind GTP. Incubation of protein kinase A with a specific protein kinase A inhibitor prior to phosphorylation prevented the inhibition. Furthermore, the hydrolysis of GTP was not required for the observed inhibition. These data suggest that phosphorylation of the RAS2 gene product by protein kinase A may function as one mechanism by which the intracellular level of cAMP in yeast is regulated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Ghany M., Kole H. K., Racker E. Effect of protein kinase P on phosphorylations catalyzed by the epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8888–8892. doi: 10.1073/pnas.84.24.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG P., JOKLIK W. K. Enzymatic phosphorylation of nucleoside diphosphates. J Biol Chem. 1954 Oct;210(2):657–672. [PubMed] [Google Scholar]
- Backer J. M., Weinstein I. B. Proteins encoded by ras oncogenes stimulate or inhibit phosphorylation of specific mitochondrial membrane proteins. Biochem Biophys Res Commun. 1986 Feb 26;135(1):316–322. doi: 10.1016/0006-291x(86)90979-4. [DOI] [PubMed] [Google Scholar]
- Ballester R., Furth M. E., Rosen O. M. Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J Biol Chem. 1987 Feb 25;262(6):2688–2695. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- Casperson G. F., Walker N., Brasier A. R., Bourne H. R. A guanine nucleotide-sensitive adenylate cyclase in the yeast Saccharomyces cerevisiae. J Biol Chem. 1983 Jul 10;258(13):7911–7914. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Field J., Broek D., Kataoka T., Wigler M. Guanine nucleotide activation of, and competition between, RAS proteins from Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jun;7(6):2128–2133. doi: 10.1128/mcb.7.6.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. On ras gene function in yeast. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4740–4744. doi: 10.1073/pnas.82.14.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hall A., Self A. J. The effect of Mg2+ on the guanine nucleotide exchange rate of p21N-ras. J Biol Chem. 1986 Aug 25;261(24):10963–10965. [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng A. Y., Srivastava S. K., Lacal J. C., Blumberg P. M. Phosphorylation of ras oncogene product by protein kinase C. Biochem Biophys Res Commun. 1987 Jun 15;145(2):782–788. doi: 10.1016/0006-291x(87)91033-3. [DOI] [PubMed] [Google Scholar]
- Kagimoto T., Uyeda K. Hormone-stimulated phosphorylation of liver phosphofructokinase in vivo. J Biol Chem. 1979 Jul 10;254(13):5584–5587. [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayor F., Jr, Benovic J. L., Caron M. G., Lefkowitz R. J. Somatostatin induces translocation of the beta-adrenergic receptor kinase and desensitizes somatostatin receptors in S49 lymphoma cells. J Biol Chem. 1987 May 15;262(14):6468–6471. [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Cameron S., Toda T., Ferguson K. M., Wigler M. Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1987 Nov;1(9):931–937. doi: 10.1101/gad.1.9.931. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Siddle K., Houslay M. D., Hall A. Interaction of the human insulin receptor with the ras oncogene product p21. FEBS Lett. 1987 Jun 15;217(2):253–259. doi: 10.1016/0014-5793(87)80673-7. [DOI] [PubMed] [Google Scholar]
- Parries G., Hoebel R., Racker E. Opposing effects of a ras oncogene on growth factor-stimulated phosphoinositide hydrolysis: desensitization to platelet-derived growth factor and enhanced sensitivity to bradykinin. Proc Natl Acad Sci U S A. 1987 May;84(9):2648–2652. doi: 10.1073/pnas.84.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Fischer E. H., Krebs E. G. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Suard Y., Perucho M., Li Y., Kamata T., Feramisco J., Stavnezer E., Fogh J., Wigler M. H. Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2112–2116. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Uesaka H., Yokoyama M., Ohtsuki K. Physiological correlation between nucleoside-diphosphate kinase and the enzyme-associated guanine nucleotide binding proteins. Biochem Biophys Res Commun. 1987 Mar 13;143(2):552–559. doi: 10.1016/0006-291x(87)91389-1. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Hunter T. Isolation and characterization of two distinct forms of protein kinase C. J Biol Chem. 1987 Apr 5;262(10):4836–4843. [PubMed] [Google Scholar]
- Yanagita Y., Abdel-Ghany M., Raden D., Nelson N., Racker E. Polypeptide-dependent protein kinase from bakers' yeast. Proc Natl Acad Sci U S A. 1987 Feb;84(4):925–929. doi: 10.1073/pnas.84.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]