Abstract
The inducible isoform of the enzyme cyclooxygenase-2 (COX2) is an immediate early gene induced by synaptic activity in the brain. COX2 activity is an important mediator of inflammation, but it is not known whether COX2 activity is pathogenic in brain. To study the role of COX2 activity in ischemic injury in brain, expression of COX2 mRNA and protein and the effect of treatment with a COX2 inhibitor on neuronal survival in a rat model of global ischemia were determined. Expression of both COX2 mRNA and protein was increased after ischemia in CA1 hippocampal neurons before their death. There was increased survival of CA1 neurons in rats treated with the COX2-selective inhibitor SC58125 {1-[(4-methylsulfonyl) phenyl]-3-trifluoro-methyl-5-[(4-fluoro)phenyl] pyrazole} before or after global ischemia compared with vehicle controls. Furthermore, hippocampal prostaglandin E2 concentrations 24 h after global ischemia were decreased in drug-treated animals compared with vehicle-treated controls. These results suggest that COX2 activity contributes to CA1 neuronal death after global ischemia.
Cyclooxygenase (prostaglandin G/H synthase) is the first committed step in the production of prostaglandins and thromboxanes. Two forms of the cyclooxygenase enzyme have been cloned. Cyclooxygenase-1 (COX1) is constitutively expressed in many tissues including platelets, gastrointestinal mucosa, and kidney (1–3). The inducible form, cyclooxygenase-2 (COX2), is primarily expressed in leukocytes and brain (4). Its expression is induced by cytokines and inhibited by glucocorticoids, and it is an important mediator of cell injury in inflammation (5–7). Transcription of COX2 mRNA does not require new protein synthesis; therefore, it is an immediate early gene (6). The rat brain COX2 is identical to the non-nervous system COX2 (8). Its mRNA expression is rapidly induced by synaptic activity but blocked by the N-methyl-d-aspartate receptor antagonist MK801 (8). This finding suggests that COX2 transcription is induced by increased intraneuronal Ca2+. COX2 is found in dendrites of neurons that receive excitatory input (9). Thus, COX2 may produce rapid neuronal responses to synaptic activity.
Neuronal excitation and increased intracellular calcium, two stimuli that induce expression of COX2, are also important in the pathophysiology of neuronal death in ischemia and a variety of neurodegenerative diseases (10, 11). In nonneural cells, COX2 activity mediates inflammatory injury (12); however, COX2 overexpression may prevent apoptosis in intestinal epithelium (13). What role COX2 expression and activity have in mediating injury after global ischemia in brain is unknown. To address this question, the expression of COX2 mRNA and protein was studied in rat brain after global ischemia, and the effect of treatment with a selective inhibitor of COX2 {SC58125; 1-[(4-methylsulfonyl)phenyl]-3-trifluoro-methyl-5-[(4-fluoro)phenyl] pyrazole} on hippocampal neuronal survival and prostaglandin E2 (PGE2) concentrations was determined.
METHODS
Animal Model.
All studies were performed under protocols approved by the University of Pittsburgh. Male Sprague–Dawley rats weighing 275–300 g (Charles Rivers Breeding Laboratories) were used for all studies. Anesthesia was induced with 3–4% halothane, then rats were intubated and ventilated with 1.5% halothane. In some experiments, a thermistor was stereotaxically placed in the right striatum and brain temperature was recorded during and for 30 min after ischemia. Samples for arterial blood gas and blood glucose analysis were withdrawn before and during induction of ischemia. Animals were warmed with a heating pad and thermostatically controlled heating lamp. Global ischemia was induced by the four-vessel occlusion method with modifications (14). The vertebral arteries were exposed and coagulated between the first and second cervical vertebrae. The paravertebral and cervical branches were also coagulated. Then, while anesthesia was maintained, the carotid arteries were exposed and transiently occluded by a surgical clip. The electorencephalogram (EEG) was monitored as described (15), and rats were excluded from the study if their EEG did not become isoelectric during ischemia.
In situ hybridization was performed by using the previously described method (16). Rats were anesthetized with isoflurane and decapitated at 0 h (sham operation), 0.5 h, 2 h, 4 h, 8 h, 24 h, or 72 h after ischemia (n = 3/group). Their brains were rapidly removed and frozen in 2-methylbutane at −30°C. Coronal sections (20 μm) were cut on a cryostat and collected on Probe-On-Slides (Fisher). An oligonucleotide probe complementary to nucleotides 1421–1460 of rat COX2 mRNA was labeled with [35S]dATP at the 3′ end by using terminal deoxynucleotidyltransferase (GIBCO/BRL). This probe hybridizes with a single size of mRNA appropriate to rat brain COX2 mRNA in samples from both normal and kainate-treated brain (17). After hybridization at 37°C for 18 h with 107 cpm/ml of the probe, the sections were rinsed in 150 mM sodium chloride and 15 mM sodium citrate (pH 7.4) at 52°C for 60 min and exposed on Kodak SB-5 film for 3 weeks. All sections from the same level were exposed on a single film.
Western Blotting.
Rats were sacrificed at 8 h, 16 h, or 24 h after 20-min ischemia (n = 3/group). Controls consisted of hippocampi from rats that had not had previous ischemia or anesthesia (naïve controls) or hippocampi removed 24 h after the identical anesthesia and surgery except that the arteries were not occluded (sham-operated animals). The hippocampi were dissected, frozen on dry ice, homogenized, and lysed. A total of 40 μg of protein from each sample was loaded onto a 12% SDS/polyacrylamide gel. Western blots were performed as described (18) by using a chemiluminescent detection system. A mouse mAb (9) was used as the primary antibody. Each gel contained lanes from each time point and control brains. Density × area measurements of each band were determined by the mcid gel analysis software program (Imaging Research, St. Catherine’s, Ontario). Density of ischemic samples was expressed in terms of their ratio to naïve control lanes. Murine recombinant COX2 protein was used as a preabsorption control as described (9).
Immunocytochemistry.
Twenty-four hours after 20 min of global ischemia or sham operation, rats received 100 units/kg heparin i.p. and were anesthetized with 8% chloral hydrate (400 mg/kg). Rats were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed, postfixed for at least 4 h, sectioned at 50 μm on a sliding microtome, and processed for immunocytochemical staining. The sections were tested for the expression of COX2 protein by using a mouse mAb (9). Immunocytochemistry was performed by using the avidin-biotin-horseradish peroxidase technique. Sections were placed in phosphate buffer (PB) containing 2% horse serum (HS), 0.2% Triton X-100, and 0.1% BSA for 2 h at room temperature. This result was followed by a 72-h incubation at 4°C in the COX2 antibody diluted 1:5000 in PB-HS, and then with a biotinylated secondary antibody for 2 h. Sections were placed in an avidin-horseradish peroxidase solution for 2 h, washed twice with PB, and treated for horseradish peroxidase by using 0.015% diaminobenzidine and 0.001% hydrogen peroxide. For controls, alternate sections were incubated in the absence of a primary antibody and murine recombinant COX2 protein was used as a preabsorption control as described (19).
PGE2 Concentration.
The hippocampi were dissected and rapidly frozen in liquid N2. Prostaglandins were isolated from tissue as described (19). The tissue was weighed and homogenized in 0.5 mM Tris⋅HCl (pH 7.4), and prostaglandins were extracted with methanol. The tissue was centrifuged and the supernatant diluted with 0.1 mM phosphate buffer (pH 4.0) and loaded on a C18 Sep-Pack (Waters). The Sep-Pak was rinsed with distilled water and hexane, then prostaglandins were eluted with ethyl alcohol. PGE2 was then determined by enzyme-linked immunoassay (Cayman Chemicals, Ann Arbor MI). Two drugs were used to study the effect of COX2 selective and nonselective COX inhibitors on PGE2 concentrations in normal brain and after ischemia. SC58125, a COX2 inhibitor that is 1,000-fold selective for COX2 compared with COX1 (20) and that penetrates the blood-brain barrier (21), was administered. Drugs of this class bind tightly to the COX2 protein (22). SC58125 has a half-life of greater than 24 h. Piroxicam also has a half-life of greater than 24 h, but is a nonselective COX inhibitor (23). To determine the effect of these drugs on basal concentrations of PGE2, 0, 0.03, 0.3, 3, or 30 mg/kg of SC58125 or piroxicam was administered by gastric lavage 2 h before sacrifice. To determine the effect on PGE2 concentrations after ischemia, drugs were administered 30 min before global ischemia and hippocampi removed 24 h after ischemia for PGE2 analysis.
Histology.
Rats were anesthetized and perfused transcardially with normal saline (200 ml) and 4% paraformaldehyde (400 ml, 0.1 M phosphate buffer at pH 7.4) and kept in the same fixative for 3 days at 4°C. Brains were cut in 2-mm-thick slices and then embedded in paraffin. Paraffin blocks were cut in 6-μm-thick coronal sections from bregma −4.5 mm and stained with cresyl violet. The number of surviving cells in three 0.41 mm2 regions in CA1a, CA1b, and CA1c sectors in the right hippocampus of each brain from three adjacent sections were counted by a blinded observer by using a described method (24). The number of surviving cells in these three regions was expressed as a number of surviving cells in CA1. Whole neurons containing nuclei were counted. Cell counts were corrected by Abercrombie’s method by using mean nuclear diameter and slice thickness (25). Neurons with ischemic changes consisting of shrunken cytoplasm and dark pyknotic nuclei, or neurons that had progressed to homogenizing changes or naked nuclei, were not counted (26).
SC58125 or 3% methylcellulose vehicle was administered by gastric lavage. Subsequent doses were given 24 and 48 h after ischemia. Rats were briefly anesthetized with 3% halothane for gastric lavage and measurement of rectal temperature. Rats were randomized in four experiments to evaluate the effect of COX2 inhibition upon histological outcome. In the first experiment, 3 or 30 mg/kg of SC58125 or vehicle was administered 30 min before, and 24 and 48 h after, 20 min of global ischemia. Histological outcome was determined at 72 hr after ischemia (n = 10/group). Brain temperature was not measured. In the second experiment, 3 mg/kg SC58125 or vehicle was administered 30 min before 15 min of global ischemia and again at 24 and 48 h after ischemia (n = 10/group). Brain temperature was measured by a temperature probe inserted into the striatum. During ischemia, a heating lamp maintained brain temperature at 35.0 ± 0.2°C. Rats were sacrificed 14 days after ischemia. In a third experiment, 3 mg/kg SC58125 or vehicle was administered 1 h after 15 min of ischemia and 24 and 48 h after ischemia (n = 6–7/group). Brain temperature was maintained at 37.0 ± 0.2°C as described (27). Rats were sacrificed 14 days after ischemia. In a fourth experiment, the effect of maximally effective doses of SC58125 and piroxicam were compared. Brain temperature was maintained at 35.0 ± 0.2°C during ischemia. A fifth group consisted of naïve controls.
RESULTS
COX2 mRNA Expression Is Increased in Hippocampus After Global Ischemia.
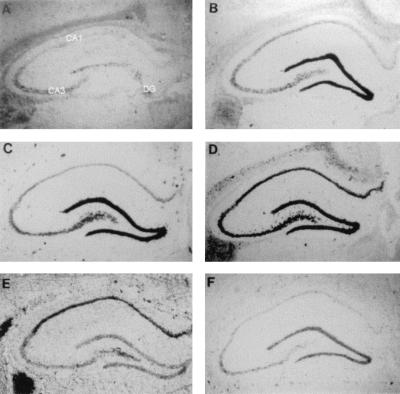
In situ hybridization was used to study COX2 mRNA expression after ischemia. In naïve control brains (Fig. 1A), there was modest expression of COX2 mRNA in the dentate gyrus (DG) granule cell neurons and in CA3, but only a faint signal in CA1, detected by in situ hybridization. COX2 mRNA expression increased rapidly in hippocampus following global ischemia (Fig. 1 B–F). At 2 h after ischemia, there was increased expression in the DG, and by 8 h there was increased expression throughout the hippocampus. Twenty-four hours after ischemia there was persistent increased expression in CA1. At 72 h after ischemia (Fig. 1F), when CA1 neurons have died, COX2 expression in CA1 was almost absent, and had returned nearly to pre-ischemia levels in DG.
Figure 1.
Dipped emulsion photomicrographs of COX2 in situ hybridization of coronal sections of the rat brain after global ischemia. Shown are nonischemic rat (A), and photos 2 h (B), 4 h (C), 8 h (D), 24 h (E), and 72 h (F) after 20 min of global ischemia.
COX2 Protein Expression Is Also Increased After Global Ischemia.
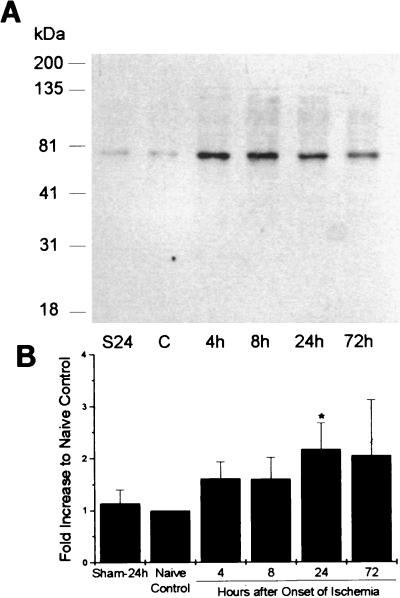
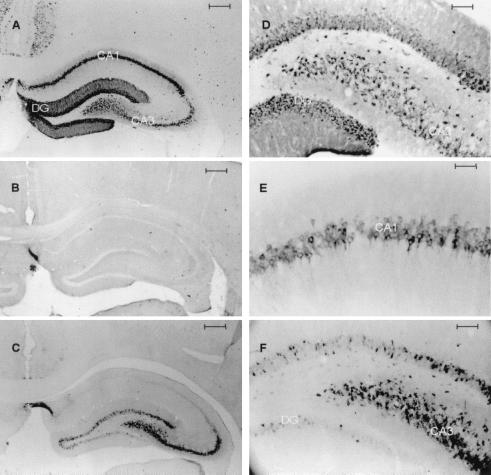
In nonischemic control brains, there was some basal expression of COX2 shown in Western blots of hippocampal homogenates (Fig. 2). There was a single major band at ≈70 kDa, corresponding to the nonglycosylated form of COX2. There was no significant difference in expression of COX2 protein between hippocampi removed 24 h after sham operation and naïve control hippocampi. After ischemia, there was significantly increased density of the band at 24 h after ischemia. When the antibody was incubated with murine recombinant COX2, no band was evident in control or ischemic samples (data not shown). Immunocytochemical staining of brain sections (Fig. 3) showed that there was constitutive expression of COX2 protein in neurons in naïve controls, but no immunoreactivity was evident in glial or endothelial cells. In brains obtained from rats 24 h after sham operation, there was constitutive expression primarily in CA3 and DG neurons within the hippocampus (Fig. 3 C and F), but only faint immunoreactivity in CA1 neurons. At 24 h after ischemia, there was increased expression in CA1, CA3 and DG (Fig. 3 A, D, and E). There was also increased immunoreactivity in many scattered neurons in superficial cortex after global ischemia (data not shown). There was no immunoreactivity within neurons when the recombinant murine COX2 protein was used to preabsorb the antibody (Fig. 3B).
Figure 2.
Protein expression. (A) Western blot analysis of protein extracts obtained from rat hippocampi at 4 h, 8 h, 24 h, and 72 h after 15 min of global ischemia and nonischemic controls (C) and hippocampi 24 h after sham operation (S24). (B) Graph summarizing results of Western blots from four to six animals/group. Changes in COX2 expression are expressed as ratio to control lane optical density × area measurements (n = 4–6/group). ∗, P < 0.05 greater than control, Mann–Whitney U test; mean ± SE.
Figure 3.
Photomicrographs of COX2 immunocytochemistry from coronal sections through anterior hippocampus: 24 h after 15 min of global ischemia (A); 24 h after global ischemia, antibody preabsorbed with recombinant murine COX2 protein (B); 24 h after sham operation (C); hylar region, 24 h after global ischemia (D); CA1 24 h after global ischemia (E); and hylar region, 24 h after sham operation (F). [Bars = 400 μm (A–C), 50 μm (D and F), and 25 μm (E).]
Treatment with SC58125 Decreases Hippocampal PGE2 Concentrations.
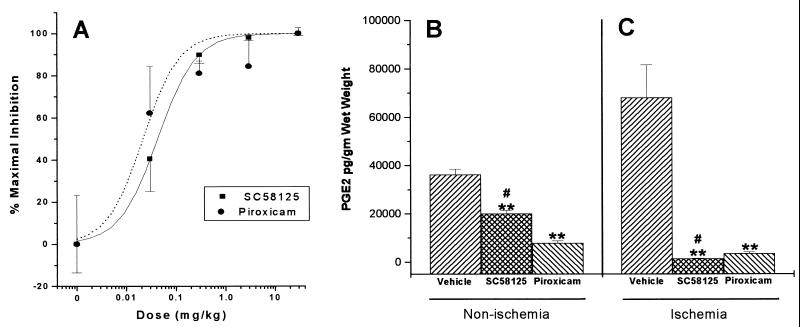
The effect of the nonselective COX1/COX2 inhibitor piroxicam on PGE2 concentration in nonischemic hippocampus was compared with the effect of the selective COX2 inhibitor SC58125. The ED50 values for piroxicam (0.02 ± 0.01 mg/kg) and SC58125 (0.04 ± 0.01 mg/kg) in reducing PGE2 in nonischemic brain were similar (Fig. 4A); however, piroxicam was more potent in reducing basal PGE2 concentrations than SC58125 (Fig. 4B). SC58125 treatment reduced mean PGE2 concentrations to about 50% of control levels, whereas piroxicam treatment almost completely abolished PGE2 content. Because both COX1 and COX2 are expressed in normal brain (28), these data are consistent with selective COX2 inhibition by SC58125. Twenty-four hours after global ischemia, there was a large increase in hippocampal PGE2. Treatment with maximally effective doses of piroxicam (30 mg/kg) and SC58125 (30 mg/kg) reduced hippocampal PGE2 content; however, SC58125-treated hippocampi contained less PGE2 than piroxicam-treated hippocampi after ischemia (Fig. 4C). These data suggest that most of the ischemia-induced increase in PGE2 originates from COX2 activity.
Figure 4.
Effect of drug treatment upon hippocampal PGE2 concentrations. (A) Dose-response relationship of SC58125 and piroxicam upon PGE2 concentrations in nonischemic brain. Data shown as percent of maximal effect. (B) Effect of treatment with maximally effective doses of SC58125 (30 mg/kg) and piroxicam (30 mg/kg) upon PGE2 concentration in nonischemic hippocampus. (C) Effect of treatment with maximally effective doses of SC58125 (30 mg/kg) and piroxicam (30 mg/kg) upon PGE2 concentrations in hippocampus after global ischemia. ∗∗, P < 0.01 different than nonischemic controls; #, P < 0.05 different than piroxicam-treated group; ANOVA; mean ± SE.
Treatment with SC58125 Decreases Neuronal Death in CA1 Hippocampus After Ischemia.
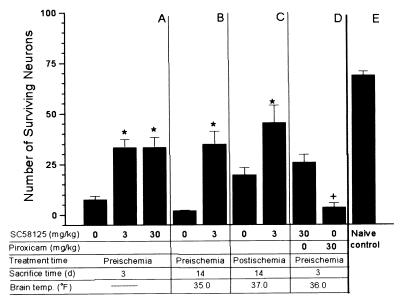
The observation that COX2 protein expression was persistently expressed in CA1 neurons that die and in DG neurons that were resistant to ischemia could be consistent with either a death-promoting or death-suppressing role for COX2 in ischemia. To investigate this question, the COX2-selective inhibitor SC58125 was used in four experiments. In experiment 1, SC58125 (0, 3, or 30 mg/kg p.o.) was given before the onset of ischemia and again 24 and 48 h after ischemia. There was almost complete loss of neurons in CA1 72 h after ischemia in vehicle-treated animals. In drug-treated animals many neurons were preserved and had a normal histological appearance, although there were occasional pyknotic neurons. More CA1 neurons survived in both the 3 and 30 mg/kg drug-treated groups compared with vehicle controls (Fig. 5A). In experiment 2, where rats survived 14 days, there was also increased survival of CA1 neurons 14 days after ischemia (Fig. 5B). There were no significant differences in brain temperature during or after ischemia between the drug- and vehicle-treated groups. In experiment 3, drug or vehicle was administered 1 h after global ischemia. There was significantly increased survival of CA1 neurons in drug-treated rats 14 days after ischemia (Fig. 5C). In experiment 4, we compared the maximally effective dose of SC58125 (30 mg/kg) with a dose of piroxicam that maximally inhibits basal PGE2 (30 mg/kg). This dose of piroxicam exceeds the single 10 mg/kg dose that maximally protected CA1 neurons in a previous global ischemia experiment (29). Treatment with SC58125 resulted in increased neuronal survival compared with piroxicam (Fig. 5D). Fig. 5E illustrates the results of counting CA1 neurons by using these methods in normal rats not subjected to ischemia or anesthesia. No treatment group reached the normal number of neurons per high power field; thus, the protection was not complete. There were no significant differences in mean arterial pressure, blood glucose, or arterial blood gases either before or during ischemia between drug- and vehicle-treated animals in any of the drug treatment experiments. Nor were there significant differences in rectal temperature during ischemia, at 24 and 48 h after ischemia, or at the time of sacrifice.
Figure 5.
Graph summarizing effect of SC58125 and piroxicam treatment upon CA1 neuronal counts in cresyl violet-stained sections. Brain temp. refers to minimum brain temperature during ischemia, if monitored. Treatment time refers to the first time that drugs were administered, either before (preischemia) or after global ischemia (postischemia). Sacrifice time refers to the time at which rats were euthanized for histology. (A) Animals were treated 15 min before and at 24 and 48 h after induction of 20 min of global ischemia. Rats were sacrificed 72 h after ischemia (n = 10/group). (B) Animals were treated 15 min before, and 24 and 48 h after, 15 min of global ischemia. Rats were sacrificed at 14 days (n = 10/group). (C) Animals were treated 1 h after 15 min of global ischemia. Rats were sacrificed at 14 days (n = 7–8/group). (D) Comparison of treatment with SC58125 30 mg/kg and piroxicam 30 mg/kg upon neuronal survival at 3 days (n = 8/group). (E) Naïve control rats (n = 5). ∗, P < 0.05 greater than vehicle-treated group; +, P < 0.05 different than SC58125-treated rats; ANOVA; mean ± SE.
DISCUSSION
Although it has been known for decades that cyclooxygenase inhibitors decrease ischemic injury, it has been assumed that prostaglandins exacerbate damage by their effects on inflammation or blood flow. Recently, treatment with a COX2 selective inhibitor has been shown to decrease damage after temporary focal ischemia (30). Because secondary hypoperfusion and inflammation are important in this model, the observed neuroprotection could be caused by these mechanisms. However, in the global ischemia model used in these studies, there is little evidence that secondary ischemia or inflammation contributes to the death of CA1 neurons (31, 32). Instead, the delayed death of neurons in CA1 appears to be the result of events that occur within the neuron (33). The observation that protein synthesis inhibitors prevent this delayed neuronal death after global ischemia suggests that new gene expression is required (34). These data demonstrate that expression of the immediate early gene COX2 is induced in vulnerable CA1 neurons. Because translation of most proteins is blocked in these neurons after ischemia (35), COX2 is one of a select few proteins that is expressed before the death of these neurons. Several other proteins that promote programmed cell death are expressed in neurons after ischemia and have been proposed to promote death of these neurons (36, 37); however, selective antagonists for these proteins do not exist. In the case of COX2, our data show that treatment with a highly specific inhibitor of COX2 activity after ischemia prevents neuronal death. Furthermore, COX2 inhibitors prevent delayed neuronal death after kainate-induced seizures and MK801 toxicity (38). These data support the hypothesis that COX2 activity directly injures neurons.
The hypothesis that COX2 could be important in the pathogenesis of neuronal death is supported by some, but not all, additional observations. High doses of the nonselective cyclooxygenase inhibitors indomethacin and piroxicam, which block both COX1 and COX2, have been reported to reduce delayed necrosis of CA1 neurons after global ischemia (29, 39). Glucocorticoids, which block induction of brain COX2 expression (8), are protective in neonatal cerebral ischemia models (40), but chronic administration of steroids exacerbates ischemic injury in adult rats (41). Not all neurons that show increased expression of COX2 after ischemia die. However, many other interactive factors may determine whether neurons die after ischemia: the regional severity of the ischemia, availability of cyclooxygenase substrates, subsequent prostaglandin metabolism, neuronal antioxidant defenses, expression of specific prostaglandin receptors, expression of death-regulatory proteins, and other factors. Thus, COX2 expression is by no means the sole determinant of neuronal survival after ischemia.
COX2 immunoreactivity in brain in vivo is found almost exclusively in neurons (42). Furthermore, COX2 expression is induced in neurons by spreading depression, which resembles in many respects the anoxic depolarization that occurs in cerebral ischemia (43). The current data show that there is dramatic induction of COX2 protein expression in neurons after ischemia. This observation suggests that the site of action of COX2 is the neuron; however, COX2 is also expressed in microglia, astrocytes, and endothelial cells in vitro (44–46). Further studies are required to determine the site of action of COX2 inhibitors in vivo.
These data suggest that SC58125 could be a more potent neuroprotectant than the nonselective COX inhibitor piroxicam. Piroxicam, like many nonselective COX inhibitors, is a more potent inhibitor of COX1 than COX2 in vitro (47). This finding could explain why piroxicam was less effective in reducing increases in hippocampal PGE2 concentrations and neuronal death induced by ischemia than SC58125. However, other factors such as the dosage paradigm used or nonspecific effects of the drugs could also explain these results. Accordingly, further studies are required to determine whether selective COX2 agents are more potent than nonselective drugs. Regardless of their potency, COX2 inhibitors may have another potential advantage: they lack the gastrointestinal and renal side effects of nonselective agents (48). These side effects may prevent tolerated doses of nonselective agents from achieving maximal inhibition of COX2 activity in brain.
The current observation that a COX2 inhibitor prevents selective neuronal death in global ischemia could be relevant to the epidemiological association between nonsteroidal anti-inflammatory use and a decreased incidence of Alzheimer’s disease. Nonsteroidal anti-inflammatory drugs such as ibuprofen (which is approximately equipotent for COX1 and COX2), but not aspirin (which inhibits COX2 only at very high concentrations), decrease the incidence of Alzheimer’s disease (47, 49).
These data and another recent study in temporary focal ischemia (30) suggest that COX2 inhibitors ameliorate ischemic injury in brain. Further studies are necessary to investigate the mechanisms of action of these drugs and to determine the role of COX2 activity in stroke and neurodegenerative diseases.
Acknowledgments
We thank Pat Strickler for secretarial support, Michele Victain for technical support, and David Greenberg for helpful comments. These results were presented at the Society for Neuroscience Annual Meeting in 1995 and the American Academy of Neurology Annual Meeting in 1996. This work was supported by the Department of Veterans Affairs Merit Review Program (S.H.G.).
ABBREVIATIONS
- COX1
cyclooxygenase-1
- COX2
cyclooxygenase-2
- SC58125
1-[(4-methylsulfonyl)phenyl]-3-trifluoro-methyl-5-[(4-fluoro)phenyl] pyrazole
- PGE2
prostaglandin E2
- DG
dentate gyrus
References
- 1.DeWitt D L, Smith W L. Proc Natl Acad Sci USA. 1988;85:1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiger M K, DeWitt D L, Schindler M S, Smith W L. Arch Biochem Biophys. 1993;301:439–444. doi: 10.1006/abbi.1993.1168. [DOI] [PubMed] [Google Scholar]
- 3.Smith, W. L., DeWitt, D. L. & Shimokawa, T. (1991) Adv. Prostaglandin Thromboxane Leukotrine Res. 77–80. [PubMed]
- 4.Kujubu D A, Fletcher B S, Varnum B C, Lim R W, Herschman H R. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 5.Kujubu D A, Herschman H R. J Biol Chem. 1992;267:7991–7994. [PubMed] [Google Scholar]
- 6.O’Banion M K, Winn V D, Young D A. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng L, Sun W, Xia Y, Tang W W, Chanmugam P, Soyoola E, Wilson C B, Hwang D. Arch Biochem Biophys. 1993;307:361–368. doi: 10.1006/abbi.1993.1601. [DOI] [PubMed] [Google Scholar]
- 8.Yamagata K, Andreasson K I, Kaufmann W E, Barnes C A, Worley P F. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann W E, Worley P F, Pegg J, Bremer M, Isakson P. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothman S M, Olney J W. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 11.Choi D W. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 12.Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, Leahy K, Perkins W, Isakson P. Agents Actions Suppl. 1995;46:41–50. doi: 10.1007/978-3-0348-7276-8_5. [DOI] [PubMed] [Google Scholar]
- 13.Tsujii M, DuBois R N. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 14.Pulsinelli W A, Brierley J B, Plum F. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 15.Lowenstein D H, Shimosaka S, So Y T, Simon R P. Epilepsy Res. 1991;10:49–54. doi: 10.1016/0920-1211(91)90094-v. [DOI] [PubMed] [Google Scholar]
- 16.Clark R S B, Chen J, Watkins S C, Kochanek P M, Chen M, Stetler R A, Loeffert J E, Graham S H. J Neurosci. 1997;17:9172–9182. doi: 10.1523/JNEUROSCI.17-23-09172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Marsh T, Zhang J S, Graham S H. NeuroReport. 1995;6:245–248. [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 19.Powell W S. Prostaglandins. 1980;20:947–957. doi: 10.1016/0090-6980(80)90144-6. [DOI] [PubMed] [Google Scholar]
- 20.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Proc Natl Acad Sci USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy, T. J., Sherman, E. L. C., Tally, J. J., Seibert, K., Isakson, P. C. & Welch, M. J. (1995) J. Nucl. Med. 36 (Suppl.), 49.
- 22.Kurumbail R G, Stevens A M, Gierse J K, McDonald J J, Stegeman R A, Pak J Y, Gildehaus D, Miyashiro J M, Penning T D, Seibert K, Isakson P C, Stallings W C. Nature (London) 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 23.Engelhardt G, Homma D, Schlegel K, Utzmann R, Schnitzler C. Inflamm Res. 1995;44:423–433. doi: 10.1007/BF01757699. [DOI] [PubMed] [Google Scholar]
- 24.Buchan A, Li H, Pulsinelli W A. J Neurosci. 1991;11:1049–1056. doi: 10.1523/JNEUROSCI.11-04-01049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abercrombie M. Anat Rec. 1946;94:274–329. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 26.Brierley J B, Meldrum B S, Brown A W. Arch Neurol. 1973;29:367–374. doi: 10.1001/archneur.1973.00490300029003. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Zhu R L, Nakayama M, Kawaguchi K, Jin K, Stetler R A, Simon R P, Graham S H. J Neurochem. 1996;67:64–71. doi: 10.1046/j.1471-4159.1996.67010064.x. [DOI] [PubMed] [Google Scholar]
- 28.Kusuhara H, Fukunari A, Matsuyuki H, Okumoto T. Brain Res Mol Brain Res. 1997;52:151–156. doi: 10.1016/s0169-328x(97)00263-5. [DOI] [PubMed] [Google Scholar]
- 29.Nakagomi T, Sasaki T, Kirino T, Tamura A, Noguchi M, Saito I, Takakura K. Stroke. 1989;20:925–929. doi: 10.1161/01.str.20.7.925. [DOI] [PubMed] [Google Scholar]
- 30.Nogawa S, Zhang F, Ross M E, Iadecola C. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirnagl U, Sixt G, Villringer A. J Cereb Blood Flow Metab. 1993;13:S130. doi: 10.1038/jcbfm.1993.70. (abstr.) [DOI] [PubMed] [Google Scholar]
- 32.Anderson M L, Smith D S, Nioka S, Subramanian H, Garcia J H, Halsey J H, Chance B. Neurol Res. 1990;12:195–204. doi: 10.1080/01616412.1990.11739943. [DOI] [PubMed] [Google Scholar]
- 33.Seisjo B K. Brain Energy Metabolism. New York: Wiley; 1978. [Google Scholar]
- 34.Tortosa A, Rivera R, Ferrer I. J Neurol Sci. 1994;121:10–17. doi: 10.1016/0022-510x(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 35.Widmann R, Kuroiwa T, Bonnekoh P, Hossmann K A. J Neurochem. 1991;56:789–796. doi: 10.1111/j.1471-4159.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- 36.Krajewski S, Mai J K, Krajewska M, Sikorska M, Mossakowski M J, Reed J C. J Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Chopp M, Zhang Z G, Zaloga C, Niewenhuis L, Gautam S. Stroke. 1994;25:849–855. doi: 10.1161/01.str.25.4.849. [DOI] [PubMed] [Google Scholar]
- 38.Graham S H, Kawaguchi K, Zhu L. Neurology. 1997;48:A152. (Abstr.). [Google Scholar]
- 39.Sasaki T, Nakagomi T, Kirino T, Tamura A, Noguchi M, Saito I, Takakura K. Stroke. 1988;19:1399–1403. doi: 10.1161/01.str.19.11.1399. [DOI] [PubMed] [Google Scholar]
- 40.Tuor U I. Ann NY Acad Sci. 1995;765:179–195. doi: 10.1111/j.1749-6632.1995.tb16574.x. [DOI] [PubMed] [Google Scholar]
- 41.Sapolsky R M, Pulsinelli W A. Science. 1985;229:1397–400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- 42.Breder C D, Dewitt D, Kraig R P. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caggiano A O, Breder C D, Kraig R P. J Comp Neurol. 1996;376:447–462. doi: 10.1002/(SICI)1096-9861(19961216)376:3<447::AID-CNE7>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Banion M K, Miller J C, Chang J W, Kaplan M D, Coleman P D. J Neurochem. 1996;66:2532–2540. doi: 10.1046/j.1471-4159.1996.66062532.x. [DOI] [PubMed] [Google Scholar]
- 45.Hla T, Maciag T. J Biol Chem. 1991;266:24059–24063. [PubMed] [Google Scholar]
- 46.Minghetti L, Levi G. J Neurochem. 1995;65:2690–2698. doi: 10.1046/j.1471-4159.1995.65062690.x. [DOI] [PubMed] [Google Scholar]
- 47.Frolich J C. Trends Pharmacol Sci. 1997;18:30–34. doi: 10.1016/s0165-6147(96)01017-6. [DOI] [PubMed] [Google Scholar]
- 48.Masferrer J L, Zweifel B S, Manning P T, Hauser S D, Leahy K M, Smith W G, Isakson P C, Seibert K. Proc Natl Acad Sci USA. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart W F, Kawas C, Corrada M, Metter E J. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]