Abstract
Aristolochic acids I and II (AA-I, AA-II) are found in all Aristolochia species. Ingestion of these acids either in the form of herbal remedies or as contaminated wheat flour causes a dose-dependent chronic kidney failure characterized by renal tubulointerstitial fibrosis. In ∼50% of these cases, the condition is accompanied by an upper urinary tract malignancy. The disease is now termed aristolochic acid nephropathy (AAN). AA-I is largely responsible for the nephrotoxicity while both AA-I and AA-II are genotoxic. DNA adducts derived from AA-I and AA-II have been isolated from renal tissues of patients suffering from AAN. We describe the total synthesis, de novo, of the dA and dG adducts derived from AA-II, their incorporation site-specifically into DNA oligomers and the splicing of these modified oligomers into a plasmid construct followed by transfection into mouse embryonic fibroblasts. Analysis of the plasmid progeny revealed that both adducts blocked replication but were still partly processed by DNA polymerase(s). Although the majority of coding events involved insertion of correct nucleotides, substantial misincorporation of bases also was noted. The dA adduct is significantly more mutagenic than the dG adduct; both adducts give rise, almost exclusively, to misincorporation of dA, which leads to AL-II-dA→T and AL-II-dG→T transversions.
INTRODUCTION
Various species of Aristolochia have been used as medicinal herbs since the time of Hippocrates to treat diverse disorders including snake-bite, fever, infection, gout, diarrhea and inflammation (1). A traditional use of this herb, as its Greek name implies, has been to assist women in childbirth (2). As part of a screening program for new anti-tumor agents, Kupchan and Doskovich (3) reported that aristolochic acid I (AA-I) (1; Figure 1), a principal chemical constituent of Aristolochia indica, was highly toxic to cells in culture; in addition, the compound proved to be nephrotoxic in Phase I clinical trials (4). Development of aristolochic acid as a drug was abandoned after Mengs reported its carcinogenicity in rodents (5). Earlier reports that Aristolochia sp. might be nephrotoxic in humans was dramatically confirmed in 1993 (6). Of more than 1800 Belgian women who had been given pills that contained, by error (7), Aristolochia fangchi as part of a slimming regimen, more than 100 women later developed chronic renal failure. Shortly thereafter, Cosyns and his colleagues (8) reported that these same patients also were at risk for urothelial carcinomas. The clinical syndrome was initially termed Chinese herbs nephropathy (CHN); later, it was suggested (9) that the generic term ‘aristolochic acid nephropathy’ (AAN) be used in place of CHN.
Figure 1.
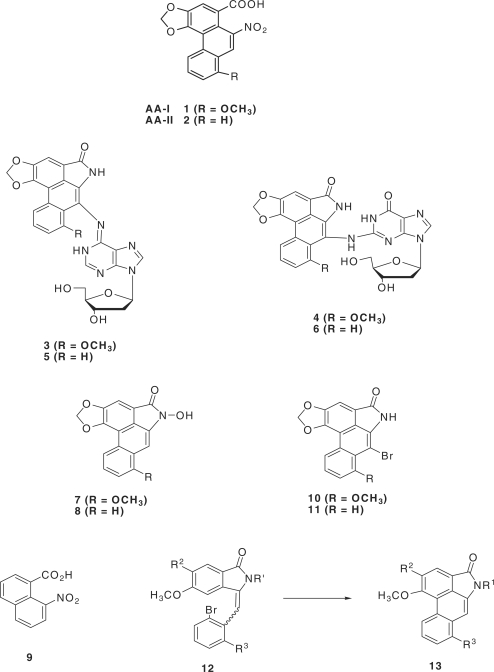
Structure of the aristolochic acids AA-I and AA-II, the dA and dG adducts of AL-I and AL-II and related compounds discussed in the text.
These observations drew attention to an endemic disease known as Balkan nephropathy (BEN), occurring exclusively in residents of farming villages in the Danube river basin (10). In a prescient report, Ivic (11) suggested that the origins of BEN might lie in the A. clematitis that grows in the wheat fields in the endemic region. Upper urinary track carcinomas develop in approximately 50% of BEN cases, often associated with renal insufficiency (12,13). That the histopathology and clinical features of BEN are nearly identical to those of the disease reported in Belgium was recognized by Cosyns and co-workers (14). Since then, several groups have used AA-I or a mixture of AA-I and AA-II to reproduce the main features of AAN in rodents (15–17), removing any doubt that the aristolochic acids are responsible for CHN. In areas where BEN is endemic, A. clematitis grows in the wheat fields, and its seeds, which contain significant quantities of the AAs, co-mingle with wheat grain contaminating the flour used for home-baked bread (18). Although most residents of an endemic village are potentially exposed to the AAs, <10% suffer from BEN due to differences in exposure or to the fact that a subset of the populace is resistant to the effects of AAs due to individual genetic variation. Sato and his associates (16) reported significant differences in tissue responses to various AAs among various strains of mice, a finding confirmed by Shibutani et al. (17).
The genotoxicity of AAs is supported by the finding of AA-derived DNA adducts in renal cortex of humans (19,20). These adducts were identified as 3 and 4, derived from the aristolactam (AL) metabolite of AA-I, and the corresponding adducts 5 and 6, derived from the AL metabolite of AA-II (21). In humans with AAN, AL-dA adducts are invariably more abundant than AL-dG (22). Adducts arise by the same metabolic pathways as do other aromatic nitro compounds (23), in which the intermediate N-hydroxyamines (in the cases under discussion, the N-hydroxylactams 7 and 8 or their O-acetylated or O-sulfonylated derivatives) are the likely pro-carcinogens. Surprisingly, C-8 purine adducts are not formed, and only products of attack at the exocyclic amino groups of dA and dG have been detected. Recently Grollman and his group (19) have shown that ‘signature’ A:T→T:A mutations predominate in the p53 tumor-suppressor gene isolated from urothelial cancers associated with BEN. However, the molecular mechanism by which AA-I, but not AA-II, induces proximal tubule damage remains a mystery.
Cases of AAN have been reported in China and in other countries where herbal remedies are widely used (24), and the disease has been described as a problem of global dimensions (25,26). Studies by Schmeiser and his associates (21), Nortier et al. (27) and more recently by Grollman and coworkers (18,19,25) strongly support the idea that the AAs play a causative role in the upper urinary track carcinomas in humans exposed to these toxins. There is a pressing need for public health authorities to take action to reduce human exposure to this powerful nephrotoxic carcinogen (28). Recently, in a comprehensive review of the subject the National Toxicology Program has designated the aristolochic acids as established human carcinogens (24).
In this article we describe the total synthesis, in quantity, of the dA and dG adducts derived from AA-II (5 and 6, respectively), allowing not only their complete chemical characterization but also their use as standards for the identification of AL-DNA adducts in human tissues by mass spectrometric methods and for their site-specific incorporation into oligomeric DNA of any designated sequence. We discuss also some of the difficulties associated with the chemistry of the AAs and the reasons that we adopted a ‘total synthesis’ route to the adducts. Finally, we present the results of site-specific mutagenesis studies in mouse embryonic cells designed to establish the mutagenic potential and specificity of these lesions in vivo.
MATERIALS AND METHODS
All reagents and solvents employed in this experimental work were reagent grade and were used as such unless otherwise specified. Melting points were taken in a Thomas-Hoover open capillary melting point apparatus and are uncorrected. 1H NMR spectra were recorded either on a Varian Gemini 300 or a Varian NOVA 400 spectrometer. Samples prepared for NMR analysis were dissolved in CDCl3 or DMSO-d6. Chemical shifts are reported in parts per million (ppm) relative to TMS. Mass spectra were recorded on either a Thermo Electron DSQ GC/MS equipped with a solid probe inlet and EI ionization or a Micromass Platform mass spectrometer using electrospray ionization. Thin-layer chromatography (TLC) was performed on silica gel sheets (Tiedel-deHaën, Sleeze, Germany). After appropriate purification all new products showed a single spot on TLC analysis in two solvent systems: (i) 30% EtOAc in hexanes and (ii) 5% MeOH in CH2Cl2. Components were visualized by UV light (λ = 254 nm) or by spraying with a solution of 2% phosphomolybdic acid in ethyl alcohol containing 5% sulfuric acid. Flash column chromatographic separations were carried out on 60 Å (230–400 mesh) silica gel (TSI Chemical Co., Cambridge, MA). All experiments dealing with moisture or air-sensitive compounds were conducted under dry nitrogen. The starting materials and reagents, unless otherwise specified, were the best grade commercially available (Sigma-Aldrich, Milwaukee, WI or Fluka Chemie GmbH, Sigma-Aldrich, Germany) and were used without further purification.
7H-Furo[3′,4′:4,5]benzo[1,2-d][1,3]dioxol-5-one (15)
Cuprous cyanide (115.2 g; 1.286 mol) was added to formamide (800 ml) containing water (19.2 g), and the mixture was heated with stirring to 100°C. 2-Bromopiperonyl alcohol 14 (147 g; 0.636 mol; mp 89–90°C) (29), easily obtained by the bromination of piperonyl alcohol in methanol at 25°C, was then added in portions over a period of 10 min. The temperature of the mixture was raised to 160°C, and a vigorous reaction set in, the temperature rising spontaneously to 178°C with the evolution of ammonia and steam. Over the next 30 min the temperature subsided to 170°C and thereafter was maintained at 165–170°C for 2 h. The mixture was allowed to cool to 100°C, poured into a solution of sodium cyanide (192 g; 3.92 mol) in water (800 ml), stirred for 30 min followed by the addition of CH2Cl2 (2 l). The mixture was filtered through diatomaceous earth, the organic phase was removed and the aqueous phase again was extracted with CH2Cl2 (400 ml). The organic extracts were combined and dried over MgSO4. Silica gel (25 g) and charcoal (5 g) were added to the solution while the drying agent was still present, the mixture was stirred for 5 min then filtered, and the filter cake was washed with boiling CH2Cl2 (200 ml). Removal of the solvent from the filtrate left a yellow–orange colored residue of crude 15 (75.6 g; yield 66.8%), mp 185–187°C—Lit. 190–191°C (30). Recrystallization from EtOAc/CH2Cl2 gave two crops, 55 g and 10 g, each as a pale yellow solid, both with mp 190–191°C. Combined yield of pure lactone 15 was 65 g (57.4%). 1H NMR (CDCl3) δ 5.22 (s, 2H), 6.16 (s, 2H), 6.87 (s, 1H), 7.26 (s, 1H).
4-Nitro-7H-furo[3′,4′:4,5]benzo[1,2-d][1,3]dioxol-5-one (16)
Concentrated sulfuric acid (320 ml) was cooled in water-ice bath (20°C), and lactone 15 (63.5 g, 35.9 mmol) was added in small portions with stirring over 10 min while keeping the temperature <20°C. The solution was cooled to 5°C, and concentrated (70%) nitric acid (24.7 ml; 37.8 mmol) was added drop-wise over a period of 30 min (exothermic) while maintaining the reaction temperature <5°C (ice-MeOH bath). Stirring was continued for 2 h at 7–10°C, then the mixture was poured on to ice (2 kg). The resulting yellow precipitate was removed by filtration and washed with water until free of acid. The product was air dried and recrystallized from EtOH to give 16 as a yellow solid (70.5 g, 88%); mp 186–187°C; 1H NMR (DMSO-d6) δ 7.39 (s, 1H), 6.41 (s, 2H), 5.26 (s, 2H); electrospray mass spectrometry (ESI-MS) (M + H)+ 224.1.
4-Amino-7H-furo[3′,4′:4,5]benzo[1,2-d][1,3] dioxol-5-one (17)
Nitro-lactone 16 (15 g, 67.3 mmol) was dissolved in DMF (120 ml), and 5% Pd/C catalyst (1.5 g) was added under nitrogen. The mixture was then shaken in a Parr hydrogenator at 60 psi overnight. The reaction mixture was then warmed to dissolve some precipitated product, and the catalyst was removed by filtration. The filtrate was concentrated under vacuum, and the residual liquid was poured into water. The resulting solid was collected and recrystallized from EtOH to give 17 as white crystals (12 g, 92%); mp 238–239°C; 1H NMR (DMSO-d6) δ 6.36 (s, 1H), 6.04 (s, 2H), 5.88 (s, 2H), 5.11 (s, 2H); EI-MS M+ 193.2.
4-Amino-8-bromo-7H-furo[3′,4′:4,5]benzo[1,2-d][1,3] dioxol-5-one (18)
Amino-lactone 17 (3.8 g, 19.8 mmol) was dissolved in dry pyridine (100 ml), and bis(N-methyl-2-pyrrolidinone)hydrogen tribromide (8 g, 23.7 mmol) was added under a nitrogen atmosphere with magnetic stirring. After 15 h, TLC analysis (EtOAc:hexanes/3:7) showed complete absence of the starting material. The pyridine was removed under reduced pressure, and the residue, after dissolution in CH2Cl2, was washed with 10% NaHCO3 solution. The organic layer was washed with water then dried over anhydrous Na2SO4. After removal of the solvent, the resulting solid was suspended in a minimum amount of CH2Cl2, triturated well, filtered and dried to give almost pure 18. Recrystallization from isopropyl ether gave the pure material (4.6 g, 86%); mp 210–212°C; 1H NMR, (DMSO-d6) δ 6.16 (s, 2H), 6.03 (s, 2H), 5.06 (s, 2H); ESI-MS (M + H)+ 272.2.
8-Bromo-7H-furo[3′,4′:4,5]benzo[1,2-d][1,3] dioxol-5-one (19)
Amino-lactone 18 (4.9 g, 18.08 mmol) was dissolved in concentrated HCl (100 ml), and the resulting solution was diluted with cold water (200 ml) then cooled to −5°C. To this mixture sodium nitrite solution (1.2 g/180 ml) was added dropwise while maintaining the internal temperature <0°C. After the addition was completed, the mixture was stirred for 2 h at 0°C, then cold hypophosphorous acid (30%) (68 ml) was added dropwise while maintaining the internal temperature at <0°C. Thereafter, stirring was continued for 2 h, then the mixture was held at 5°C overnight. The pale pink precipitate was removed by filtration, washed with cold water and dried to give the pure desired product 19 (4.3 g, 73.7%); mp. 173–174°C; 1HNMR (DMSO-d6) δ 7.27 (s, 1H), 6.28 (s, 2H), 5.19 (s, 2H); ESI-MS (M + H)+ 257.2.
2-(7-Oxo-5,7-dihydro-furo[3′,4′:4,5]benzo[1,2-d][1,3] dioxol-4-yl)-benzaldehyde (21)
A solution of bromolactone 19 (10 g, 39 mmol) in dioxane (150 ml) was degassed with nitrogen for 10 min and 1,1′-bis(diphenylphosphino)ferrocene]palladium dichloride catalyst (0.85 g) was added. Degassing was continued for an additional 10 min, then a solution of Na2CO3 (3.5 g) in water (120 ml; previously degassed with nitrogen) was added, and nitrogen was bubbled through reaction mixture for an additional 30 min. To this mixture 2-formylphenylboronic acid 20 (7.02 g, 46.8 mmol) was added, and the mixture was refluxed for 6 h after which TLC analysis (EtOAc: hexanes/3:7) showed the reaction to be complete. The mixture was cooled, diluted with EtOAc and then filtered. Solvent removal gave a solid residue which was dissolved in CH2Cl2, and the solution was washed with water then dried over anhydrous Na2SO4. The isolated product was purified by column chromatography over silica gel (elution with hexane:EtOAc/70:30) which afforded pure compound 21 (7 g, 63.7%); mp 145–146°C; 1H NMR (CDCl3) δ 9.94 (s, 1H), 8.08–8.03 (m, 1H), 7.73–7.66 (m, 1H), 7.42–7.39 (m,1H), 7.30 (s, 1H), 6.13–6.09 (d, 2H), 5.20–4.88 (dd, 2H); ESI-MS (M + H)+ 283.3.
6H-Benzo[f]1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd] furan-5-one:Aristolactone II (22)
The lactonic aldehyde 21 (7.3 g, 25.9 mmol) was dissolved in anhydrous THF, and anhydrous potassium t-butoxide (5.7 g) was added under nitrogen. The mixture was refluxed with stirring for 4 h, then cooled and the solvent removed under reduced pressure. The residue was taken up in MeOH (100 ml), acidified with 12 N HCl (20 ml) and the solution was refluxed for 1 h. After cooling and removal of the MeOH, the residue was dissolved in CH2Cl2, and the solution was washed with water (50 ml), then with saturated NaHCO3 solution (50 ml), again with water (2 × 50 ml) and finally with brine (50 ml). The solution was dried over anhydrous Na2SO4, filtered and evaporated to dryness. The resulting yellow solid was purified by column chromatography on silica gel. Elution with 1% MeOH in CH2Cl2 gave the pure desired product AA-II lactone 22 (3.7 g, 55%); mp 183–184°C (31); 1H NMR (DMSO-d6) δ 8.50–8.47, (m, 1H), 8.05–8.04 (m, 1H), 7.86 (s, 1H), 7.69–7.68 (m, 2H), 7.50 (s,1H), 6.56 (s, 2H); ESI-MS (M + H)+ 265.3.
6H-Benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd] indol-5-one:Aristolactam II (23)
A mixture of lactone 22 (600 mg, 2.3 mmol), concentrated aqueous ammonium hydroxide (8 ml), sodium sulfite (450 mg) and ammonium chloride (350 mg) dissolved in water (1 ml) was heated in a sealed tube at 140°C overnight. The lactone went into solution at 110–115°C, then gradually, as the reaction proceeded, a solid separated. The mixture was cooled, filtered and the solid product was washed with water then dried to give an almost quantitative yield of pure AL-II (23); mp 297–298°C (32); 1H NMR (DMSO-d6) δ 10.72 (s, 1H), 8.60–8.58 (d, 6 Hz, 1H), 7.94–7.92 (d, 6 Hz, 1H), 7.61 (s, 1H), 7.60–7.58 (m, 2H), 7.09 (s, 1H), 6.46 (s, 1H) essentially identical with the published spectrum (32); ESI (M + H)+ 264.3.
7-Bromo-6H-benzo[f][1,3]dioxolo[4′,5′:4,5] benzo[1,2,3-cd]indol-5-one (11)
To a solution of 23 (1.5 g, 5.7 mmol) dissolved in glacial HOAc (10 ml) and cooled in an ice bath was added anhydrous NaOAc (500 mg, 6 mmol) followed by the drop-wise addition of bromine (1.18 g) in HOAc (5 ml). After the addition was complete, the reaction mixture was stirred for 15 min and filtered. The collected solid was washed with CH2Cl2, then water and dried to give compound 11 (1.9 g, quantitative) mp 301–302°C. It was virtually insoluble in any of the usual organic solvents and very sparingly soluble in hot DMSO. 1H NMR (hot DMSO-d6) δ 11.19 (s, 1H), 8.61–8.59 (d, 6 Hz, 1H), 7.82–7.80 (m, 3H), 6.52 (s, 2H); EI-MS M+ 341.2.
7-Bromo-6-(tert-butyl-dimethylsilanyloxymethyl)-6H-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-5-one (24)
To a solution of sodium hydride (120 mg, 3.2 mmol; 60% suspended in mineral oil) in dry DMF (20 ml), AA-II bromolactam (11), (900 mg, 2.6 mmol) was added, and the mixture was heated at 50°C for 30 min under a nitrogen atmosphere. After cooling to ∼5°C, freshly prepared (33) tert-butylchloromethoxydimethylsilane (0.5 g, 3 mmol) was added by syringe. The ice bath was removed after 10 min, and the reaction mixture was stirred at 24°C for 0.5 h when TLC analysis (EtOAc: hexanes/2:8) showed completion of the reaction. The DMF was removed under reduced pressure, and the residue taken up in CH2Cl2 (50 ml).This solution was washed with water, dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure to give the crude product. This was purified by column chromatography (EtOAc: hexanes/2:8) on silica gel to afford the pure desired N-protected lactam 24 (800 mg, 63%). mp 199–200°C; 1H NMR (CDCl3) δ 8.62 (dd, 6H, 1H), 8.5 (dd, 6 Hz, 1H), 7.79 (m, 2H), 7.6 (s, 1H), 6.01 ((s, 2H), 5.95 (s, 2H), 0.0 (s, 9H), 5.95 (s, 6H); ESI-MS (M + H)+ 486.5.
7-{9-[4-(tert-Butyl-dimethyl-silanyloxy)-5-(tert-butyl-dimethyl-silanyloxymethyl)-tetrahydro-furan-2-yl]-1,9-dihydro-purin-6-ylideneamino}-6-(tert-butyl-dimethyl-silanyloxymethyl)-6H-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-5-one (26)
An oven-dried 100 ml two-necked flask was charged with the protected dA 25 (920 mg, 1.9 mmol), cesium carbonate (1.82 g, 5.6 mmol), trisdibenzylideneacetone dipalladium catalyst (220 mg, 0.24 mmol), xantphos (460 mg, 0.79 mmol), the protected 7-bromo AL-II (24, 465 mg, 0.96 mmol) and toluene (30 ml). This mixture was stirred under a nitrogen atmosphere for 30 min at room temperature and then heated at 100°C for 5 h. After cooling to room temperature, the solids were removed by filtration and washed with EtOAc. The filtrate was evaporated to dryness, and the residual crude product was purified by chromatography over silica gel using 5% EtOAc in CH2Cl2 as the eluent to afford pure compound 26 as a colorless glassy solid (750 mg, 88%). 1H NMR (CDCl3): δ 8.67 (d, 1H), 8.58 (s, 1H), 8.32 (d, 1H), 8.25 (br s, 1H), 7.89 (d, 1H), 7.45–7.58 (m, 3H), 6.51 (t, 1H), 6.46 (s, 2H), 5.80 (s, 1H), 5.19 (s, 1H), 4.66 (m, 1H), 4.04 (m, 1H), 3.95–3.78 (m, 2H), 2.70 (m, 1H), 2.47 (m, 1H), 0.94–0.90 (m, 27H), −0.10 to 0.21 (m, 18H); ESI-MS (M + H)+ 885.5.
7-[9-(4-Hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1,9-dihydro-purin-6-ylideneamino]-6H-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-5-one (5)
To an ice-cold solution of the silyl-protected compound 26, (700 mg, 0.81 mmol) in pyridine (15 ml) was added 70% HF in pyridine (2 ml) over a period of 3 min. The mixture was stirred at room temperature overnight, then poured into aqueous 10% NaHCO3 (50 ml) and stirred for 2 h. The solid that precipitated was collected, washed with water and then added to THF (10 ml) and concentrated aqueous ammonia (5 ml). This mixture was heated at 70°C in a closed vial overnight, then taken to dryness, and the remaining solid was chromatographed over silica gel using 7.5% MeOH in CH2Cl2 to elute the pure deprotected adduct 5 (0.35 g; 84%) whose UV absorbance spectrum was identical to that published by Pfau et al. (34). 1H NMR (DMSO-d6): δ 10.80 (s, 1H), 9.83 (br s, 1H), 8.70 (m, 2H), 8.41 (s, 1H), 8.00 (m, 1H), 7.55 (m, 3H), 6.59 (s, 2H), 6.49 (t, 1H), 5.19 (br s, 1H), 4.49 (m, 1H), 3.39 (br s, 1H), 3.75–3.51 (m, 2H), 3.21 (m, 1H), 2.83 (m, 1H), 2.37 (m, 1H); ESI-MS (M + H)+ 513.3.
7-(9-{5-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-hydroxy-tetrahydro-furan-2-yl}-1,9-dihydro-purin-6-ylideneamino)-6H-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-5-one (27)
To a solution of compound 5 (165 mg, 0.32 mmol) in pyridine (5 ml) was added solid dimethoxytrityl (DMT) chloride (165 mg, 0.48 mmol), and the mixture was stirred at room temperature for 3 h at which time TLC analysis (CH2Cl2/MeOH 92:8) showed the reaction to be ∼90% complete. MeOH (5 ml) was added, and the mixture was stirred for 30 min at room temperature, then concentrated under reduced pressure. To the residue was added aqueous 10% NaHCO3 (25 ml), and the mixture was extracted with CH2Cl2. The extract was dried over anhydrous MgSO4, filtered and concentrated to give the crude product which was purified by chromatography over silica gel. Elution with 5–10% MeOH in CHCl2 afforded pure DMT-protected compound 27 again a glassy solid (120 mg; 66% based on unrecovered 5; 50 mg of 5 was recovered). 1H NMR (CDCl3): δ 9.80 (br s, 1H), 8.58 (s, 1H), 8.23 (s, 1H), 8.08 (s, 1H), 8.01 (br s, 1H), 7.59 (m, 2H), 7.42 (m, 3H), 7.38–7.18 (m,8H), 6.79 (m, 4H), 6.38 (t, 1H), 6.23 (s, 2H), 4.70 (br s, 1H), 4.20 (m, 1H), 3.79 (s, 6H), 3.40 (m, 3H), 2.81 (m, 1H), 2.47 (m, 1H); ESI-MS (M + H)+ 815.5.
Diisopropyl-phosphoramidous acid, 2-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-5-[6-(5-oxo-5,6-dihydro-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-7-ylimino)-1,6-dihydro-purin-9-yl]-tetrahydro-furan-3-yl ester, 2-cyano-ethyl ester (28)
DMT compound 27 (110 mg, 0.14 mmol) was co-evaporated with dry toluene (3 × 10 ml) and then dissolved in dry CH2Cl2 (10 ml). Tetrazole (11.3 mg, 0.16 mmol) was added to the solution followed by 2-cyanoethyl N,N,N′,N′-tetraisopropylphosphordiamidite (57 mg, 0.19 mmol). The reaction mixture was stirred at room temperature for 2 h under nitrogen and then diluted with CH2Cl2 (25 ml) containing 2% TEA. The CH2Cl2 layer was washed with aqueous saturated NaHCO3 solution, dried over Na2SO4, filtered and concentrated under reduced pressure to obtain the desired product 28 as a viscous oil (145 mg, 100%), which was used as such in the preparation of DNA oligomers. 1H NMR (CDCl3): δ 9.95 (br s, 1H), 8.60 (s, 1H), 8.25 (s, 1H), 8.09 (s, 1H), 7.99 (br s, 1H), 7.62–7.22 (m, 13H), 6.81 (m, 4H), 6.40 (t, 1H), 6.23 (s, 2H), 4.68 (m, 1H), 3.92 (m, 2H), 3.81 (s, 6H), 3.42 (m, 3H), 2.99 (m, 2H), 2.81 (m, 1H), 2.62 (m, 2H), 2.47 (m, 1H), 1.08 (m, 12H); 31P NMR (CDCl3): 150.2, 149.8.
7-{6-Benzyloxy-9-[4-(tert-butyldimethylsilyloxy)-5-(tert-butyldimethylsilyloxymethyl)-tetrahydrofuran-2-yl]-9H-purin-2-ylamino}-6-(tert-butyldimethylsilyloxymethyl)-6H-benzo[f][1,3]dioxolo[4′5′-4,5]benzo[1,2,3-cd] indol-5-one (30)
A dry 100 ml two-necked flask was charged with the fully protected dG derivative 29, (418 mg: 0.71 mmol), cesium carbonate (0.785 g, 2.4 mmol), trisdibenzylideneacetone dipalladium catalyst (164 mg, 0.18 mmol), xantphos (343 mg, 0.59 mmol), compound 24 (450 mg, 0.93 mmol) and toluene (25 ml) under a nitrogen atmosphere. This mixture was stirred for 30 min at room temperature and then heated at 100°C for 6 h. After cooling to room temperature, the mixture was filtered, and the collected solids were washed with EtOAc. After vacuum evaporation of the solvent, the residue was purified by chromatography over silica gel. Elution with 5% EtOAc in CHCl2 afforded pure compound 30 as a solid glass (703 mg, 75%). 1H NMR (CDCl3): δ 8.75 (d, 1H), 8.13 (d, 2H), 8.04 (br s, 1H), 7.70 (s, 1H), 7.60 (m, 2H), 7.09–7.18 (m, 5H), 6.42 (s, 2H), 6.29 (t, 1H), 5.93 (s, 1H), 5.19 (s, 3H), 4.42 (m, 1H), 3.98 (m, 1H), 3.78 (m, 2H), 2.62 (m, 1H), 2.27 (m, 1H), 1.05–0.95 (m, 27H), 0.31–0.12(m, 18H); ESI-MS (M + H)+ 991.5.
7-[6-Benzyloxy-9-(4-hydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-9H-purin-2-ylamino]-6H-benzo[ f ][1,3]dioxolo[4′,5′-4,5]benzo[1,2,3-cd]indol-5-one (31)
To an ice-cold solution of compound 30 (530 mg, 0.53 mmol) in pyridine (10 ml) was added 70% HF in pyridine (1.7 ml) over a period of 3 min. The mixture was stirred at room temperature overnight, poured into ice-cold aqueous 10% NaHCO3 solution and again stirred for 2 h. The separated solids were filtered, washed with water and added to THF (10 ml) and concentrated ammonia (5 ml). The mixture was then heated overnight at 70°C in a closed vial. The solvents were removed by vacuum evaporation, and the residue was purified chromatographically over silica gel. Elution with CH2Cl2/MeOH (9:1) gave pure coupled product 31 as a glassy solid (300 mg, 91%). 1H NMR (DMSO-d6): δ 10.62 (s, 1H), 9.21 (s, 1H), 8.98 (br s, 1H), 8.19 (s, 1H), 8.05 (m, 1H), 7.79 (m, 1H), 7.59 (m, 2H), 7.02–7.18 (m, 5H), 6.45 (s, 2H), 6.20 (t, 1H), 5.20 (br s, 1H), 4.79 (br s, 1H), 4.20 (m,1H), 3.76 (m, 2H), 2.60 (m, 1H), 2.19 (m, 1H); ESI-MS (M + H)+ 619.4.
7-[9-(4-Hydroxy-5-hydroxymethyl-tetrahydrofuran-2-yl)-6-oxo-6,9-dihydo-1H-purin-2-ylamino]-6H-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-5-one (6)
To a solution of 31 (50 mg, 0.08 mmol) in methanol (50 ml) was added a 10% palladium-on-carbon catalyst. The flask was evacuated (50 torr) and flushed with hydrogen thrice. The reaction mixture was hydrogenated for 16 h at 50 psi with stirring. It was then filtered through a pad of Celite, the Celite bed was washed with DMF (20 ml), and the filtrate was concentrated under reduced pressure. The residue was diluted with water (20 ml); the separated solid was filtered and dried in a vacuum oven overnight (26 mg, 78%). 1H NMR (DMSO-d6): δ 12.04 (br s, 1H), 10.98 (br s, 1H), 10.82 (br s, 1H), 8.61 (d, 1H), 8.02 (m, 1H), 7.93 (s, 1H), 7.67–7.61 (m, 3H), 6.50 (s, 2H), 6.22 (s, 1H), 5.83 (t, 1H), 5.05 (m, 1H), 4.59 (br s, 1H), 3.98 (br s, 1H), 3.59 (m, 1H), 3.17 (br s, 1H), 2.44 (m,1H), 1.99 (m,1H); MALDI-MS (M + H)+ 529.1.
7-(6-Benzyloxy-9-{5-[bis(4-methoxyphenyl)-phenylmethoxymethyl]-4-hydroxy-tetrahydrofuran-2-yl}-9H-purin-2-ylamino)-6H-benzo[f][1,3]dioxolo[4′,5′-4,5] benzo[1,2,3-cd]indole-5-one (32)
To a solution of compound 31 (225 mg, 0.36 mmol) in pyridine (8 ml) was added solid DMT chloride (200 mg; 0.59 mmol), and the mixture was stirred at room temperature for 3 h. TLC (CH2Cl2/MeOH 92:8) showed the reaction to be ∼90% complete. The product was then worked up as in the case of 27, noted above, and purified by chromatography on silica gel. Elution with 5–10% MeOH in CH2Cl2 gave the pure DMT-protected compound 32 as a colorless glass (190 mg, 57% based on unrecovered 31; recovered 31 amounted to 99 mg). 1H NMR (CDCl3): δ 9.62 (br s, 1H), 8.60 (s, 1H), 7.90 (d, 1H), 7.83 (s, 1H), 7.56 (m, 2H), 7.38 (m, 5H), 7.28–7.12 (m, 11H), 6.77 (m, 4H), 6.36 (t, 1H), 6.22 (s, 2H), 5.48 (s, 2H), 4.55 (br s, 1H), 4.23 (m, 1H), 3.73 (s, 6H), 3.20 (m, 2H), 2.62 (m, 2H); ESI-MS (M + H)+ 921.3.
7-(9-{5-[bis(4-Methoxyphenyl)phenylmethoxymethyl]-4-hydroxy-tetrahydrofuran-2-yl}-6-oxo-6,9-dihydro-1H-purin-2-ylamino)-6H-benzo[f][1,3]dioxolo[4,′5′-4,5] benzo[1,2,3-cd]indol-5-one (33)
To a solution of compound 32 (280 mg, 0.30 mmol) in EtOAc/MeOH (1:1, 20 ml) was added 10% Pd/C catalyst (50 mg). The flask was evacuated (50 torr), flushed thrice with hydrogen and then shaken under hydrogen for 16 h at 50 psi. The resulting solution was filtered through a pad of celite and concentrated under reduced pressure. The residue was purified by silica gel column chromatography. Elution with 7–10% MeOH in CHCl2 afforded pure debenzylated product 33 (220 mg, 91%). 1H NMR (CDCl3): δ 12.40 (br s, 1H), 10.62 (br s, 1H), 9.82 (br s, 1H), 8.49 (m, 1H), 8.02 (m, 1H), 7.66 (s, 1H), 7.59 (m, 1H), 7.27 (m, 6H), 6.90 (m, 5H), 6.86 (m, 4H), 6.60 (s, 2H), 6.28 (m, 1H), 5.57 (m, 1H), 3.74 (m, 9H), 2.62 (m, 1H), 2.45 (m, 1H); ESI-MS (M + H)+ 831.0.
Diisopropylphosphoramidous acid, 2-[bis(4-methoxyphenyl)phenylmethoxymethyl]-5-[6-oxo-2-(5-oxo-5,6-dihydrobenzo[f][1,3]dioxolo[4,′,5′-4,5]benzo[1,2,3-cd]indol-7-ylamino)-1,6-dihydropurin-9-yl]tetrahydrofuran-3-yl ester-2-cyano-ethyl ester (34)
The DMT derivative 33 (180 mg, 0.22 mmol) was co-evaporated with dry toluene (3 × 10 ml), and the residue was dissolved in dry CH2Cl2 (10 ml). Tetrazole (18.2 mg, 0.26 mmol) was added, followed by 2-cyanoethyl N,N,N′,N′-tetraisopropylphosphordiamidite (91.6 mg, 0.3 mmol), and the reaction mixture was stirred at room temperature for 2 h under nitrogen after which CH2Cl2 containing 2% TEA (25 ml) was added. This solution was then washed with aqueous saturated NaHCO3 solution (50 ml), dried over Na2SO4, filtered and concentrated under reduced pressure to yield the desired product 34 (245 mg, 100% yield) as a viscous oil. This was used directly in the synthesis of DNA oligomers. 1H NMR (CDCl3): δ 12.38 (br s, 1H), 10.62 (br s, 1H), 9.89 (br s, 1H), 8.62 (m, 1H), 8.42 (m, 1H), 8.02 (m, 1H), 7.99 (s, 1H), 7.82 (m, 2H), 7.42 (m, 2H), 7.40–7.05 (m, 9H), 6.66 (m, 4H), 6.37 (s, 2H), 6.22 (m, 1H), 5.60 (m, 1H), 4.62 (m, 1H), 4.02 (m, 2H), 3.83 (s, 6H), 2.97 (m, 2H), 2.60–2.31 (m, 4H), 1.07 (m, 12H); 31P NMR (CDCl3): 149.5, 149.4.
DNA synthesis
All oligomers were synthesized at the 1.0 µmol scale using an Applied Biosystems 394 DNA Synthesizer (Foster City, CA) with normal phosphoramidite reagents (Glen Research). Individual oligomers were liberated from the controlled pore glass (CPG) support by treatment with aqueous 28% ammonia at 55°C overnight which also removed all of the nucleobase-protecting groups. Purification of the DNA was accomplished in two stages. In the first the deprotected oligomers having a terminal DMT group were separated from failure sequences by means of a Waters HPLC system using a Luna phenyl-hexyl column (10 × 250 mm, 5 µm, Phenomenex, Torrence, CA) at a solvent flow rate of 4 ml/min. A solvent gradient of 16–36% acetonitrile in 0.1 M TEAA buffer (pH 6.8) was employed over 35 min. In the second stage the DMT group was removed by treatment with HOAc containing 20% water, and the naked DNA was again purified on the same column using a gradient of 5 to 15% acetonitrile in 0.1 M TEAA over a period of 45 min. Quality control was achieved using two methods. First, the masses of all of the deprotected oligomers were measured by ESI-MS using a Micromass Platform LC/MS. Each oligomer was infused directly into the ESI source via the autosampler without an HPLC column present. Second, selected oligomers were digested to the deoxynucleosides using a published procedure (35) and analyzed by a Thermo Quantum Ultra LC/MS/MS. Deoxynucleosides were separated on a Hewlett Packard 1100 HPLC system with an Aquasil C18 column (0.5 mm × 250 mm) at a flow rate of 12 µl/min. The solvent gradient was 0% B to 100% B over 20 min (solvent A—90:10 water:acetonitrile and 0.05% formic acid; solvent B—95:5 acetonitrile:water with 0.05% formic acid).
Construction of site-specifically modified plasmids
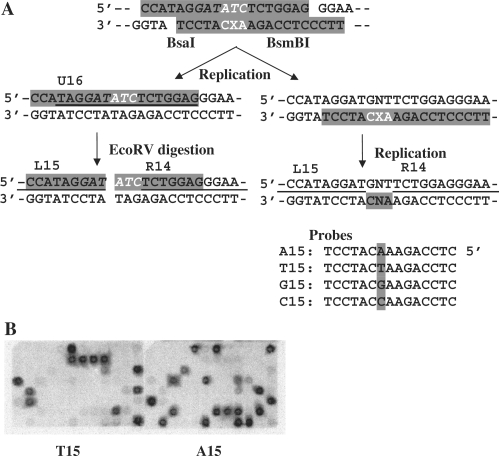
The methods for the construction of the shuttle vector (pMTEX4) and modified plasmid and the strategy for the site-specific mutagenesis experiments have been published (36). A modified 19-mer, 5′-TTCCCTCCAGAAXCATCCT, where X represents either the AL-II-dA or AL-II-dG adduct, and its complementary 19-mer, 5′-CCATAGGATATCTCTGGAG, were annealed following phosphorylation at their 5′-ends to form 4-nucleotide overhangs on both termini (5′-CCAT and 5′-TTCC) and mismatches on both sides of and opposite the adduct (5′-ATC/3′-CXA) (Figure 2A). The duplex oligodeoxynucleotide was ligated at 4°C overnight to the pMTEX4 vector, which had been digested with BsaI and BsmBI. Closed circular constructs containing a site-specific, single DNA adduct were purified by ultracentrifugation in a CsCl-ethidium bromide solution. The amount of modified construct was quantified by a UV spectrophotometer using a published procedure (36).
Figure 2.
Outline of experimental strategy (36). (A) Modified and unmodified 19 mers (highlighted), which form 3 consecutive base mismatches shown in white (X represents adduct) upon annealing, are ligated to pMTEX4 vector (40) digested with BsaI and BsmBI. The construct is introduced into MEFs and allowed to replicate. Progeny plasmids are recovered from transfected MEFs and analyzed for translesional events. Progeny derived from the unmodified strand are detected by the hybridization probe, U16 (underlined) while those derived from the modified strand are detected by the probes, A15, T15, G15 and C15, which identify nucleotides T, dA, dC and dG, respectively, inserted opposite the adduct. The partial sequences of probes (L15 and R14), which detect the sequence of the oligonucleotides ligated, are also underlined. Their full sequences are provided in the text. Refer to (B) for examples of hybridization. The ratio of progeny derived from the modified and unmodified strands, presented in Table 2 as a percentage, apparently reflects the blocking effects of DNA adducts on DNA synthesis. To facilitate the analysis of translesional coding events, recovered plasmid was digested with EcoRV restriction enzyme (the recognition sequence is italicized), which inactivates progeny derived from the unmodified strand. This digested sample was used to transform E. coli, and transformants were analyzed for the coding specificity at the adduct site, using A15, T15, G15 and C15 probes. (B) Filters probed with 32P-lablelled T15 (left panel) and A15 (right panel). “N” represents one of the four normal deoxynucleotides.
Introduction of the modified plasmid into mouse embryonic fibroblasts and recovery of the progeny plasmid
Immortalized mouse embryo fibroblasts (MEFs) were cultured in Dulbecco’s modified Eagle’s medium supplemented with fetal bovine serum (10%), penicillin (100 units/ml) and streptomycin (100 µg/ml) under 5% CO2 at 37°C. Cells (1 × 106) were plated in a 25-cm2 flask and cultured overnight after which they were transfected overnight with 500 ng of a modified construct by the FuGENE6 (Roche) method according to the manufacturer’s instruction. The next day, cells were detached by treating with trypsin–EDTA, seeded in a 150-cm2 flask and cultured for 4 days. Progeny plasmids were recovered by the method of Hirt (37) and analyzed for translesional events as described below.
Analysis of progeny plasmid for translesion events
To the recovered plasmids, 5 ng of pVgRXR (Invitrogen), which coded for zeocin resistance, was added. This plasmid served as an internal control for DpnI digestion. The mixture was treated with Dpn I (1 unit) for 1 h at 37°C to remove residual nonreplicated input DNA and then used to transform Escherichia coli DH10BMax electrocompetent cells (Invitrogen) by an E. coli Pulser (Bio Rad). Varying portions of a transformation mixture were plated on YT (1×) agar plates with ampicillin (100 µg/ml medium) and blasticidin S (50 µg/ml medium) or with zeocin (25 µg/ml medium). Since the adduct was located close to the blasticidin S resistance gene (36), transformants containing progeny plasmids with large deletions around the adduct site should not grow on a blasticidin-containing plate and were thus excluded from analysis. A marked reduction in the number of colonies on a zeocin-containing plate assured efficient digestion of nonreplicated plasmids by DpnI. E. coli transformants were picked individually and subjected to oligonucleotide hybridization as described in detail previously (38). Examples of hybridization results are shown in Figure 2B. Oligonucleotide probes (Figure 2A) were used to determine the DNA sequence at the adduct site. L15 (5′-GAGGAGCCATAGGAT) and R14 (5′-TCTGGAGGGAAGGG) probes were used to confirm the presence of the oligonucleotide insert and to detect untargeted mutations and small deletions around the adduct site. Plasmids that did not hybridize to both L15 and R14 probes were omitted from a further analysis. U16 (5′-TAGGATATCTCTGGAG) detected progeny derived from the unmodified complementary strand. A15, T15, G15 and C15 (5′-CTCCAGAANCATCCT, where N represents A, T, G or C) detected AL-II adduct→dA, →T, →dG and →dC base substitutions, respectively. When plasmids did not hybridize to any of these four probes, DNA sequencing was conducted. Thus, this strategy detected all types of events including base substitutions, frameshifts, deletions and insertions without any bias.
RESULTS AND DISCUSSION
The chemistry of AA-I and AA-II and total synthesis of the dA and dG adducts 5 and 6 derived from AA-II
AA-I (1) and AA-II (2) (Figure 1) occur together naturally in many Aristolochia species and are commercially available as a mixture. However, they cannot be separated cleanly by crystallization or by simple column chromatography, and their separation by HPLC is an extremely tedious procedure applicable only to small quantities. By contrast, the corresponding methyl esters (formed by the action of diazomethane) can be separated chromatographically, albeit with difficulty, but when basic hydrolysis is attempted, <6% of the acid is recovered (39). This unusual chemistry is a reflection of the steric strain—the aromatic version of 1,3-allylic strain (40)—that exists between the nitro and carboxyl functions and parallels identically the group compression that exists in 8-nitronaphthoic acid 9. The latter acid cannot be esterified by the standard Fischer–Speier method, and its methyl ester (formed by diazomethane) undergoes hydrolysis extremely slowly under forcing conditions (41). Similarly, attempts to form the acid chloride (SOCl2) of 9 leads to 8-chloronaphthoyl chloride and other decomposition products (42). Based on such aberrant chemistry, the possibility of obtaining multi-gram quantities of the individual AAs from natural sources seemed limited. We elected, therefore, to attempt to develop a new synthetic approach that would not only allow us to obtain the desired DNA adducts in quantity, but also would permit the synthesis of a wide range of AA-related substances potentially including the AAs themselves. The synthesis of AA-I had previously been accomplished by Kupchan and Wormser (43), but the method is impracticable; too many steps are involved, yields at some stages are low, the route lacks versatility and the final ester hydrolysis is extremely low-yielding as noted above. In addition the route did not seem practical for the synthesis of the 7- bromolactams 10 and 11, which we saw as the critical intermediates for the synthesis of the corresponding DNA adducts. Satisfactory synthetic routes to this class of lactam have been published by Estevez et al. (44,45) and by Couture and associates (46). The first method by the Estevez group involves a rather low-yielding benzyne cyclization step, whereas both of the other approaches involve the linking of the B and D rings using a tributyltin hydride radical reaction as a critical step in the synthesis of the basic phenanthrene. In addition the latter approach utilizes an isoindolenone intermediate (12) which is always obtained as a mixture of geometric isomers that have to be separated before conversion to the required penultimate lactam (13) is possible. These methods are suitable for the preparation of the aristolactams but not for the synthesis of the aristolochic acids themselves, one of the ultimate goals of our research. The approach that we have taken involves reversing the order of the assembly, first by linking the two principal aromatic rings (B and D) by means of a Suzuki reaction (tolerant of a nitro group), then cyclizing the resultant biphenyl intermediate to the desired phenanthrene. This route avoids the problem of the double bond isomerism and has proven to be both efficient and versatile for our purposes. In this report we present the successful application of the method to the synthesis of the naturally occurring AA-II adducts of dA and dG. The dA adduct derived from AA-II had been synthesized earlier at the milligram level by Pfau and coworkers (34) by the solvolysis of N-chloroaristololactam II in the presence of a large excess of 2′-deoxyadenosine. The method, although effective, requires an extensive purification procedure that is not adaptable to larger-scale work.
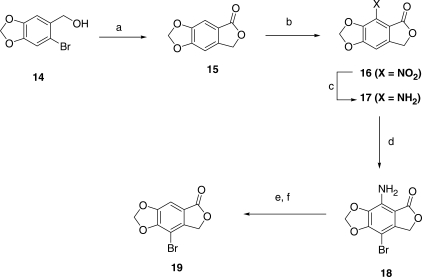
The key intermediate in the early phase of the synthetic work is the bromolactone 19, which was prepared according to Figure 3. Phthalide 15 had previously been prepared by a multi-step process (30), but in our hands was accomplished in a single step by heating 14 with cuprous cyanide at 160°C in 3% aqueous formamide. This has the advantages of (i) giving 15 directly without having to isolate the intermediate nitrile and (ii) avoiding contamination of the product with solvent by using formamide instead of DMF as the reaction medium (formamide has virtually no solubility in non-polar organic solvents). Surprisingly perhaps, electrophilic reactions of 15 lead dominantly to substitution at the 7-position (ortho to the carbonyl group), thus blocking a one-step conversion to 19. Thus, we followed literature methods that are based largely on the work of Manske and co-workers (47) in the parallel veratrole series but with modifications which made the overall route relatively efficient and manageable on a larger scale. Nitration of 15 gave 16 (88%) which on catalytic reduction led to 17 (92%). The latter, on bromination with bis(N-methylpyrrolidin-2-one) hydrogen tribromide (48), led to compound 18 (86%), which then was converted to the desired bromolactone 19 (74%) by a standard diazotization/deamination procedure. The overall yield for the conversion of 14 to 19 was 37%, acceptable for further large-scale work.
Figure 3.
Synthetic scheme for the preparation of 8-bromo-7H-furo[3′,4′:4,5]benzo[1,2-d][1,3]dioxol-5-one (19). Reagents: (a) CuCN/HCONH2/H2O; (b)HNO3/H2SO4; (c) H2/Pd; (d) bis(N-methylpyrrolidinone) HBr3 and (e) NaNO2/H+; (f) H3PO2.
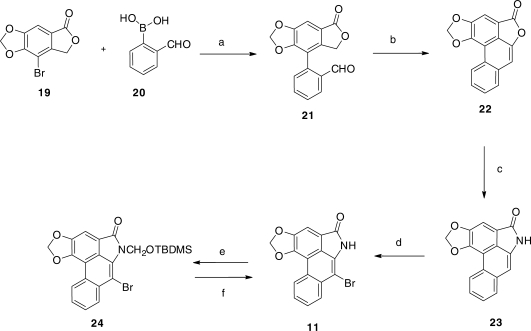
The synthesis of the required bromolactam 11 was accomplished according to Figure 4. Coupling of the bromolactone 19 with the commercially available boronic acid 20, under Suzuki coupling conditions, afforded the biphenyl derivative 21 in 64% yield, and the latter, under the influence of potassium t-butoxide in boiling t-butanol, led to the lactonic phenanthrene 22 in 55% yield. This was converted by means of the Bucherer reaction (49) to AL-II (23; ∼100%), identical in all respects with the natural product (32). Bromination of 23 then led smoothly to a quantitative yield of the desired but extremely insoluble bromolactam 11. The absence of absorption at or near 7.09 ppm in the 1H NMR spectrum of 11 (in hot DMSO-d6), characteristic of the 7-hydrogen atom in AL-II (23), confirmed that bromination had occurred at this location.
Figure 4.
Synthetic scheme for the preparation of 7-bromo-6-(tert-butyl-dimethylsilanyloxymethyl)-6H-benzo[f][1,3]dioxolo[4′,5′:4,5]benzo[1,2,3-cd]indol-5-one (24). Reagents: (a) PD(dpf)Cl2/K2CO3; (b) KOBut; (c) NaHSO3/NH3; (d) Br2; (e)TBDMSOCH2Cl/NaH/DMF and (f) HF/Pyr, then NH3/H2O.
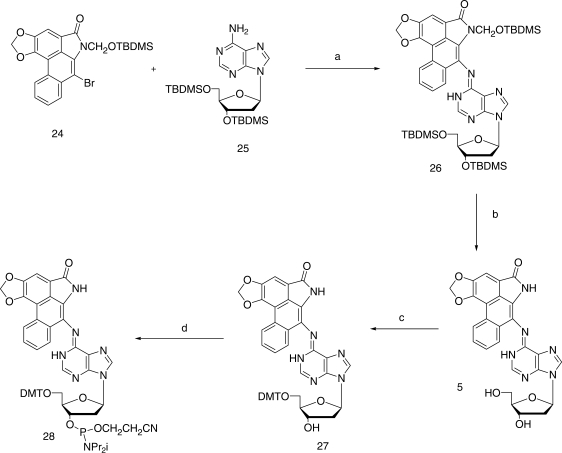
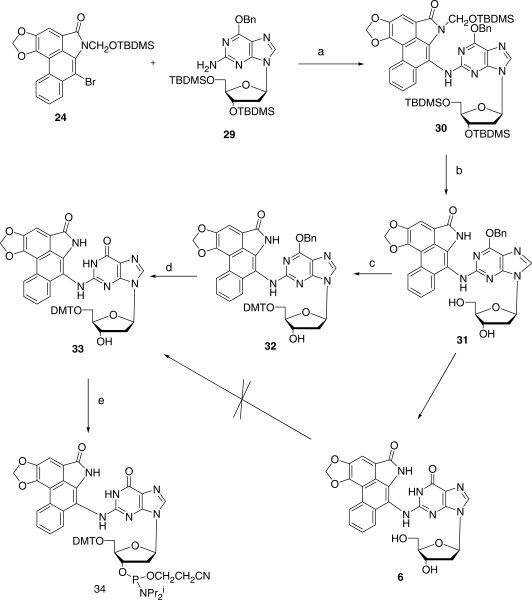
In order to couple 11 with the protected forms 25 and 29, respectively, of dA and dG (Figures 5 and 6) under Buchwald–Hartwig conditions (50), the nitrogen of the lactam ring needed to be protected by a group that not only would block any possible reaction at this site during the coupling process, but would also provide increased solubility in common solvents and be easily removable without causing collateral damage once coupling was complete. After more than a dozen attempts to accomplish this with a series of well-established protecting groups, success was finally achieved with the little-used t-butyldimethylsilyloxymethyl (TBDMSOM) protecting group (51). Treatment of the bromolactam 11 with sodium hydride in dry DMF at 50°C followed by the addition of t-butyl chloromethyloxydimethylsilane at 0°C for 30 min gave, after chromatographic purification, a 63% yield of the easily soluble, protected lactam 24. This compound when coupled (Figures 5 and 6) with either 25 or 29 under Buchwald–Hartwig conditions (50) using Xantphos as the palladium-chelating agent gave excellent yields of the protected forms 26 and 30 of the dA and dG adducts 5 and 6, respectively, of AL-II. Deprotection of the silyl-protecting groups was accomplished in two steps. Treatment with HF in pyridine removes all of the TBDMS groups but leaves a hydroxymethyl residue on the lactam nitrogen. This residue was then easily removed by heating with aqueous ammonia to give 5 in the case of 26, and 31 in the case of 30. Catalytic hydrogenolysis of the benzyl group in 31 then led to 6 almost quantitatively. The UV absorption spectrum of 5 proved to be identical with that of the published spectrum (34). Also, the 1HNMR spectral data of 5 was identical to those already published (34), and in agreement it contained a peak at 9.83 that was assigned by previous workers to the hydrogen at the 1 position of the purine ring. Surprisingly, however, no evidence for geometrical isomerism was noted at the N6 position.
Figure 5.
Synthetic scheme for the preparation of AL-II-dA (5) and its 5′-DMT protected phosphoramidite (28). Reagents: (a) PdCl2/Xantphos; (b) HF/Pyr then NH3/H2O; (c) DMTCl/Pyr and (d) CIP(OCH2CH2CN)N(i-Pr)2/Triazole.
Figure 6.
Synthetic scheme for the preparation of AL-II-dG (6) and its 5′-DMT protected phosphoramidite (34). Reagents: (a) PdCl2/Xantphos; (b) HF/Pyr then NH3/H2O; (c) DMTCl/Pry; (d) Pd/H2 and (e) CIP(OCH2CH2CN)N(i-Pr)2/Triazole.
Although it proved possible to convert 5 smoothly to its DMT derivative (27) and subsequently to the desired phosphoramidite 28 by standard procedures, insolubility problems plagued adduct 6 and its 5′-O-DMT derivative could not be prepared directly. This difficulty was overcome by introducing the DMT group at an earlier stage. Reaction of 31 with DMT chloride in pyridine afforded the much more soluble 5′-O-DMT derivative 32. Removal of the benzyl group from 32 by catalytic hydrogenolysis followed by phosphoramidite formation then afforded the required compound 34. Thus, the DMT-phosphoramidites 28 and 34 of adducts 5 and 6, respectively, became available for site-specific incorporation into oligomeric DNA by automated solid-state methods. Further applications of this methodology to AL-I and related substances are under study.
Synthesis of DNA oligomers containing adducts 5 and 6
Both DMT-phosphoramidites (28 and 34) were used successfully in the synthesis of a series of oligomers. Table 1 contains the sequences and masses obtained by ESI/MS for the oligomers containing respectively the xenonucleosides AL-II-dA (entries 1–7) and AL-II-dG (entries 8–14) adducts. All these oligomers were synthesized at the 1.0 µmol scale on an Applied Biosystems 394 DNA Synthesizer (Foster City, CA). In all cases the coupling time was 15 min for the modified deoxynucleoside phosphoramidites, and coupling efficiencies at the point of introduction varied from 93 to 98%. To verify that the modified deoxynucleosides were incorporated without further modification by reagents during DNA synthesis, two HPLC-purified oligomers were digested enzymatically to the deoxynucleosides using previously published procedures (35). The first was entry #5 in Table 1; the second was the same sequence in which the AL-II-dA was replaced by AL-II-dG. Products for both reactions were analyzed by LC/ESI/MS/MS, and in both cases the retention time and the MS/MS spectrum for the modified deoxynucleoside matched that for the synthetic standard (data not shown) indicating that AL-II-dA and AL-II-dG were stable to the conditions of DNA synthesis and were present in the oligomers.
Table 1.
Sequence and mass data for the synthesized DNA oligomers
| Entry | Sequence | Calc Mass (Da) | Meas Mass (Da) |
|---|---|---|---|
| 1. | 5′-CTC CTC A*AT ACC T-3′ | 4091 | 4090.2 |
| 2. | 5′-TTC CCT CCA GAA A*CA TCC T-3′ | 5929 | 5927.3 |
| 3. | 5′-CCA TTC ACA CA*A TCC-3′ | 4702 | 4701.1 |
| 4. | 5′-TTT TTA* TTT T-3′ | 3251 | 3250.3 |
| 5. | 5′-CCT TCA* CTT CTT TCC TCT CCC TTT-3′ | 7345 | 7344.2 |
| 6. | 5′-TCT TCT TCT GTG CA*C TCT TCT TCT-3′ | 7439 | 7439.7 |
| 7. | 5′-TCT TCT TCT GCA* GAC TCT TCT TCT-3′ | 7448 | 7447.9 |
| 8. | 5′-CGT ACG* CAT GC-3′ | 3579 | 3577.6 |
| 9. | 5′-TTG* TTT-3′ | 2050 | 2048.9 |
| 10. | 5′-CTC CTC G*AT ACC T-3′ | 4107 | 4106.1 |
| 11. | 5′-TTC CCT CCA GAA G8CA TCC T-3′ | 5945 | 5944.0 |
| 12. | 5′-CCA TTC ACA CG*A TCC-3′ | 4718 | 4717.6 |
| 13. | 5′-TCT TCT TCT GCG*TAC TCT TCT TCT-3′ | 7439 | 7438.6 |
| 14. | 5′-TCT TCT TCT GTG* CAC TCT TCT TCT-3′ | 7439 | 7436.9 |
A*, AL-II-dA; G*, AL-II-dG.
Blocking of DNA synthesis in cells
For the biological experiments, lesions were positioned in the middle of three consecutive base mismatches. This made it possible to determine the number of progeny plasmids derived from modified and unmodified strands; the ratio of progeny reflects the degree to which DNA synthesis is blocked. In the absence of blocking, the ratio should be 50:50, as revealed with a construct containing three base mismatches without a lesion (52). DNA repair (removal of a DNA lesion and the two flanking mismatches followed by gap-filling synthesis) converts the three nucleotide sequence of the modified strand to the sequence complementary to the unmodified strand, thus losing the strand tag. Thus, DNA repair could influence the apparent blocking effect of a DNA adduct in experiments using repair-proficient MEFs. This possibility should be considered in determing the ratio of progeny. Nevertheless, translesion DNA synthesis (TLS) occurred, giving rise to a progeny plasmid from the modified strand. Both adducts block DNA synthesis strongly. When fractions of progeny for the AL-II-dG and AL-II-dA adducts were compared, the dA adduct yielded about half of the progeny produced by the dG adduct. This suggests that the dA adduct is more effective at blocking DNA synthesis than is the dG adduct.
Miscoding properties of the two adducts
In MEFs, the major coding events were the insertion of the correct nucleotides opposite the adducts: T and dC for the dA and dG adducts, respectively. However, substantial frequencies of misincorporation were observed for both adducts; 22% for the dA adduct and 9% for the dG adduct. The nucleotide mis-inserted opposite both adducts was almost exclusively dA, leading to AL-II-dA→T and AL-II-dG→T transversions. The insertion of T opposite the dG adduct was observed once, leading to an AL-II-dG→dA transition.
The Schmeiser group reported, using an in-vitro primer extension system, that both dA and dG adducts strongly blocked DNA synthesis, mainly one nucleotide 3′ to the adduct, and that dA and T were inserted equally well opposite the dA adduct whereas the dG adduct primarily directed insertion of the correct dC (53). In general, our site-specific mutagenesis results for the dA adduct are in accord with the findings obtained in the in-vitro system and other studies in cells, animals and humans (19,24,54–56). However, we find that the dG adduct is less miscoding than is the dA adduct in cells (Table 2).
Table 2.
Translesional events induced by a site-specific AL-II-dA and AL-II-dG adducts in mouse cells
| DNA adduct | No. of progeny from |
Nucleotide inserted opposite adducta |
MFb (%) | Others | ||||
|---|---|---|---|---|---|---|---|---|
| UMSc | MSc | T | A | C | G | |||
| AAII-dA | 429 (95)d | 22 (5) | 191 (78) | 53 (22) | 0 | 0 | 22 | 3e |
| AAII-dG | 416 (91) | 41 (9) | 1 (0.4) | 25 (8.8) | 257 (90.8) | 0 | 9 | 2f |
aNumbers were determined following removal of progeny derived from the unmodified strand by EcoRV treatment.
bMF, miscoding frequency.
cUMS, unmodified strand; MS, modified strand; numbers were determined before removal of progeny derived from the unmodified strand by digesting with EcoRV.
dThe numbers in parentheses represent percentages.
eTGXTT → AGTTT, TATGT, TATAT.
fTGXTT → TAACT, TACTT.
SUMMARY
The aristolochic acids I and II have been implicated in the development of urothelial cancer via DNA adduction of their metabolites. We have developed a method for the large-scale synthesis of the dA and dG adducts derived from AA-II and, after facile conversion to the 5′-dimethoxytrityl-protected phosphoramidites, have incorporated these adducts into DNA oligomers using automated synthesis techniques. Adducts were chemically stable to the conditions of oligomer synthesis and were isolated intact from selected oligomers by enzymatic digestion. After rigorous HPLC purification, DNA oligomers containing dA or dG adducts were used for site-specific mutagenesis studies in mouse embryonic cells designed to establish the mutagenic potential and specificity of these lesions in vivo. Both adducts block DNA synthesis, but the dA adduct is the more effective inhibitor. The major coding events are the insertion of the correct nucleotides opposite the dA or dG adducts; however, misincorporation is also observed, and the nucleotide mis-inserted opposite both adducts is almost exclusively dA, leading to AL-II-dA→T and AL-II-dG→T transversions.
FUNDING
National Institute for Environmental Health Sciences grant [ES04068]. Funding for open access charge: National Institute for Environmental Health Sciences grant [ES04068].
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors would like to thank Mr Robert Rieger for obtaining mass spectra of intermediates and final products, Ms Naomi Suzuki and Dr Shinya Shibutani for assistance in obtaining UV spectra and Dr Robert J. Turesky for the enzymatic digestion and MS/MS analysis of two synthetic oligomers.
REFERENCES
- 1.Dawson WR. Birthwort: a study of the progress of medical botany through twenty-two centuries. Pharm. J Pharmacist. 1927:396–397. 427–430. [Google Scholar]
- 2.Grieve M. A Modern Herbal: the Medicinal, Culinary, Cosmetic and Economic Properties, Cultivation and Folk-lore of Herbs, Grasses, Fungi, Shrubs and Trees with all their Modern Scientific Uses. New York: Dover Pubs; 1971. p. 104. [Google Scholar]
- 3.Kupchan SM, Doskovitch RW. Tumor inhibitors I. Aristolochic acid, the active principle of Aristolochia indica. J. Med. Pharm. Chem. 1962;91:657–659. doi: 10.1021/jm01238a029. [DOI] [PubMed] [Google Scholar]
- 4.Jackson L, Kofman S, Weiss A, Brodovsky H. Aristolochic acid (NSC-50413): Phase I clinical study. Cancer Chemother. Rep. 1964;42:35–37. [PubMed] [Google Scholar]
- 5.Mengs U. Tumor induction in mice following exposure to aristolochic acid. Arch Toxicol. 1988;61:504–505. doi: 10.1007/BF00293699. [DOI] [PubMed] [Google Scholar]
- 6.Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D, Verbeelen D, Vanhaelen-Fastre R, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 7.Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem JL. Identification of aristolochic acid in Chinese herbs. Lancet. 1994;343:174. doi: 10.1016/s0140-6736(94)90964-4. [DOI] [PubMed] [Google Scholar]
- 8.Cosyns JP, Jadoul M, Squifflet JP, Wese FX, van Ypersele de Strihou C. Urothelial lesions in Chinese herb nephropathy. Am. J. Kidney Dis. 1999;33:1011–1017. doi: 10.1016/S0272-6386(99)70136-8. [DOI] [PubMed] [Google Scholar]
- 9.Dillerot G, Jadoul M, Arlt VM, van Ypersele De Strihou C, Schmeiser HH, But PPH, Bierler CA, Cosyns J. Aristolochic acid nephropathy in a Chinese patient: time to abandon the term “Chinese herbs nephropathy”. Am J. Kidney Dis. 2001;38:E26. doi: 10.1053/ajkd.2001.28624. [DOI] [PubMed] [Google Scholar]
- 10.Djukanović L, Radovanović Z. In: Balkan Endemic Nephropathy in Clinical Nephrotoxins. 2nd edn. De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. pp. 588–601. [Google Scholar]
- 11.Ivic M. The problem of etiology of endemic nephropathy. Lijetc Vjes. 1969;91:1278–1281. [PubMed] [Google Scholar]
- 12.Nikolić J. Epidemic Nephropathy and Upper Urothelial Tumors. 2006. Izdavačko preduzeće Belgrade, Serbia.
- 13.Petronić V. Tumors of the upper urothelium and endemic nephropathy. In: Radovanovic Z, Sindic M, Polenakovic M, Djukanović L, Petronic V, editors. Endemic Nephropathy. Belgrade, Serbia: Zavod Za Udzbenike I Nastavna Sredstva; 2000. pp. 350–439. [Google Scholar]
- 14.Cosyns JP, Jadoul M, Squifflet JP, De Plaen JF, Ferluga D, van Ypersele de Strihou C. Chinese herbs nephropathy: a clue to Balkan endemic nephropathy? Kidney Int. 1994;45:1680–1688. doi: 10.1038/ki.1994.220. [DOI] [PubMed] [Google Scholar]
- 15.Cosyns JP, Dehoux JP, Guiot Y, Goebbels RM, Robert A, Bernard AM, Van Ypersele De Strihou C. Chronic aristolochic acid toxicity in rabbits: a model of Chinese herb nephropathy? Kidney Int. 2001;59:2164–2173. doi: 10.1046/j.1523-1755.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Takahashi D, Chen SM, Tsuchiya R, Mukoyama T, Yamagata S. Acute nephrotoxicity of aristolochic acids in mice. J. Pharm. Pharmacol. 2004;56:221–229. doi: 10.1211/0022357023051. [DOI] [PubMed] [Google Scholar]
- 17.Shibutani S, Dong H, Suzuki N, Ueda S, Miller F, Grollman AP. Selective toxicity of aristolochic I and II. Drug Metab. Dispos. 2007;35:1217–1222. doi: 10.1124/dmd.107.014688. [DOI] [PubMed] [Google Scholar]
- 18.Hranjec T, Kovac A, Kos J, Mao W, Chen JJ, Grollman AP, Jelakovic B. Endemic nephropathy: the case for chronic poisoning by aristolochia. Croat. Med. J. 2005;46:116–125. [PubMed] [Google Scholar]
- 19.Grollman AP, Shibutani S, Moryia M, Miller F, Wu L, Moll U. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl Acad. Sci. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmeiser HH, Bieler CA, Weissler M, van Ypersele de Strihou C, Cosyns JP. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res. 1996;56:2025–2028. [PubMed] [Google Scholar]
- 21.Arlt VM, Stiborova M, Schmeiser HH. Aristolochic acid as a probable human hazard in herbal remedies: a review. Mutagenesis. 2002;17:265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- 22.Pfau W, Schmeiser HH, Wiessler M. 32P-postlabeling analysis of the DNA adducts formed by aristolochic acid I and II. Carcinogenesis. 1990;11:1627–1633. doi: 10.1093/carcin/11.9.1627. [DOI] [PubMed] [Google Scholar]
- 23.Stiborová M, Frei E, Arlt VM, Schmeiser HH. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat. Res. 2008;658:55–67. doi: 10.1016/j.mrrev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Jameson CW, Lunn R, Jeter S, Garner S, Atwood S, Carter G, Levy D, Cosyns J-P. 12th edn. Public Health Service, Research Triangle Park, NC: US Department of Health and Human Services; 2008. Background document for aristolochic acid-related exposures, National Toxicology Program Report on Carcinogens; pp. 1–228. [Google Scholar]
- 25.Grollman AP, Scarborough J, Jelakovic B. Aristolochic acid nephropathy: an environmental and iatrogenic disease. Adv. Mol. Tox. 2009;3:211–227. [Google Scholar]
- 26.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 27.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, DePauw L, Abramowicz D, Vereerstraeten P, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N. Engl. J. Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 28.Grollman AP, Jelakovic B. Role of environmental toxins in endemic (Balkan) nephropathy. J. Am. Soc. Nephrol. 2009;18:2817–2823. doi: 10.1681/ASN.2007050537. [DOI] [PubMed] [Google Scholar]
- 29.Bressy C, Menant C, Piva O. Synthesis of polycyclic lactams and sultams by a cascade ring-closure metathesis/isomerization and subsequent radical cyclization. Synlett. 2005;4:577–582. [Google Scholar]
- 30.Sinhababu AK, Borchardt RT. General method for the synthesis of phthaldehydic acids from o-bromobenzaldehydes. J Org. Chem. 1983;48:2356–2358. [Google Scholar]
- 31.Schmeiser HH, Frei E, Wiessler M, Stiborova M. Comparison of DNA adduct formation by aristolochic acids in various in vitro activation systems by 32P-post-labelling: evidence for reductive activation by peroxidases. Carcinogenesis. 1997;18:1055–1062. doi: 10.1093/carcin/18.5.1055. [DOI] [PubMed] [Google Scholar]
- 32.Priestap HA. Seven aristololactams from Aristolochia argentina. Phytochemistry. 1985;24:849–852. [Google Scholar]
- 33.Cosyns JP. Aristolochic acid Chinese herbs nephropathy. A review of the evidence to date. Drug Safety. 2003;26:33–48. doi: 10.2165/00002018-200326010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Pfau W, Schmeiser HH, Wiessler M. N6-Adenylarylation of aristolochic II and a synthetic model for the putative proximate carcinogen. Chem. Res. Toxicol. 1991;4:581–586. doi: 10.1021/tx00023a015. [DOI] [PubMed] [Google Scholar]
- 35.Goodenough AK, Schut HAJ, Turesky RJ. Novel LC-ESI/MS/MSn method for the characterization and quantification of 2′-deosyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem. Res. Toxicol. 2007;20:263–276. doi: 10.1021/tx0601713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang IY, Hashimoto K, de Wind N, Blair IA, Moriya M. Two distinct translesion synthesis pathways across a lipid peroxidation-derived DNA adduct in mammalian cells. J. Biol. Chem. 2009;284:191–198. doi: 10.1074/jbc.M806414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 38.Stein S, Lao Y, Yang IY, Hecht SS, Moriya M. Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1,N2-propanodeoxyguanosine DNA adducts in human cells. Mutation Res. 2006;608:1–7. doi: 10.1016/j.mrgentox.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Pailer M, Schleppnik A. Plantzliche naturstoffe mit einer nitrogruppe II. Die konstitution der aristolochiasaure II. Monat. 1957;88:367–387. [Google Scholar]
- 40.Johnson F. Allylic strain in six-membered rings. Chem. Rev. 1968;68:374–413. [Google Scholar]
- 41.Acevedo S, Bowden K. Transmission of polar effects. Part 16. Ionization of 8-Substituted 1-Naphthoic acids and Alkaline Hydrolysis of their Methyl Esters. J. Chem. Soc. Perkin Trans II. 1986:2049–2050. [Google Scholar]
- 42.Rule HG, Barnett AJG. Displacement of the nitro group in 8-nitro-1-naphthoic acid by thionyl halides to form 8-chloro- and 8-bromonaphthoic acids. J. Chem. Soc. 1932:175–79. [Google Scholar]
- 43.Kupchan SM, Wormser HC. Tumor inhibitors X. Photochemical synthesis of phenanthrenes. Synthesis of aristolochic acid and related compounds. J. Org. Chem. 1965;30:3792–3800. doi: 10.1021/jo01022a046. [DOI] [PubMed] [Google Scholar]
- 44.Estevez JC, Estevez RJ, Castedo L. The intramolecular aryne cycloaddition approach to aporphinoids. A new total synthesis of aristolactams and phenanthrene alkaloids. Tetrahedron. 1995;51:10801–10810. [Google Scholar]
- 45.Estevez JC, Villaverede MC, Estevez RJ, Castedo L. Tributyltin (IV) hydride mediated free-radical syntheses of dehydrodibenzochromanones, dihydrodibenzocoumaranones and aristolactams. Tetrahedron. 1995;14:4075–4082. [Google Scholar]
- 46.Couture A, Deniau E, Grandclaudon P, Rybalko-Rosen H, Leonce S, Pfeiffer B, Renard P. Synthesis and biological evaluation of aristolactams. Bioorg. Med. Chem. Lett. 2002;12:3557–3559. doi: 10.1016/s0960-894x(02)00794-1. [DOI] [PubMed] [Google Scholar]
- 47.Manske RH, McRae JA, Moir RY. 3-Bromometameconine. Can. J. Chem. 1951;29:526–535. doi: 10.1139/v51-061. [DOI] [PubMed] [Google Scholar]
- 48.Daniels WE, Chiddix ME, Glickman SA. Lactam complexes with bromine-hydrogen bromide. J. Org. Chem. 1963;28:573–574. [Google Scholar]
- 49.Bucherer HT. Effect of sulfurous acid salts on aromatic amido and hydroxyl compounds. J. Prakt. Chem. 1904;69:49–91. [Google Scholar]
- 50.De Riccardis F, Bonala RR, Johnson F. A general method for the synthesis of the N2- and N6-carcinogenic amine adducts of 2′-deodyguanosine and 2′-deoxyadenosine. J. Am. Chem. Soc. 1999;121:10453–10460. [Google Scholar]
- 51.Benneche T, Gundersen LL, Undheim K. (tert-Butyldimethylsilyloxy)methyl chloride: synthesis and use as N-protecting group in pyrimidinones. Acta Chem. Scand. 1988;42:384–389. [Google Scholar]
- 52.Yang IY, Johnson F, Grollman AP, Moryia M. Genotoxic mechanism for the major acrolein-derived deoxyguanosine adduct in human cells. Chem. Res. Toxicol. 2002;15:160–164. doi: 10.1021/tx010123c. [DOI] [PubMed] [Google Scholar]
- 53.Broschard TH, Wiessler M, von der Lieth CW, Schmeiser HH. Translesional synthesis on DNA templates containing site-specifically placed deoxyadenosine and deoxyguanosine adducts formed by the plant carcinogen aristolochic acid. Carcinogenesis. 1994;15:2331–2340. doi: 10.1093/carcin/15.10.2331. [DOI] [PubMed] [Google Scholar]
- 54.Kohara A, Suzuki T, Honma M, Ohwada T, Hayashi M. Mutagenicity of aristolochic acid in the lambda/lacZ transgenic mouse (MutaMouse) Mutation Res. 2002;515:63–72. doi: 10.1016/s1383-5718(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Mei N, Yao L, Chen T. Mutations induced by carcinogenic doses of aristolochic acid in kidney of Big Blue transgenic rats. Toxicol Lett. 2006;165:250–256. doi: 10.1016/j.toxlet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Nedelko T, Arlt VM, Phillips DH, Hollstein M. TP53 mutation signature supports involvement of aristolochic acid in the aetiology of endemic nephropathy-associated tumours. Int. J. Cancer. 2009;124:987–990. doi: 10.1002/ijc.24006. [DOI] [PubMed] [Google Scholar]