Abstract
Phage λ propagation in Escherichia coli host cells requires transcription antitermination on the λ chromosome mediated by λN protein and four host Nus factors, NusA, B, E (ribosomal S10) and G. Interaction of E. coli NusB:NusE heterodimer with the single stranded BoxA motif of λnutL or λnutR RNA is crucial for this reaction. Similarly, binding of NusB:NusE to a BoxA motif is essential to suppress transcription termination in the ribosomal RNA (rrn) operons. We used fluorescence anisotropy to measure the binding properties of NusB and of NusB:NusE heterodimer to BoxA-containing RNAs differing in length and sequence. Our results demonstrate that BoxA is necessary and sufficient for binding. We also studied the gain-of-function D118N NusB mutant that allows λ growth in nusA1 or nusE71 mutants. In vivo λ burst-size determinations, CD thermal unfolding measurements and X-ray crystallography of this as well as various other NusB D118 mutants showed the importance of size and polarity of amino acid 118 for RNA binding and other interactions. Our work suggests that the affinity of the NusB:NusE complex to BoxA RNA is precisely tuned to maximize control of transcription termination.
INTRODUCTION
Phage λ-mediated antitermination in Escherichia coli enables RNA polymerase (RNAP) to read through early transcription termination sites on the phage chromosome (1). Antitermination is regulated via the direct interaction of N protein and the transcription elongation complex (TEC) formed by RNA, RNAP and the Nus (N utilization substance) host factors NusA, NusB, NusE (S10 ribosomal protein) and NusG (2–4). N-mediated antitermination is coupled to transcription of the phage λ nut RNA sites, each consisting of the single stranded BoxA and the palindromic stem–loop BoxB linked by a spacer sequence to which NusA binds (5). N interacts with BoxB and converts the TEC to a termination-resistant form (6,7). Binding of λ N to BoxB results in an indirect interaction with RNAP through NusA (8,9). NusB interacts with the nut site by binding to BoxA, an interaction that is ∼10-fold strengthened upon NusE:NusB heterodimer formation (10–13). The NusB:NusE:RNA ternary complex is proposed to associate with RNAP through NusE (1,14,15). A similar complex in which λ N is replaced by phage HK022 Nun protein induces transcription arrest on the λ chromosome (7).
In addition to its involvement in transcription, NusE participates in translation as part of the 30S ribosomal subunit (16–18).
A termination-resistant TEC also assembles during transcription of rrn operons in E. coli and other bacteria (19,20). In addition to Nus factors, ribosomal proteins S4, S2, L4 and L13 participate in transcription regulation (21,22). BoxA is highly conserved in all seven E. coli rrn operons. A promoter-proximal BoxB-like element is present but is not required for rrn antitermination (23). As is the case with λ, formation of the ternary NusB:NusE:BoxA complex is a key step during rrn processive antitermination (13).
The structure of the NusB:NusEΔloop complex, in which the 22 residue ribosome-binding loop of NusE was deleted, has recently been determined (24). Analysis of this structure and other data (25) suggest that NusE is the active partner of the complex and that NusB mainly acts as a loading factor for NusE, a notion that is supported by the fact that NusE in a NusB deletion background could still support N antitermination and Nun termination (24). Although UV-crosslinking studies indicate that both NusB and NusEΔloop contact BoxA RNA, detailed structural information about the RNA binding of NusB and the ternary NusB:NusE:RNA complex is not currently available.
Several nutR BoxA mutants abolish N-mediated antitermination or Nun-mediated transcription arrest in vivo and/or in vitro, namely nutR BoxA5 (G35U) (26), nutR BoxA16 (C38A) (27), nutR BoxA (U39G) (28). Oddly, the 9-bp transversion mutant nutR BoxA69 has little effect on N-mediated antitermination except to make it NusB-independent, and it was proposed that NusB competed for BoxA binding with an as yet unidentified inhibitor of N activity (14).
NusB101 (D118N) presents an intriguing gain of function variant that suppresses a block in N-mediated antitermination by NusA1 (L183R) and NusE71 (A86D) at 42°C (29,30). NusBD118N has enhanced affinity for rrn and λ nut BoxA (29); for example, NusBD118N:NusE can be UV-crosslinked to BoxA-containing RNAs more efficiently than wt NusB:NusE (24). However, whether the increased affinity originates from a charge effect, from different direct contacts of the amino acid at position 118 to the RNA, or from a combination of effects is not clear.
In the present study, we used biophysical and genetic approaches to delineate identity elements of nut RNA that are recognized by NusB and by heterodimeric NusB:NusE complex. Furthermore, we studied NusB D118 mutants to clarify the role of this amino acid in NusB:RNA and NusB:NusE:RNA interactions.
MATERIALS AND METHODS
Cloning, expression and protein purification of Nus-factors
The nusB gene was cloned via BamH1 and Nde1 restriction sites into the E. coli expression vector pET29b (Novagen, Madison, WI, USA). Escherichia coli strain BL21(DE3) (Novagen, Madison, WI, USA) harboring the recombinant plasmid was grown at 37°C in LB (Luria–Bertani) medium containing kanamycin (30 µg/ml) until an OD600 = 0.5 was reached, then the temperature was reduced to 20°C for 30 min and the cells were induced by 1 mM isopropyl 1-thio-β-d-galactopyranoside (IPTG). Cells were harvested 4 h after induction, resuspended in four times the pellet weight of lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM DTT, pH 7.5), and lysed by using a micro-fluidizer (Microfluidics, Newton, MA, USA). After centrifugation the supernatant was dialyzed for 4 h against lysis buffer without NaCl and afterwards applied to a HeparinFF column (GE Healthcare, Munich, Germany) using a step gradient with increasing NaCl concentrations (0–1 M). For further purification the eluted fractions containing NusB were pooled and concentrated with Vivaspin concentrators (Vivascience, MWCO 5000 Da). The concentrated sample was applied to an S75 gel filtration column (GE Healthcare). The fractions containing NusB were pooled and dialyzed against buffer as used for fluorescence measurements (25 mM HEPES, 100 mM potassium acetate, pH 7.5). The identity and structural integrity of the purified protein was analyzed by 19% SDS–PAGE and NMR spectroscopy.
NusB mutations
For NusBD118N (NusB101), NusBD118R, NusBD118A, NusBD118E and NusBD118K the mutation primers shown in Supplementary Table S1 were used. Mutations were introduced by using the QuikChange protocol (Stratagene, La Jolla, CA, USA). Expression and purification was as described for wildtype NusB. NusBK2E was inherent in the original NusB pETM11-plasmid (24).
NusE:NusB complex
NusE was cloned via BamH1 and EcoR1 restriction sites into the E. coli expression vector pGEX-6P (GE Healthcare) (24). The recombinant plasmid encoded a GST-NusE fusion protein with an internal PreScission cleavage site following the GST-tag. Escherichia coli strain BL21(DE3) (Novagen) harboring the recombinant plasmid was grown at 37°C in LB medium containing ampicillin (100 µg/ml) until an OD600 = 0.5 was reached, then the temperature was reduced to 20°C for 30 min and the cells were induced by 1 mM IPTG. After induction overnight, the cells were harvested and resuspended in four times the pellet weight of lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM DTT, pH 7.5). At this point, the NusB cell extract solved in the same buffer was added. After mixing for 20 min the cells were lysed with a micro-fluidizer (Microfluidics, Newton, MA, USA), and additional mixing was performed for 1 h to ensure correct formation of the NusB:NusE dimer. After centrifugation the dimer was purified from the supernatant via a GSTrap-FF column (GE Healthcare) using a one step elution (lysis buffer with 15 mM reduced gluthathione). The GST-NusE fusion protein was cleaved by PreScission protease while dialyzing against lysis buffer at 4°C overnight. The cleaved protein was reapplied to a GSTrap-FF column using the same step elution procedure, but this time collecting the flow-through. For further purification the eluted fractions containing NusB:NusE were pooled and concentrated with Vivaspin concentrators (Vivascience, MWCO 5000 Da). The concentrated sample was applied to an S75 gel filtration column (GE Healthcare). The fractions containing NusB:NusE were pooled and dialyzed against buffer as used for fluorescence measurements (25 mM HEPES, 100 mM potassium acetate, pH 7.5). The identity and structural integrity of the purified protein complexes were analyzed by 19% SDS–PAGE.
NusBD118E–NusEΔloop production and purification for crystallization
Cloning of the genes encoding NusB and NusEΔloop has been described (24). Mutations were introduced by using the QuikChange protocol (Stratagene, La Jolla, CA, USA). To produce protein for crystallographic analysis, plasmids containing the genes of interest were co-transformed into E. coli strain BL21(DE3) by electroporation. The cells were grown in auto-inducing medium (31) in the presence of the appropriate antibiotics to an OD600 of 0.5 at 37°C, and then incubated for an additional 16 h at 20°C. After harvesting at 4°C, the cell pellets were washed with binding buffer (50 mM Tris, pH 7.5, 150 mM NaCl) and stored at −80°C. Purification of the NusBD118N:NusEΔloop complex followed a double affinity chromatography procedure as described for the NusB:NusEΔloop complex (24).
Crystallographic analysis
NusBD118N:NusEΔloop complex (NusB101:NusEΔloop; 16 mg/ml) was crystallized at 20°C via the sitting drop vapor diffusion method by mixing 1 ml of sample with 1 ml of reservoir solution (0.2 M potassium citrate, 20% PEG 3350). Crystals could be flash frozen in liquid nitrogen after transfer into 60% reservoir plus 40% glycerol. Diffraction data were collected at 100 K on beamline PXII (SLS, Villigen, Switzerland) using a MarCCD 225 mm detector. The data were processed with the XDS package (32).
The structure of the NusBD118N:NusEΔloop complex was solved by molecular replacement using the coordinates of the NusB:NusEΔloop complex [PDB ID 3D3B; (24)]. The model was manually rebuilt using COOT (33) and refined by standard methodology using Refmac5 including TLS refinement (34). Each protein molecule in the crystallographic asymmetric unit represented a separate TLS group.
In vivo assays
nusB::Cam nusA+ or nusB::Cam nusA1 mutants carrying λcI857 prophage were constructed. Wild-type and mutant NusB were supplied from a pBAD30 plasmid. Phage burst size after thermal induction was determined according to standard protocols.
Fluorescence equilibrium measurements
Various RNA sequences corresponding to the nut regions of the λ genome or to the rrnG BoxA of the E. coli genome (Supplementary Table S2) were used. Fluorescence equilibrium titrations were performed using an L-format Jobin–Yvon Horiba Fluoromax fluorimeter (Edison, NJ, USA). Extrinsic fluorescence measurements with 3′-6-carboxy-fluorescein (6-FAM)-labeled RNA were performed in fluorescence buffer as above in a total volume of 1 ml using a 10 × 4 mm quartz cuvette (Hellma, Müllheim, Germany). The excitation wavelength was 492 nm, and the emission intensity was measured at 516 nm applying a 500 nm cutoff filter. Anisotropic measurements were performed with slit widths of 4 nm and 3 nm for excitation and emission, respectively. All titration measurements were carried out at 25°C with 50 nM of 6-FAM-labeled RNA. Following sample equilibration, six data points with an integration time of 0.8 s were collected for each titration point.
Data fitting
Anisotropic data were fitted to a two-state binding model to determine the equilibrium dissociation constant (Kd) using standard software. The anisotropy was calculated from:
| 1 |
where A, Acomplex, ARNA are anisotropies and fcomplex, fRNA are fractional intensities. The change in fluorescence intensity has to be taken into account, so that the bound fraction is given by
| 2 |
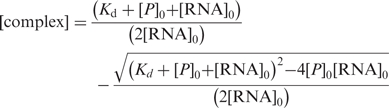 |
3 |
with A, anisotropy; ARNA, initial free anisotropy; Acomplex, anisotropy of the protein–RNA complex; P0, RNA0, total protein and RNA concentration, respectively; R, ratio of intensities of bound and free forms.
CD measurements
Far UV CD measurements were performed on a J-810 S spectropolarimeter with a CDF-426S temperature control unit (JASCO International, Tokyo, Japan). Samples were prepared by dialyzing protein solutions against 10 mM sodium phosphate buffer, pH 7.5. Spectra were recorded at 25°C in a wavelength range of 185–260 nm with 50 nm/min scanning speed in a 1 mm path length quartz cuvette (Hellma, Müllheim, Germany) at a protein concentration of 10 μM. Buffer spectra were subtracted and ten spectra were accumulated. In order to normalize the measured ellipticity the mean residue molar ellipticity was calculated as:
| 4 |
Θ, measured ellipticity; MRW, mean residue mass; c, protein concentration; d, path length; N, number of amino acids.
Thermal stability was analyzed by monitoring the CD signal at 222 nm during heating from 25°C to 90°C with a heating rate of 1°C/min. Quartz cuvettes with 1 cm path length equipped with a stirrer were used at a protein concentration of 2.5 μM. Both baselines and the transition region were fitted simultaneously:
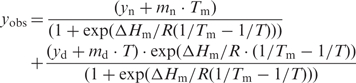 |
5 |
yobs, observed ellipticity; yn, yd y-intercepts of the baselines of native and denatured protein; mn, md, baseline slopes. ΔHm is the enthalpy at the temperature of the melting point (TM) (35,36). Evaluations were based on the assumption that the unfolding transition is a two-state reaction. The temperature dependence of ΔGD (free energy of unfolding) can be predicted at any temperature from the modified Gibbs–Helmholtz equation:
| 6 |
Over the narrow temperature range of the transition effects of ΔCP (change in heat capacity) are negligible. Therefore, the equilibrium constant of the unfolding reaction, K, is defined as
| 7 |
| 8 |
where T is the temperature in Kelvin, R is the gas constant and ΔSm is the entropy of unfolding at the melting point (TM). When observing a two-state process with an experimental observable, yobs, the equilibrium constant for the reaction is
| 9 |
By combining equations 7 and 9, ΔGD can be calculated (36,37).
RESULTS
Fluorescence anisotropy measurements were performed to determine the dissociation constants of NusB or NusB:NusE and various RNA constructs (Tables 1 and 2).
Table 1.
Dissociation constants for NusB monomer variants in nano molar
| NusB | NusBD118N | NusBD118A | NusBD118E | NusBD118K | NusBD118R | |
|---|---|---|---|---|---|---|
| rrnG BoxA-spacer | 130 ± 20 | 24 ± 4 | 230 ± 140 | 370 ± 30 | 240 ± 40 | 500 ± 130 |
| rrnG BoxA | 200 ± 10 | 50 ± 15 | 1100 ± 100 | 150 ± 20 | 450 ± 50 | 800 ± 80 |
| nutR BoxA-spacer | 1200 ± 600 | 900 ± 300 | nb | 3200 ± 800 | nb | nb |
| nutL BoxA-spacer | 2200 ± 800 | 1400 ± 500 | ||||
| nutR BoxA | 1600 ± 100 | 290 ± 15 | 500 ± 140 | 1400 ± 100 | 600 ± 25 | 1000 ± 70 |
| nutR-spacer | nb | nb | ||||
| nutL-spacer | nb | nb | ||||
| nutR BoxA5-spacer | 3100 ± 1600 | 12 200 ± 1600 | ||||
| nutR BoxA16-spacer | 5100 ± 1400 | 6500 ± 2000 | ||||
| nutR BoxA(U39G)-spacer | 1600 ± 800 | 9000 ± 3400 | ||||
| nutR BoxA69-spacer | nb | nb |
25 mM HEPES, 100 mM potassium acetate, pH 7.5.
nb = no binding detectable; empty cell = not determined. At least two independent experiments were performed per Kd. The relative molecular weights of the amino acids at residue 118 are A < N < D < E < K < R.
Table 2.
Dissociation constants for NusB:NusE heterodimer variants in nano molar
| NusB:NusE | NusBD118N: NusE | NusBD118A: NusE | NusBD118E: NusE | NusBD118K: NusE | NusBD118R: NusE | NusB: NusEΔloop | |
|---|---|---|---|---|---|---|---|
| rrnG BoxA-spacer | 8 ± 2 | 24 ± 4 | 30 ± 5 | 125 ± 20 | 42 ± 6 | 26 ± 5 | 22 ± 2 |
| rrnG BoxA | 5 ± 1 | 25 ± 7 | 60 ± 5 | 135 ± 5 | 90 ± 4 | 65 ± 6 | 50 ± 5 |
| nutR BoxA-spacer | 90 ± 37 | 32 ± 11 | 700 ± 100 | 290 ± 90 | 110 ± 20 | 110 ± 28 | 360 ± 50 |
| nutL BoxA-spacer | 80 ± 22 | 40 ± 10 | 600 ± 60 | ||||
| nutR BoxA | 83 ± 8 | 9 ± 1 | 60 ± 5 | 200 ± 20 | 75 ± 3 | 31 ± 2 | 160 ± 20 |
| nutR Spacer | nb | ||||||
| nutL Spacer | nb | ||||||
| nutR BoxA5-spacer | 5200 ± 1300 | ||||||
| nutR BoxA16-spacer | 3400 ± 1800 | ||||||
| nutR BoxA(U39G)-spacer | 1900 ± 700 | ||||||
| nutR BoxA69-spacer | nb |
25 mM HEPES, 100 mM potassium acetate, pH 7.5.
nb = no binding detectable; empty cell = not determined. At least two independent experiments were performed per Kd. The relative molecular weights of the amino acids at residue 118 are A < N < D < E < K < R.
Formation of the dimeric NusB:NusE complex enhances RNA binding affinity
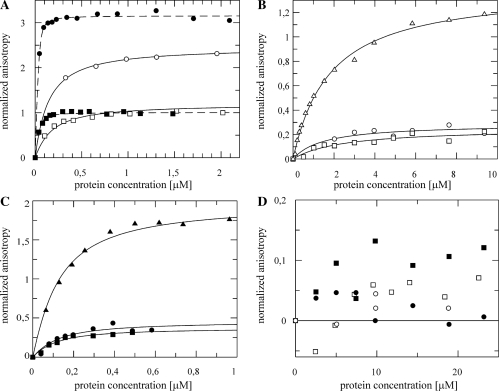
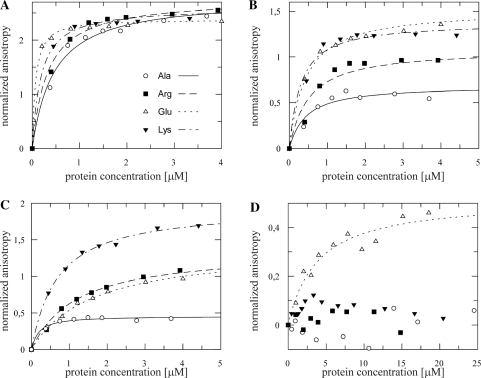
NusB bound rrnG BoxA-spacer and rrnG BoxA with nearly identical efficiencies (dissociation constants Kd of 130 ± 20 nM and 200 ± 10 nM, respectively; Figure 1A, Table 1). The efficiency of NusB binding to λ nutR BoxA-spacer, nutL BoxA-spacer and nutR BoxA sequences was significantly lower (Kd values of ∼1.5 μM; Figure 1B). The higher anisotropy of nutR BoxA relative to the other constructs reflects the different rotational correlation time relative to the large RNA constructs. Preformed NusB:NusE heterodimer bound to all RNAs tested with affinities more than an order of magnitude greater than NusB alone, consistent with the findings that both NusB and NusE in the NusB:NusE complex make RNA contacts (24). Kd values for the rrnG BoxA-spacer and the rrnG BoxA were 8 ± 2 and 5 ± 1 nM, respectively (Figure 1A; Table 2), and 90 ± 37 nM for nutR BoxA-spacer, 80 ± 22 nM for nutL BoxA-spacer, and 83 ± 8 nM for nutR BoxA (Figure 1C; Table 2). These data also indicate that contacts of NusE to the spacer region previously seen by UV-induced crosslinking (24) do not significantly increase the RNA affinity of the complex.
Figure 1.
Fluorescence anisotropy titration of fluorescein labeled λ nut and rrnG RNAs with NusB (open markers) and NusB:NusE complex (filled markers). (A) A 50 nM rrnG BoxA-spacer (squares) and 50 nM rrnG BoxA (circles) titrated with NusB and NusB:NusE. (B) Fifty nanomolar nutR BoxA-spacer (circles), nutL BoxA-spacer (squares) and nutR BoxA (triangles) titrated with NusB. (C) A 50 nM nutR BoxA-spacer (circles), nutL BoxA-spacer (squares) and nutR BoxA (triangles) titrated with NusB/NusE. (D) A 50 nM nutR spacer (circles) and 50 nM nutL spacer (squares) titrated with NusB and NusB/NusE. Solid lines represent the best fit to equation (3).
Protein RNA interaction takes place predominantly via BoxA
The binding of NusB and NusB:NusE to λ nut BoxA-spacer and nutR BoxA sequences with virtually identical affinities suggests that the spacer regions, shown previously to bind NusA (5), do not bind NusB or NusB:NusE. To confirm this, we measured binding to spacer alone. Specific binding of NusB or NusB:NusE to nutL spacer and nutR spacer was not observable in fluorescence titrations (Figure 1D). A slight increase of the fluorescence anisotropy signal with the NusB:NusE nutR spacer titration is consistent with unspecific binding with a Kd-value in the upper micromolar range.
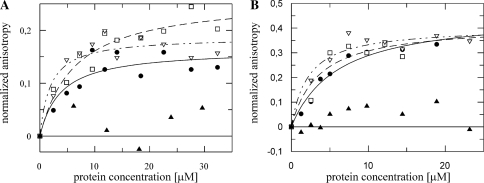
BoxA mutations decrease binding affinity
Several BoxA mutations (Supplementary Table S2) affect λ N antitermination and HK022 Nun transcription arrest (14,26–28). We studied the effect of these mutations on NusB and NusB:NusE binding affinities. No interaction with the boxA transversion mutant nutR BoxA69-spacer and either NusB or NusB:E heterodimer was detected (Figure 2A and B; Tables 1 and 2), clearly indicating that the binding of these factors is BoxA RNA sequence-dependent. This result supports the in vivo finding of Patterson et al. (14) that N antitermination on BoxA69 fusions is NusB-independent. We next tested BoxA point mutants that inhibit N and Nun (Figure 2A and B; Tables 1 and 2). The nutR BoxA5 mutation (G35U) reduced NusB binding 2- to 3-fold (Kd = 3.1 ± 1.6 µM). Interestingly, the affinity of NusB:NusE heterodimer for the mutant RNA was essentially identical to that of NusB (Kd = 5.2 ± 1.3 µM). NusB and NusB:NusE bound nutR BoxA16 (C38A) with Kd values of 5.1 ± 1.4 µM and 3.4 ± 1.8 µM, respectively. The dissociation constants of NusB and NusB:NusE for nutR BoxA (U39G), which inhibits N, were 1.6 ± 0.8 µM and 1.9 ± 0.7 µM, respectively. Judged from their effects on the NusB and NusB:NusE binding affinities, G35 and C38 participate more tightly in protein binding than U39. The equivalent binding affinities of NusB and NusB:NusE heterodimer to these BoxA point mutants stands in sharp contrast to the enhanced binding of the heterodimer to wild-type BoxA sequences.
Figure 2.
Fluorescence anisotropy, λnut BoxA variants and NusB (A), NusB:NusE (B). Each titration was performed with 50 nM of fluorescein labeled RNA. nutR BoxA5-spacer (filled circle, fit: solid line), nutR BoxA16-spacer (open square, fit: dashed line), nutR BoxA69-spacer (filled triangle) and nutR BoxA(U39G)-spacer (open triangle, fit: dotted line). Lines represent the best fit to equation (3). For nutR BoxA69-spacer no interaction was observable, therefore no fitting was performed.
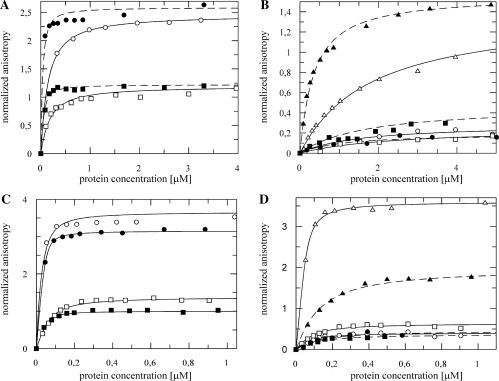
The NusBD118N (NusB101) mutation affects RNA binding affinity
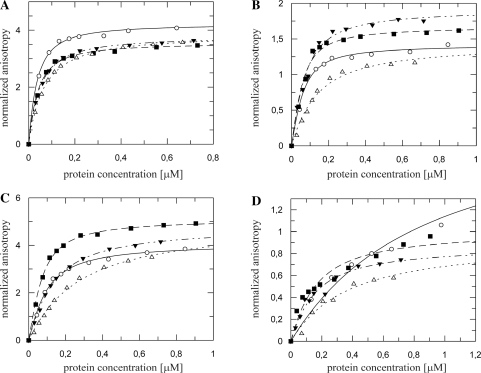
NusBD118N is a gain- of-function mutation that enables NusB to override mutations in NusE and NusA that abrogate λ N-mediated antitermination (29,30). The dissociation constants for NusBD118N complexes with rrnG BoxA-spacer and rrnG BoxA were 5- to 6-fold lower compared to wild-type NusB (Figure 3A; Table 3). Only small reductions in Kd were observed for the nutL BoxA-spacer and nutR BoxA-spacer complexes, whereas the dissociation constant for the complex of NusBD118N with nutR BoxA decreased significantly from ∼1600 nM to ∼290 nM (Figure 3B; Table 1). In contrast, the mutation increased the Kd values about 3-fold for heterodimer complexes with rrnG BoxA-spacer and rrnG BoxA (Figure 3C, Table 2). NusBD118N:NusE complexes with nutR BoxA-spacer and nutL BoxA-spacer sequences displayed 3- and 5-fold, respectively, lower Kd values for the mutant relative to the wild-type heterodimer (Figure 3D, Table 3). For the NusBD118N:NusE complex with nutR BoxA, the Kd decreased by nearly an order of magnitude to 9 ± 1 nM (Figure 3D; Table 2). Thus, NusE did not further enhance the binding of NusBD118N to rrnG BoxA or rrnG BoxA-spacer. However, NusE strongly stimulated the binding of NusBD118N to sequences derived from nutL and nutR.
Figure 3.
Fluorescence anisotropy measurements with fluorescein-labeled λnut and rrnG RNAs towards NusBD118N (A and B) and NusBD118N:NusE complex (C and D) (filled markers) compared to the wild-type NusB and NusB:NusE complex (open markers, data as in Figure 1A and C). (A) Fifty nanomolar rrnG BoxA-spacer (squares) and 50 nM rrnG BoxA (circles) titrated with NusBD118N and NusB; (B) 50 nM nutR BoxA-spacer (circles), nutL BoxA-spacer (squares) and nutR BoxA alone (triangles) titrated with NusD118N and NusB. (C) A 50 nM rrnG BoxA-spacer (squares) and 50 nM rrnG BoxA (circles) titrated NusBD118N:NusE and NusB:NusE; (D) 50 nM nutR BoxA-spacer (circles), nutL BoxA-spacer (squares) and nutR BoxA (triangles) titrated with NusBD118N:NusE and NusB:NusE. Dashed lines represent the best fit to equation (3) for NusBD118N and NusBD118N/NusE, respectively. Solid lines the similar fit for wild-type NusB and NusB:NusE.
Table 3.
Melting temperatures (TM), free reaction enthalpy at the melting point (ΔHM,D) and Gibbs free energy of the unfolding reaction at 328K (ΔGD) values for NusB variants (10 mM potassium phosphate, pH 7.5)
| TM (K) | ΔHM,D[TM] (kJ/mol) | ΔGD [328K] (kJ/mol) | |
|---|---|---|---|
| NusB | 337.8 ± 0.1 | 280 ± 4 | 8.3 ± 0.2 |
| NusBD118N | 333.6 ± 0.1 | 174 ± 3 | 2.7 ± 0.2 |
| NusBD118A | 333.5 ± 0.1 | 340 ± 4 | 5.6 ± 0.2 |
| NusBD118R | 331.0 ± 0.1 | 298 ± 4 | 2.4 ± 0.2 |
| NusBD118E | 334.5 ± 0.1 | 263 ± 3 | 5.8 ± 0.2 |
| NusBD118K | 330.8 ± 0.1 | 244 ± 3 | 1.6 ± 0.2 |
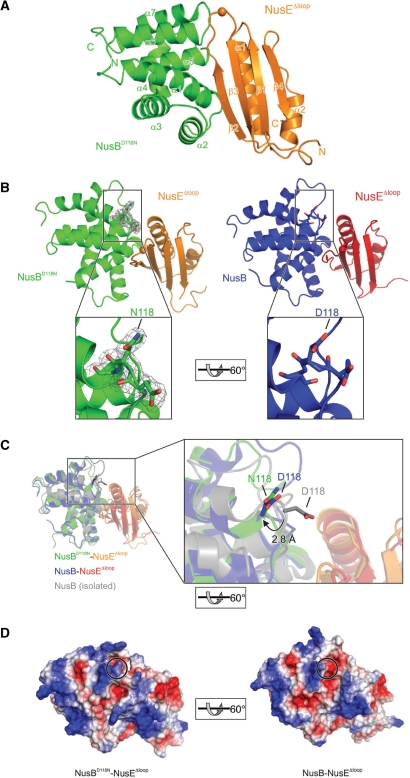
The structure of NusBD118N:NusEΔloop closely resembles the structure of NusB:NusEΔloop
NusEΔloop is a derivative of NusE that binds NusB and retains transcriptional but not translational activity (24). The NusB:NusEΔloop complex binds RNA that includes a BoxA sequence, although with lower efficiency than NusB:NusE [(24); Figure S1; Table 2]. Previous studies demonstrated that NusBD118N:NusEΔloop bound RNA more tightly than NusB:NusEΔloop. To ask if the increased RNA affinity of the NusBD118N:NusEΔloop complex correlated with structural rearrangements compared to the NusB:NusEΔloop complex, we solved the crystal structure of the NusBD118N:NusEΔloop complex by molecular replacement at 2.5 Å resolution (Figure 4). The structure was refined to Rwork and Rfree factors of 20.4% and 25.6%, respectively (Table 4). An asymmetric unit of the crystal contained three molecules each of NusBD118N and NusEΔloop, which formed three NusBD118N:NusEΔloop complexes. Two of these complexes exhibited well-defined electron density, but the electron density map of the third complex was fragmentary: In that complex, residues 60–77 and 127–139 of NusBD118N and residues 45–47 and 60–72 of NusEΔloop could not be unambiguously traced. The following discussion therefore refers to the structures of the two well defined complexes, which closely resemble each other [RMSD of 0.75 Å for 220 Cα atoms; calculated with SSM (38)].
Figure 4.
Structure of the NusBD118N:NusEΔloop complex. (A) Ribbon plot of the E. coli NusBD118N:NusEΔloop complex. NusBD118N, green; NusEΔloop, orange. Secondary structure elements and termini are labeled. The orange sphere marks the site at which the ribosome-binding loop of NusE has been replaced by a single serine. (B) Comparison of the NusBD118N:NusEΔloop complex (left) with the NusB:NusEΔloop complex [right, PDB ID 3D3B; (24)]. Insets: closeup views of the residue 118 regions. The orientation relative to (A) is indicated. Gray mesh, final 2Fo − Fc electron density of the NusBD118N:NusEΔloop structure contoured at the 1σ level and covering N118 and neighboring residues. The orientation relative to (A) is indicated. (C) Superimposition of the NusB:NusEΔloop complex [blue and red, PDB ID 3D3B; (24)] and of NusB [grey, PDB ID 1EY1; (39)] on the NusBD118N:NusEΔloop complex (green and orange). Residues at position 118 are shown as sticks and a magnified view of the residue 118 region is provided (carbon, as the respective molecule; oxygen, red; nitrogen, blue). The orientation relative to (A) is indicated. (D) Comparison of the electrostatic surface potentials of the complexes. Blue, positive charge; red, negative charge. Left, NusBD118N:NusEΔloop complex. Right, NusB:NusEΔloop complex. The positions of residue 118 are circled. The orientations are the same as in (B).
Table 4.
Crystallographic data
| NusBD118N − NusEΔloop | |
|---|---|
| Data collection | |
| Wavelength (Å) | 0.9788 |
| Temperature (K) | 100 |
| Space group | I4122 |
| Unit cell parameters (Å, °) | a = 112.64, b = 112.64, c = 263.25 |
| Resolution (Å) | 30.0–2.5 (2.6–2.5)a |
| Reflections | |
| Unique | 29 761 (3263) |
| Completeness (%) | 100 (100) |
| Redundancy | 7.22 (7.42) |
| I/σ(I) | 18.1 (4.1) |
| Rsym(I)b | 8.6 (72.5) |
| Refinement | |
| Resolution (Å) | 30.0–2.5 (2.56–2.50) |
| Reflections | |
| Number | 29 753 (2178) |
| Completeness (%) | 100 (100) |
| Test set (%) | 5.0 |
| Rworkc | 20.4 (23.1) |
| Rfreec | 25.6 (29.1) |
| Contents of AUd | |
| Protein molecules/refined atoms | 3 NusBAsp118Asn, 3 NusEΔloop/5313 |
| Water oxygen | 155 |
| Ions | 1 K+ |
| Mean B-factors (Å2) | |
| Wilson | 52.7 |
| Protein | 60.5 |
| Water | 22.8 |
| Ions | 29.5 |
| Ramachandran plote | |
| Favored (%) | 97.12 |
| Allowed (%) | 2.43 |
| Outliers (%) | 0.45 |
| RMSD from target geometry | |
| Bond lengths (Å) | 0.01 |
| Bond angles (°) | 1.22 |
| RMSD B-factors (Å2) | |
| Main chain bonds | 0.41 |
| Main chain angles | 0.82 |
| Side chain bonds | 1.43 |
| Side chain angles | 2.48 |
| PDB ID | 3IMQ |
aData for the highest resolution shell in parentheses
bRsym(I) = ΣhklΣiIi(hkl) < I(hkl) > | ΣhklΣi | Ii(hkl) |; for n independent reflections and i observations of a given reflection; <I(hkl)> – average intensity of the i observations
cR = Σhkl||Fobs| – |Fcalc||/Σhkl|Fobs|; Rwork – hkl ∉ T; Rfree – hkl ∈ T; T, test set.
dAU, asymmetric unit.
eCalculated with MolProbity (http://molprobity.biochem.duke.edu/) (40) RMSD, root-mean-square deviation.
The global structure of NusBD118N in complex with NusEΔloop is very similar to that of wild-type NusB in isolation [PDB ID 1EY1; (39); rmsd of 2.54 Å for 110 Cα atoms; Figure 4]. Furthermore, the structure of the NusBD118N:NusEΔloop complex is virtually identical to that of the NusB:NusEΔloop complex (RMSD of 0.85 Å for 220 Cα atoms; Figure 4B), demonstrating that the D118N mutation has no global conformational consequences. In particular, the positions and conformations of NusB residue N118 in the mutant and of residue D118 in the parent complex are essentially identical. Irrespective of the amino acid at position 118, the neighboring region undergoes identical adjustments upon NusEΔloop binding, during which the Cα position of residue 118 is repositioned by 2.8 Å (Figure 4C, inset). However, the D118N exchange induces a significant difference in the local electrostatic surface properties of the complex (Figure 4D). This observation is consistent with the idea that the increased RNA affinity of NusBD118N or its complex with NusEΔloop is at least in part due to the replacement of a negatively charged residue with an uncharged residue at the RNA binding site, thus reducing repulsion with the negatively charged sugar-phosphate backbone of the RNA. Alternatively, or in addition, introduction of an asparagine for an aspartate at position 118 may result in additional hydrogen bonds to the RNA.
The overall structures of various NusB118 mutants are highly similar
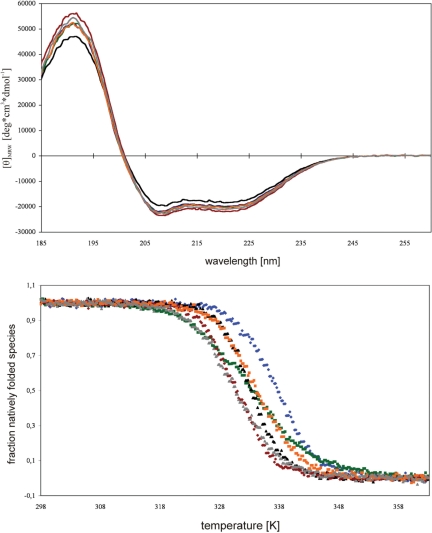
To investigate further the effect of amino acid variations at position 118, several point mutations with positively charged, negatively charged, and apolar amino acids were examined. All NusB variants show CD-spectra typical of α-helices, i.e. minima at 208 nm and 222 nm as well as a maximum at 190 nm (Figure 5A), with only minor differences from the wild-type protein spectrum. The melting temperatures of NusB and NusB D118 variants were determined by thermal unfolding. The CD signal at 222 nm, which we used to indicate melting, was reduced by all mutations, particularly by substitutions with positively charged residues. The Gibbs free energy of the unfolded species (ΔGD) at 328 K thus ranges from 8.3 ± 0.2 kJ/mol for wild-type NusB to 1.6 ± 0.2 kJ/mol for NusBD118K. The NusB variants, with the notable exception of NusBD118N, show very similar unfolding transitions. The broader transition of NusBD118N indicates a lower value for the Gibbs free energy of the unfolding reaction at 328 K and for the free reaction enthalpy at the melting point [(35,36); Figure 5 and Table 3]. These relatively small differences indicate that the D118 point mutations do not lead to global NusB misfolding or to unstable NusB proteins.
Figure 5.
(A) Overlay of the CD-spectra for the NusB variants. ΘMRW versus wavelength in nm. ΘMRW was according to equation (4). (B) Thermal unfolding for the NusB variants. Fraction of the unfolded species versus absolute temperature. NusB (blue), NusBD118N (green), NusBD118A (black), NusBD118R, (red), NusBD118E (orange) and NusBD118K (grey).
Effects of other NusB D118 mutations on RNA binding
The binding properties of different NusB mutants dependent on the RNA were tested. Thus the binding of NusBD118A to rrnG BoxA-spacer was approximately as tight as NusB+, whereas the affinity of the mutant for rrnG BoxA was 20% that of NusB+ (Figure 6; Table 1). NusBD118E bound rrnG BoxA with wild-type efficiency but associated with rrnG BoxA-spacer ∼2-fold less well than NusB+. We considered the possibility that replacing D118 with a positively charged residue might enhance binding through ionic interactions with the RNA ligand. This was not the case. Neither NusBD118K nor NusBD118R bound rrnG RNA with wild-type efficiency. The higher volumes of the lysine and arginine residues may induce unfavorable steric interactions, canceling out positive contributions to binding by electrostatic interactions with RNA. Consistent with this idea is our observation that NusBD118R bound rrn or nutR BoxA less efficiently than NusBD118K, reflecting, perhaps, the larger volume of the former substitution. Of the various D118 substitutions, interaction with the nutR BoxA-spacer could be detected only for NusBD118N and NusBD118E (Figure 6 and Table 3). NusBD118E bound nutR BoxA as well as NusB+. NusBD118A and NusBD118K bound nutR BoxA significantly better than wild-type NusB (Figure 6 and Tables 1 and 2).
Figure 6.
Fluorescence anisotropy measurements with fluorescein labeled λnut or rrnG RNAs and NusBD118A (open circle, fit: bold line), NusBD118R (filled square, fit: dashed line), NusBD118E (open triangle, fit: pointed line) and NusBD118K (filled triangle, fit: dashed/pointed line). Lines represent the best fit to equation (3). (A) Fifty nanomolar rrnG BoxA; (B) 50 nM rrnG BoxA-spacer; (C) 50 nM nutR BoxA; and (D) 50 nM nutR BoxA-spacer.
NusE improves binding of NusB mutants
With the exception of NusBD118E, NusE significantly enhanced mutant NusB binding to rrnG RNA, although no mutant except NusBD118N bound as well as NusB+:NusE. NusE also enhanced binding to nutR sequences. Binding of NusB mutants to nutR BoxA was, except for NusBD118E, at least as strong as NusB+. Thus, NusE may foster additional RNA contacts, rendering NusB-mediated interactions less dominant (Figure 7 and Tables 1 and 2).
Figure 7.
Fluorescence anisotropy measurements with fluorescein labeled λnut and rrnG RNAs to NusBD118A:NusE (open circle, fit: solid line), NusBD118R:NusE (filled square, fit: dashed line), NusBD118E:NusE (open triangle, fit: pointed line) and NusBD118K:NusE (filled triangle, fit: dashed/pointed line). Lines represent the best fit to equation (3). (A) Fifty nanomolar rrnG BoxA; (B) 50 nM rrnG BoxA-spacer; (C) 50 nM nutR BoxA; and (D) 50 nM nutR BoxA-spacer.
NusBD118N, NusBD118K and NusBD118R suppress the nusA1 (nusAL183R) mutation in vivo
We next tested the NusB D118 mutants for suppression of nusA1 (nusAL183R). nusAL183R prevents phage λ growth at 42°C by blocking λ N antitermination. Over-expression of NusB D118 mutants in a nusA+ λ cI857 lysogen had modest negative effects on phage burst size (Table 5). The negatively charged NusBD118E was most inhibitory, reducing burst size to 32% of wild-type levels. In the nusAL183R background, all mutants except NusBD118E increased burst size >100-fold. NusBD118N suppressed nusAL183R with greatest efficiency, increasing burst size from <0.0l% to 4.9% relative to nusA+. NusBD118K and NusBD118R also significantly enhanced λ growth (to 2.2% and 3.3%, respectively, of nusA+ titers). Suppression by NusBD118A was the least effective (0.5%). Taken together, these data suggest that replacing the negatively charged asp118 with an uncharged asparagine residue or a positively charged lysine or arginine residue significantly restores λ N activity in a nusAL183R strain. Poor suppression by the alanine substitution may reflect the lower molecular weight of this aminoacid relative to aspartate. Similarly, the inability of NusBD118E to restore λ growth may also be caused by steric effects due to the bulky glutamate side chain, in spite of its negative charge.
Table 5.
Strains are W3110 nusB::Cam λcI857 lysogens
| nusB plasmid | nusA+ | nusA1 | Suppression (%) |
|---|---|---|---|
| − | 103 | 0.0004 | <0.01 |
| + | 137 | 2 | <0.01 |
| D118N | 85 | 4.2 | 4.9 |
| D118A | 74 | 0.4 | 0.5 |
| D118E | 44 | 0.005 | <0.01 |
| D118K | 113 | 2.5 | 2.2 |
| D118R | 125 | 4.2 | 3.3 |
NusB is carried on pBAD30 and induced with 0.2% arabinose. Cells were grown overnight at 32°C in LB + ampicillin (50 µg/ml), diluted 1:100 into the same medium + 0.2% arabinose, and grown at 32°C for 1 h. λ was induced by temperature shift to 42°C for 90 min. Burst size was determined by plating the lysate on W3110 at 37°C. Cells were titered at 32°C prior to temperature shift; titers were equivalent for all strains. Values represent an average of two experiments; variation was <8 %.
DISCUSSION
Processive transcription antitermination depends on formation of a multi-factorial ribonucleoprotein complex on the surface of RNAP in response to nut signaling sequences in the untranslated leader regions of transcripts. These factors include NusA, NusB, NusE and NusG (2–4). In the case of λ nut, λ N protein forms part of the complex, whereas the complex that forms at rrn nut includes the Nus proteins and a number of additional host factors (21,22). Protein–protein and protein–RNA interactions in these complexes are cooperative in the sense that a stable complex is assembled based on numerous, but often weak, binary interactions. While some of these binary interactions have been mapped within the complexes, little is known about their relative strengths and how important these are for generating functional complexes. Here, we have used a combination of biophysical and functional studies to explore interactions between NusB or NusB:NusE complex and the nut BoxA RNA signaling sequence to which it binds.
We confirm that the affinity of NusB:NusE for BoxA is an order of magnitude higher than that of NusB alone (13), reflecting, presumably, the additional contacts that NusE makes with BoxA. We find that mutations in λ nut BoxA that inhibit λ N antitermination reduce NusB binding. However, in contrast to λ nut BoxA+, the affinities of NusB and NusB:NusE for the mutant BoxA sequences are essentially identical. The mutations lie throughout BoxA and do not define the known sites of NusE–RNA interactions (24).
The Kd values reported above for the association of NusB or NusB:NusE with λ nut BoxA are similar to those reported by Greive and coworkers based on fluorescence anisotropy experiments (13). We note, however, a large difference with respect to rrnG BoxA-spacer binding. These authors report a Kd of 850 nM for NusB and 200 nM for NusB:NusE, whereas we observe values of 130 nM and 8 nM, respectively. Since the RNA sequences tested were identical, we suggest that the lower affinity seen by Greive et al. reflects the 5′ location of the fluorescent label on their RNA compared to the 3′ location used in our experiments. The N-terminus of NusB contacts both rrn BoxA and nut BoxA at their 5′-ends, and interference with these contacts by a 5′ label could increase the Kd values, although in an RNA sequence-dependent manner. Indeed, we find that the K2E mutation increased the Kd of the NusB rrn BoxA-spacer complex from 130 nM to 3600 nM, but raised the Kd of NusB binding to nutL BoxA-spacer only 2-fold (from 2200 nM to 5100 nM).
We have also explored the phenotype of the NusBD118N (NusB101) mutation. NusBD118N suppresses mutations in NusA and NusE that inhibit λ N antitermination function (29,30). It has been proposed that an increase in the affinity of NusBD118N for nut RNA compared to NusB+ may compensate for weaker RNA (or protein) contacts of the mutant Nus proteins (24, 29). We show here that the D118N mutation enhances NusB binding to both rrn BoxA and λ nut BoxA. The mutation reduces binding to λ nut BoxA mutants, BoxA5 and BoxA(U39G), but has no effect on binding to BoxA16. It will be interesting to test whether the D118N mutation further reduces λ N antitermination on BoxA mutant templates in vivo. D118N also enhances NusB binding to nut BoxA in complex with NusE. However, we find that the affinity of NusBD118N:NusE for rrn BoxA is only 20% that of NusB+:NusE. These data imply that D118N, although it optimizes the formation of the λ N antermination complex, interferes with the assembly of antitermination complexes on rrn operons.
The enhanced affinity of NusBD118N to BoxA is not due to gross distortions in the shape of the NusE complex. Thus, the structure of NusBD118N:NusEΔloop complex is virtually identical to NusB+:NusEΔloop, although the electrostatic surface properties are strongly affected (Figure 4D). In fact, CD spectroscopic analyses (Figure 5) show that NusB also structurally tolerates a number of other mutations at position 118. Thus, the effects of the D118 mutations on RNA binding are local.
D118 is located close to NusB residues that interact with BoxA RNA. It was suggested that removal of the negatively charged aspartate with the neutral asparagine residue might extend the NusB RNA-binding surface and that this might stabilize the antitermination complex and account for the suppression of nusA1 and nusE71. Our results in general support this notion, although we find that the size of the D118 substitution also plays a role in the affinity of NusB for RNA.
The differential RNA affinities of the mutant NusB:NusE complexes roughly correlate with their in vivo suppression activity. We measured the burst sizes after induction of a λ cI857 lysogen in nusA+ and nusA1 hosts. This assay, which reflects the ability of λ N to antiterminate, showed that the order of nusA1 suppression efficiency was D118N > D118R > D118K > D118A. D118E failed to increase λ burst size over that seen for NusB+. The binding affinities to nut BoxA for the mutants in complex with NusE was D118N > D118 > D118R > D118A > D118K > D118E. Note that the Kd of NusB+ for nut BoxA (83 nM) was not significantly different from that of NusBD118K (75 nM), yet the mutant NusB increased the λ burst size in a nusA1 host at least 100-fold above the wild-type NusB level. This disjunction between RNA binding and N activation is, as yet, unexplained. It implies, however, that D118 may make functionally important contacts within NusB or with NusE.
We propose that D118 does not contact BoxA, but that removal of the negative charge permits such interaction. The location of position 118 opposite the NusB:NusE interaction surface renders an effect of mutations at this position on the NusB:NusE interaction unlikely. However, it cannot be ruled out completely, and a more definitive verdict needs further structural analysis of the NusB:NusE:BoxA complex. Study of how NusB mutations affect complex formation with NusEΔloop and alter its RNA binding properties might shed further light on the role of the NusE loop in RNA binding.
The results from our combined biophysical and genetic investigations illustrate how a particular protein–RNA interaction is fine-tuned with respect to other interactions within a functional ribonucleoprotein complex to achieve processive transcriptional antitermination. Furthermore, our results refine the mechanism by which NusB acts as a NusE RNA loading factor (24). NusB:NusE:RNA complex formation is entirely mediated by the BoxA sequences, whereas the spacer regions are necessary and sufficient for NusA binding (5). Sterically, simultaneous binding of these Nus factors to nut and rrn should be possible. There is, however, no evidence that NusA and NusB:NusE interact at nut, and no increase in nut binding by NusB:NusE was observed on NusA addition (15).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Deutsche Forschungsgemeinschaft (Ro617/16-1 to P.R.; Wa1126/3-1 to M.C.W.); National Institutes of Health (GM037219 to M.E.G.). Funding for open access charge: Universität Bayreuth.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Ramona Heissmann for excellent technical assistance.
REFERENCES
- 1.Greenblatt J, Nodwell JR, Mason SW. Transcriptional antitermination. Nature. 1993;364:401–406. doi: 10.1038/364401a0. [DOI] [PubMed] [Google Scholar]
- 2.Das A. Control of transcription termination by RNA-binding proteins. Annu. Rev. Biochem. 1993;62:893–930. doi: 10.1146/annurev.bi.62.070193.004333. [DOI] [PubMed] [Google Scholar]
- 3.Borukhov S, Lee J, Laptenko O. Bacterial transcription elongation factors: New insights into molecular mechanism of action. Mol. Microbiol. 2005;55:1315–1324. doi: 10.1111/j.1365-2958.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JW, Shankar S, Filter JJ. RNA polymerase elongation factors. Annu. Rev. Microbiol. 2008;62:211–233. doi: 10.1146/annurev.micro.61.080706.093422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasch S, Jurk M, Washburn RS, Gottesman ME, Wöhrl BM, Rösch P. RNA-binding specificity of E. coli NusA. Nucleic Acids Res. 2009;37:4736–4742. doi: 10.1093/nar/gkp452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chattopadhyay S, Garcia-Mena J, DeVito J, Wolska K, Das A. Bipartite function of a small RNA hairpin in transcription antitermination in bacteriophage λ. Proc. Natl Acad. Sci. USA. 1995;92:4061–4065. doi: 10.1073/pnas.92.9.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert J, Sloan SB, Weisberg RA, Gottesman ME, Robledo R, Harbrecht D. The remarkable specificity of a new transcription termination factor suggests that the mechanisms of termination and antitermination are similar. Cell. 1987;51:483–492. doi: 10.1016/0092-8674(87)90644-1. [DOI] [PubMed] [Google Scholar]
- 8.Prasch S, Schwarz S, Eisenmann A, Wöhrl BM, Schweimer K, Rösch P. Interaction of the intrinsically unstructured phage λ N protein with E. coli NusA. Biochemistry. 2006;45:4542–4549. doi: 10.1021/bi0523411. [DOI] [PubMed] [Google Scholar]
- 9.Bonin I, Mühlberger R, Bourenkov GP, Huber R, Bacher A, Richter G, Wahl MC. Structural basis for the interaction of Escherichia coli NusA with protein N of phage λ. Proc. Natl Acad. Sci. USA. 2004;101:13762–13767. doi: 10.1073/pnas.0405883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nodwell JR, Greenblatt J. Recognition of boxA antiterminator RNA by the E. coli antitermination factors NusB and ribosomal protein S10. Cell. 1993;72:261–268. doi: 10.1016/0092-8674(93)90665-d. [DOI] [PubMed] [Google Scholar]
- 11.Lüttgen H, Robelek R, Mühlberger R, Diercks T, Schuster SC, Kohler P, Kessler H, Bacher A, Richter G. Transcriptional regulation by antitermination. Interaction of RNA with NusB protein and NusB/NusE protein complex of Escherichia coli. J. Mol. Biol. 2002;316:875–885. doi: 10.1006/jmbi.2001.5388. [DOI] [PubMed] [Google Scholar]
- 12.Mason SW, Li J, Greenblatt J. Host factor requirements for processive antitermination of transcription and suppression of pausing by the N protein of bacteriophage λ. J. Biol. Chem. 1992;267:19418–19426. [PubMed] [Google Scholar]
- 13.Greive SJ, Lins AF, von Hippel PH. Assembly of an RNA-protein complex. Binding of NusB and NusE (S10) proteins to boxA RNA nucleates the formation of the antitermination complex involved in controlling rRNA transcription in Escherichia coli. J. Biol. Chem. 2005;280:36397–36408. doi: 10.1074/jbc.M507146200. [DOI] [PubMed] [Google Scholar]
- 14.Patterson TA, Zhang Z, Baker T, Johnson LL, Friedman DI, Court DL. Bacteriophage lambda N-dependent transcription antitermination. Competition for an RNA site may regulate antitermination. J. Mol. Biol. 1994;236:217–228. doi: 10.1006/jmbi.1994.1131. [DOI] [PubMed] [Google Scholar]
- 15.Mason SW, Greenblatt J. Assembly of transcription elongation complexes containing the N protein of phage λ and the Escherichia coli elongation factors NusA, NusB, NusG, and S10. Genes Dev. 1991;5:1504–1512. doi: 10.1101/gad.5.8.1504. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima S, Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970;226:1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- 17.Wimberly BT, Brodersen DE, Clemons WMJ, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 18.Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, et al. Structure of functionally activated small ribosomal subunit at 3.3 Å resolution. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 19.Li SC, Squires CL, Squires C. Antitermination of E. coli rRNA transcription is caused by a control region segment containing lambda nut-like sequences. Cell. 1984;38:851–860. doi: 10.1016/0092-8674(84)90280-0. [DOI] [PubMed] [Google Scholar]
- 20.Quan S, Zhang N, French S, Squires CL. Transcriptional polarity in rRNA operons of Escherichia coli nusA and nusB mutant strains. J. Bacteriol. 2005;187:1632–1638. doi: 10.1128/JB.187.5.1632-1638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres M, Condon C, Balada JM, Squires C, Squires CL. Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination. EMBO J. 2001;20:3811–3820. doi: 10.1093/emboj/20.14.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres M, Balada JM, Zellars M, Squires C, Squires CL. In vivo effect of NusB and NusG on rRNA transcription antitermination. J. Bacteriol. 2004;186:1304–1310. doi: 10.1128/JB.186.5.1304-1310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg KL, Squires C, Squires CL. Ribosomal RNA operon anti-termination. Function of leader and spacer region box B-box A sequences and their conservation in diverse micro-organisms. J. Mol. Biol. 1989;209:345–358. doi: 10.1016/0022-2836(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Hsiao HH, Bubunenko M, Weber G, Court DL, Gottesman ME, Urlaub H, Wahl MC. Structural and functional analysis of the E. coli NusB-S10 transcription antitermination complex. Mol. Cell. 2008;32:791–802. doi: 10.1016/j.molcel.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVito J, Das A. Control of transcription processivity in phage lambda: Nus factors strengthen the termination-resistant state of RNA polymerase induced by N antiterminator. Proc. Natl Acad. Sci. USA. 1994;91:8660–8664. doi: 10.1073/pnas.91.18.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson ER, Tomich CS, Friedman DI. The nusA recognition site: Alteration in its sequence or position relative to upstream translation interferes with the action of the N antitermination function of phage lambda. J. Mol. Biol. 1984;180:1053–1063. doi: 10.1016/0022-2836(84)90270-5. [DOI] [PubMed] [Google Scholar]
- 27.Robledo R, Gottesman ME, Weisberg RA. λ nutR mutations convert HK022 nun protein from a transcription termination factor to a suppressor of termination. J. Mol. Biol. 1990;212:635–643. doi: 10.1016/0022-2836(90)90226-c. [DOI] [PubMed] [Google Scholar]
- 28.Baron J, Weisberg RA. Mutations of the phage lambda nutL region that prevent the action of nun, a site-specific transcription termination factor. J. Bacteriol. 1992;174:1983–1989. doi: 10.1128/jb.174.6.1983-1989.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Court DL, Patterson TA, Baker T, Costantino N, Mao X, Friedman DI. Structural and functional analyses of the transcription-translation proteins NusB and NusE. J. Bacteriol. 1995;177:2589–2591. doi: 10.1128/jb.177.9.2589-2591.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward DF, DeLong A, Gottesman ME. Escherichia coli nusB mutations that suppress nusA1 exhibit lambda N specificity. J. Mol. Biol. 1983;168:73–85. doi: 10.1016/s0022-2836(83)80323-4. [DOI] [PubMed] [Google Scholar]
- 31.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Chrystallogr. 1993;26:795–800. [Google Scholar]
- 33.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Chrystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 34.Winn MD, Musshudov GN, Papiz MZ. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 2003;374:300–321. doi: 10.1016/S0076-6879(03)74014-2. [DOI] [PubMed] [Google Scholar]
- 35.Santoro MM, Bolen DW. Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl α-chymotrypsin using different denaturants. Biochemistry. 1988;27:8063–8068. doi: 10.1021/bi00421a014. [DOI] [PubMed] [Google Scholar]
- 36.Swint L, Robertson AD. Thermodynamics of unfolding for turkey ovomucoid third domain: Thermal and chemical denaturation. Protein Sci. 1993;2:2037–2049. doi: 10.1002/pro.5560021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayr LM, Landt O, Hahn U, Schmid FX. Stability and folding kinetics of ribonuclease T1 are strongly altered by the replacement of cis-proline 39 with alanine. J. Mol. Biol. 1993;231:897–912. doi: 10.1006/jmbi.1993.1336. [DOI] [PubMed] [Google Scholar]
- 38.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Chrystallogr. D. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 39.Altieri AS, Mazzulla MJ, Horita DA, Coats RH, Wingfield PT, Das A, Court DL, Byrd RA. The structure of the transcriptional antiterminator NusB from Escherichia coli. Nat. Struct. Biol. 2000;7:470–474. doi: 10.1038/75869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis IW, Murray LW, Richardson JS, Richardson DC. MOLPROBITY: Structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 2004;32:W615–W619. doi: 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.