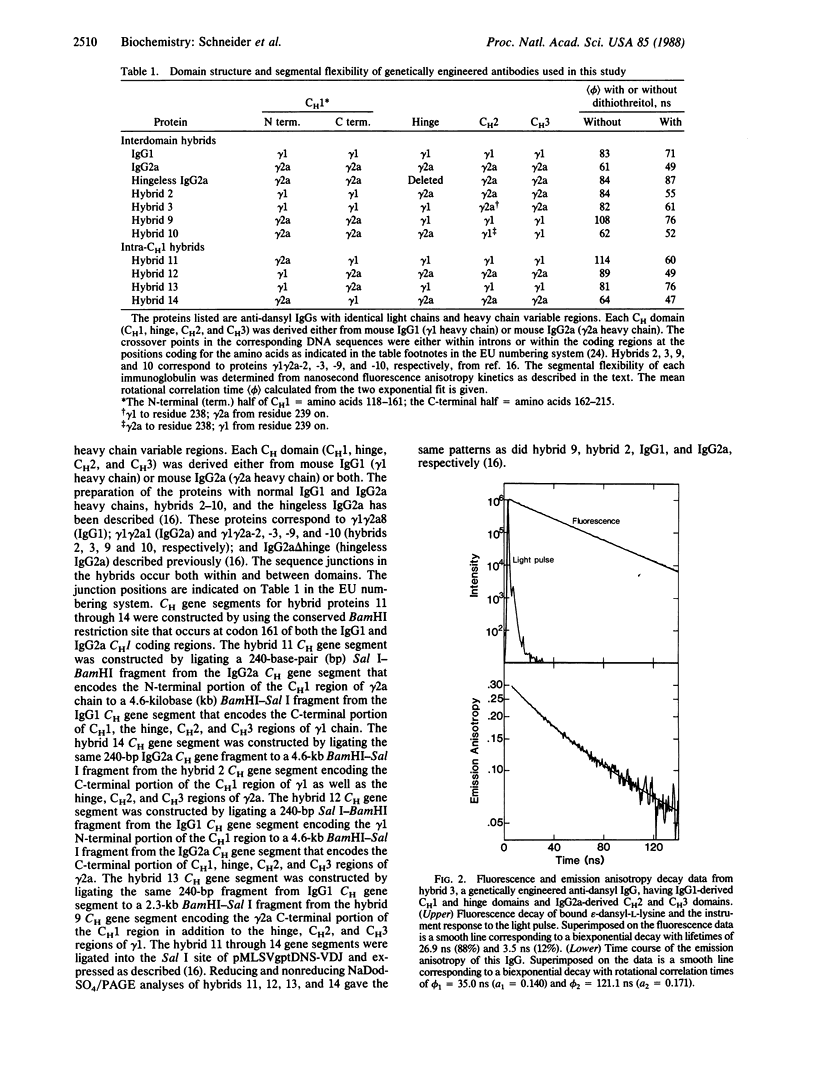
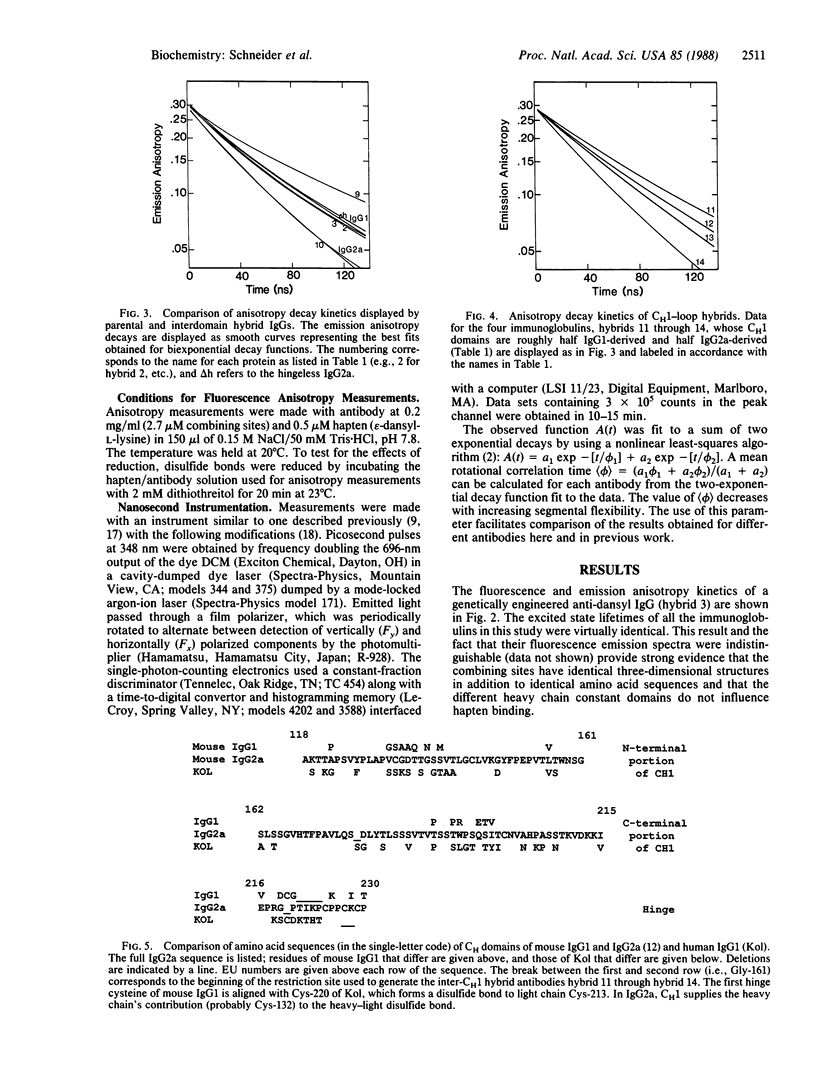
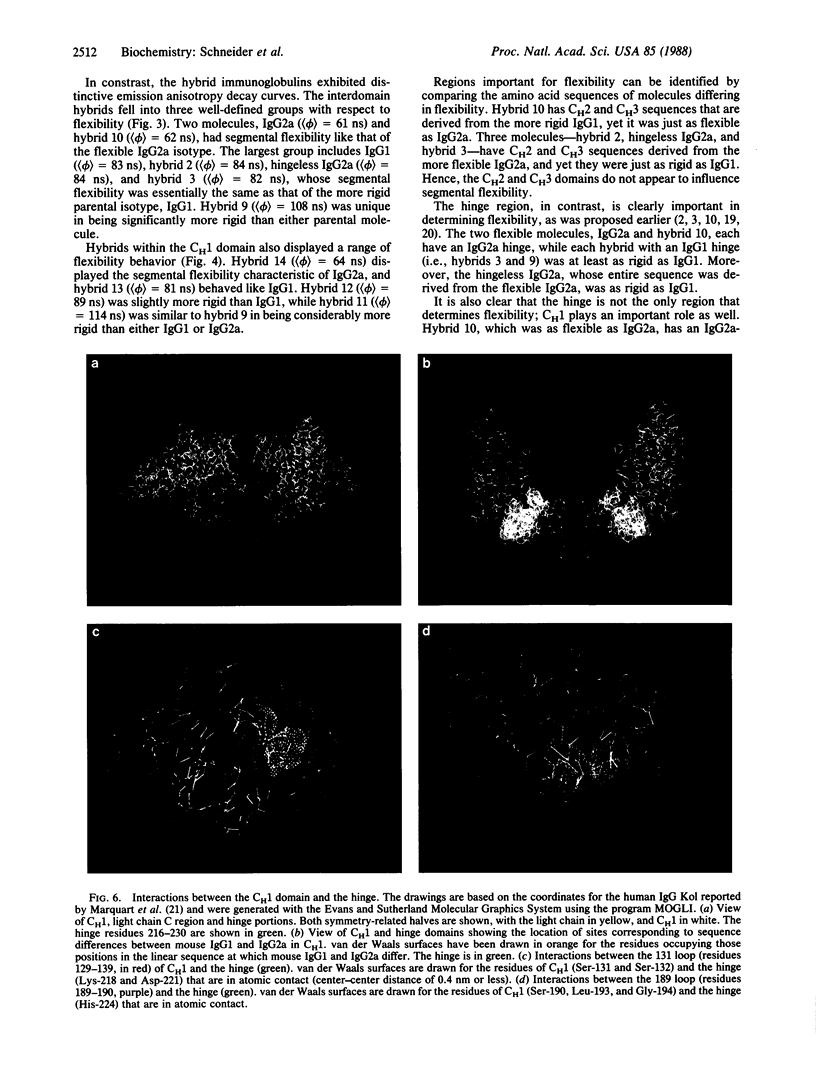
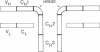
Abstract
We have carried out nanosecond fluorescence polarization studies of genetically engineered immunoglobulins to determine the structural features controlling their segmental flexibility. The proteins studied were hybrids of a relatively rigid isotype (mouse IgG1) and a relatively flexible one (mouse IgG2a). They have identical light chains and heavy chain variable regions and have the same combining sites for epsilon-dansyl-L-lysine, a fluorescent hapten. The fluorescence of the bound dansyl chromophore was excited at 348 nm with subnanosecond laser pulses, and the emission in the nanosecond time range was measured with a single-photon-counting apparatus. The emission anisotropy kinetics of the hybrid antibodies revealed that segmental flexibility is controlled by the heavy chain constant region 1 (CH1) as well as by the hinge. In contrast, the CH2 and CH3 domains did not influence segmental flexibility. The hinge and CH1 domains must be properly matched to allow facile movement of the Fab units. Studies of hybrids of IgG1 and IgG2a within CH1 showed that the loop formed by residues 131-139 is important in controlling segmental flexibility. X-ray crystallographic studies by others of human IgG1 have shown that this loop makes several van der Waals contacts with the hinge.
Full text
PDF