Abstract
Screening mammography can distort estimated effects in breast cancer risk models due to associations with other risk factors. Mammography information was available in the Nurses’ Health Study from 1988, and 1,815 incident breast cancers were accrued through 2000 among 55,625 women with risk factor data. Logistic models were fit for screening mammography, and inverse probability weighting was used to adjust parameters in an established breast cancer risk model. Approximately 80% of women in each 2-year follow-up period had screening mammograms, which were positively associated with history of benign breast disease, family history of breast cancer, hormone therapy, alcohol use, physical activity, multivitamins, and calcium supplements, and negatively associated with postmenopause, current smoking, and body mass index. Markers of medical attention, including hypertension, high cholesterol, and osteoarthritis, were positively associated, while cardiovascular disease was negative. Inverse probability weighting led to small changes in effects of benign breast disease, family history, and hormone therapy. An apparent reduced risk associated with current smoking in unadjusted models was eliminated after weighting. Thus, several risk factors for breast cancer and cancer diagnosis are associated with mammographic screening. Adjustment for screening had some impact on breast cancer prediction in this cohort, especially for hormone therapy and smoking.
Keywords: breast neoplasms, hormone replacement therapy, mammography, mass screening, probability weighting, risk factors
Mammographic screening for breast cancer is widespread in the United States, with approximately 75% of women 40 years of age or older having a mammogram per 2-year period (1). Possibly because of concerns about potential increased risk of breast cancer (2, 3), mammography screening rates are higher among women taking hormones (4–6). Mammography may be associated with other risk factors, as well as health-seeking (or -avoiding) behaviors, such as weight, smoking, or other lifestyle factors (5, 7–9).
Such differences in exposures by screening may distort their estimated effects on breast cancer itself (10). Screening may lead to the earlier detection of tumors before symptoms develop, resulting in detection bias, and an increased estimated risk among those who are screened. Although this most obviously impacts effect estimates for mammography itself (11), the same distortion could potentially occur for risk factors associated with mammography. Weiss (12) describes 2 conditions in which screening could be a source of confounding: 1) when screening identifies treatable premalignant conditions and 2) when the number of cases included in the study would have been smaller but for the presence of screening. Joffe (13) argues that screening mammography can take the role of confounder, intermediate variable, or effect modifier.
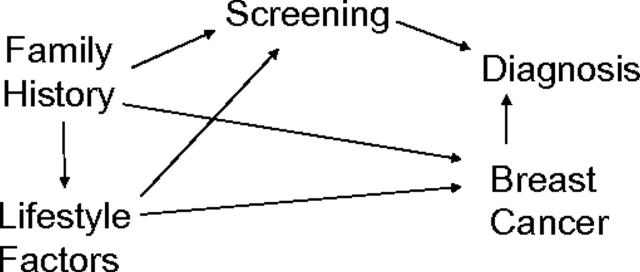
Models for breast cancer incidence may similarly be influenced by screening bias. Such models (14–17) are in wide use and can have clinical impact and affect treatment decisions. Because these models include risk factors associated with screening, bias in both risk factor estimates and predicted risk of disease can potentially occur unless there is universal and uniform screening in the population. Models developed for purposes of prediction are not necessarily intended to contain purely causal effects. If models attempt to estimate underlying associations of risk predictors with cancer, however, including screening mammography in the model is problematic, since screening may be on the causal pathway between risk factors and breast cancer diagnosis (Figure 1).
Figure 1.
Directed acyclic graph illustrating the relation among potential risk factors and screening mammography with breast cancer incidence and diagnosis.
We estimated the extent of such potential bias on data from the Nurses’ Health Study. We first modeled predictors of screening mammography using traditional risk factors for breast cancer, as well as other lifestyle exposures. We applied weights based on these models to estimate the direct effects of risk factors in a predictive model for breast cancer (15).
MATERIALS AND METHODS
Study population
The Nurses’ Health Study cohort was established in 1976, when 121,700 female registered nurses aged 30–55 years completed a mailed questionnaire including items on known or suspected risk factors for cancer and cardiovascular disease. Follow-up questionnaires were mailed every 2 years to update information on risk factors and to ascertain occurrence of major medical events. Deaths were identified by a family member, the postal service, or the National Death Index. Each questionnaire asked whether breast cancer had been diagnosed within the previous 2 years and, if so, the date of diagnosis. All women who reported breast cancer, or their next-of-kin if deceased, were contacted for permission to obtain relevant medical records. Self-reports were confirmed by pathology reports in 98% of reported cases. Because of the high confirmation rates, the few self-reported cases for whom medical records were unavailable were included in the analysis.
Information on age, weight, smoking status, family history of cancer, use of hormone therapy, and personal history of other diseases was updated on the biennial follow-up questionnaires. Additional information included age at menarche, parity, age at first birth, pregnancy history, age at menopause, type of menopause, and history of benign breast disease. Body mass index was calculated as weight (kg)/height (m)2. Alcohol intake was assessed by using questions on the consumption of beer, wine, and liquor on a food frequency questionnaire sent every 2–4 years.
Information on mammography was collected beginning in 1988. Women were asked if they ever had a mammogram and how many years since their most recent mammogram. Details on the reasons for mammography, including whether it was conducted for screening purposes or due to symptoms, were collected first in 1990 when women were asked whether they had a mammogram within the past 2 years. Other potential correlates of screening included lifestyle behaviors, such as smoking and exercise, use of aspirin or vitamin or mineral supplements, including multivitamins and calcium supplements, and markers for medical attention, including high blood pressure, high cholesterol, and history of cardiovascular disease, arthritis, osteoporosis, and diabetes. Altogether, 57,859 women provided data from 1988 onward and had complete information on the factors in the breast cancer incidence model (15). Of these, 55,625 women were free of cancer, had complete data on risk factors for breast cancer and potential predictors of screening mammography and information on breast cancer incidence in at least one 2-year period, and provided 493,763 person-years of information.
Statistical analysis
Predictors of screening mammography.
Logistic regression was used to determine predictors of screening mammography during each 2-year period. Predictors were assessed at the start of the interval, and screening within the intervening 2 years was assessed at the end of each 2-year interval. Mammograms conducted due to symptoms were excluded from these analyses. Logistic regression models were fit for each 2-year time period from 1988 through 2000. The predictors remained relatively stable over time and, for descriptive purposes, these were combined into a single model by using generalized estimating equations with an unstructured covariance matrix in SAS PROC GENMOD (18). This was based on 241,994 intervals of 2 years each, during which 208,906 screening mammograms occurred.
Inverse probability weighted breast cancer incidence model.
The log-incidence model of breast cancer has been presented previously (15, 19). Details are provided in the Web Appendix. (This information is described in a supplementary appendix that is posted on the Journal’s website (http://aje.oxfordjournals.org/).) The use of screening mammography by a portion of the cohort, however, may introduce bias. Because screening is influenced by breast cancer risk factors, including demographics, lifestyle behaviors, and medical attention, and because screening is associated with breast cancer diagnosis, the estimated effects of cancer risk predictors may be distorted. A simple directed acyclic graph (20) representing the relation among risk factors, screening, and breast cancer and its diagnosis is shown in Figure 1. Mammography screening is associated with risk predictors and is an intermediate variable for breast cancer diagnosis, so that control for or conditioning on screening is not optimal (21). Our goal was to estimate the direct or marginal effects of predictors, rather than those conditional on screening, or to estimate the effect on breast cancer itself if screening were independent of the risk factors. Operationally, we used weighting to create a hypothetical population in which screening was uncorrelated with all the potential risk factors considered.
We used inverse probability weighting to eliminate the relation between risk factors and screening. Weights were based on the estimated probability of a screening mammogram within each 2-year period separately by using exposures assessed at the start of the interval. The population was reweighted to an overall proportion of 80% of the women screened, similar to the Nurses’ Health Study cohort. Stabilized weights were used (22, 23), with the weight at time t for individual i equal to 0.8/P(screenit) for those with a screening mammogram in the interval from t to t + 1 and equal to 0.2/(1 − P(screenit)) for those with no mammogram. Note that mammograms conducted because of symptoms were considered part of the diagnostic process and were not controlled. Because the great majority of women reporting mammograms for symptoms were not ultimately diagnosed with breast cancer (94%), weights for these women were set to 0.8/P(screenit). In a sensitivity analysis, results demonstrated little change when these women were given a weight of 1.0 (i.e., no weight) instead. The probability of screening used in the denominator of the weights was computed from the logistic regression models fit in each time interval separately.
RESULTS
In 1988, 45,281 women had information on screening mammography and all risk factors for breast cancer. The average age was 53.9 years (standard deviation, 7.2). Thirty-five percent remained premenopausal, 15% had a surgical menopause, and 34% of postmenopausal women currently used hormone therapy. Body mass index was 30 or more in 24%, 17% were current smokers, and 21% had at least 1 drink per day. Thirty-eight percent reported a history of benign breast disease, 11% reported a family history of breast cancer, and 40% reported a family history of other cancer types. Seventy-seven percent of women had a screening mammogram within the next 2-year period (1988–1990), and 4% had one for symptoms. The percent having a screening mammogram increased to 85% in 1994–1996 and to 92% in 1998–2000, with 2% having a mammogram for symptoms in these years.
Predictors of screening mammography
Several potential predictors of breast cancer and other health factors were related to screening mammography in 1988–1990 (Table 1). Compared with those with no mammogram, those with a screening mammogram were less likely to have had a natural menopause, to be obese (body mass index, ≥30 kg/m2), to smoke currently, or to abstain from alcohol. They were more likely to exercise and to use multivitamins and calcium supplements. They were also more likely to report a personal history of benign breast disease, a family history of breast cancer, or a family history of other types of cancer. Among postmenopausal women, those with a screening mammogram were more likely to use hormone therapy.
Table 1.
Baseline Characteristics in 1988 of Women in the Nurses’ Health Study With Use of Screening Mammography in 1988–1990, Crude and Weighted by Inverse Probability of Screening Mammographya
| Unweighted | Weighted | |||||||||
| No Mammogram (n = 8,558) | Screening Mammogram (n = 34,754) | P Value | No Mammogram (n = 8,558) | Screening Mammogram (n = 34,754) | P Value | |||||
| No. | % | No. | % | No. | % | No. | % | |||
| Age, years (mean (SD)) | 54.1 (7.4) | 53.9 (7.1) | 0.02 | 53.9 (7.3) | 54.0 (7.2) | 0.65 | ||||
| Age, years | ||||||||||
| 40–44 | 1,067 | 12.5 | 3,652 | 10.5 | 0.022 | 959 | 11.1 | 3,783 | 10.9 | 0.72 |
| 45–49 | 1,639 | 19.2 | 7,355 | 21.2 | 1,801 | 20.7 | 7,202 | 20.8 | ||
| 50–54 | 1,628 | 19.0 | 7,541 | 21.7 | 1,861 | 21.4 | 7,340 | 21.2 | ||
| 55–59 | 1,665 | 19.5 | 6,702 | 19.3 | 1,674 | 19.3 | 6,691 | 19.3 | ||
| 60–64 | 1,874 | 21.9 | 6,965 | 20.0 | 1,745 | 20.1 | 7,058 | 20.4 | ||
| 65–69 | 685 | 8.0 | 2,539 | 7.3 | 651 | 7.5 | 2,574 | 7.4 | ||
| Menopausal status | ||||||||||
| Premenopausal | 2,880 | 33.6 | 11,911 | 34.5 | <0.0001 | 2,949 | 33.9 | 11,903 | 34.4 | 0.38 |
| Natural menopause | 4,520 | 52.8 | 17,290 | 49.8 | 4,356 | 50.1 | 17,426 | 50.3 | ||
| Surgical menopause | 1,158 | 13.5 | 5,473 | 15.8 | 1,385 | 15.9 | 5,319 | 15.4 | ||
| Hormone therapy (if postmenopausal) | ||||||||||
| Never | 3,550 | 62.5 | 9,497 | 41.7 | 2,582 | 45.0 | 10,422 | 45.8 | ||
| Current estrogen + progesterone | 207 | 3.6 | 3,135 | 13.8 | <0.0001 | 689 | 12.0 | 2,674 | 11.8 | 0.60 |
| Current estrogen only | 573 | 10.1 | 4,009 | 17.6 | <0.0001 | 939 | 16.4 | 3,667 | 16.1 | 0.68 |
| Current other | 144 | 2.5 | 1,471 | 6.5 | <0.0001 | 330 | 5.8 | 1,293 | 5.7 | 0.85 |
| Past estrogen + progesterone | 92 | 1.6 | 625 | 2.8 | <0.0001 | 149 | 2.6 | 573 | 2.5 | 0.75 |
| Past estrogen only | 537 | 9.5 | 1,890 | 8.3 | 0.005 | 513 | 8.9 | 1,950 | 8.6 | 0.37 |
| Past other | 575 | 10.1 | 2,136 | 9.4 | 0.088 | 540 | 9.4 | 2,166 | 9.5 | 0.77 |
| Parity | ||||||||||
| None | 504 | 5.9 | 2,047 | 5.9 | 0.44 | 490 | 5.6 | 2,045 | 5.9 | 0.68 |
| 1 | 684 | 8.0 | 2,426 | 7.0 | 644 | 7.4 | 2,479 | 7.2 | ||
| 2 | 2,287 | 26.7 | 10,270 | 29.6 | 2,507 | 28.8 | 10,048 | 29.0 | ||
| ≥3 | 5,083 | 59.4 | 20,011 | 57.6 | 5,049 | 58.1 | 20,076 | 57.9 | ||
| Age at first birth, years | ||||||||||
| None | 504 | 5.9 | 2,047 | 5.9 | 0.39 | 490 | 5.7 | 2,045 | 5.9 | 0.41 |
| <25 | 4,103 | 47.9 | 16,755 | 48.2 | 4,313 | 49.6 | 16,681 | 48.1 | ||
| 25–29 | 3,116 | 36.4 | 12,738 | 36.6 | 3,056 | 35.2 | 12,683 | 36.6 | ||
| ≥30 | 835 | 9.8 | 3,214 | 9.2 | 832 | 9.6 | 3,239 | 9.4 | ||
| Body mass index, kg/m2 | ||||||||||
| <25 | 4,139 | 48.4 | 18,668 | 53.7 | <0.0001 | 4,507 | 51.9 | 18,228 | 52.6 | 0.23 |
| 25–<30 | 2,010 | 23.5 | 8,202 | 23.6 | 2,078 | 23.9 | 8,172 | 23.6 | ||
| ≥30 | 2,409 | 28.2 | 7,884 | 22.7 | 2,106 | 24.2 | 8,248 | 23.8 | ||
| Smoking status | ||||||||||
| Never | 3,593 | 42.0 | 15,723 | 45.2 | <0.0001 | 3,894 | 44.8 | 15,447 | 44.6 | 0.99 |
| Past | 2,746 | 32.1 | 13,720 | 39.5 | 3,264 | 37.6 | 13,167 | 38.0 | ||
| Current | 2,219 | 25.9 | 5,311 | 15.3 | 1,533 | 17.6 | 6,034 | 17.4 | ||
| Alcohol use | ||||||||||
| None | 3,359 | 39.2 | 11,329 | 32.6 | <0.0001 | 2,924 | 33.6 | 11,750 | 33.9 | 0.81 |
| <1 drink/day | 3,492 | 40.8 | 15,805 | 45.5 | 3,901 | 44.9 | 15,435 | 44.6 | ||
| ≥1 drinks/day | 1,707 | 20.0 | 7,620 | 21.9 | 1,867 | 21.5 | 7,463 | 21.5 | ||
| Physical activity, MET hours/week | ||||||||||
| <10 | 4,916 | 57.4 | 17,876 | 51.4 | <0.0001 | 4,618 | 53.1 | 18,235 | 52.6 | 0.63 |
| 10–19 | 1,542 | 18.0 | 7,078 | 20.4 | 1,683 | 19.4 | 6,888 | 19.9 | ||
| ≥20 | 2,100 | 24.5 | 9,800 | 28.2 | 2,390 | 27.5 | 9,525 | 27.5 | ||
| Benign breast disease | ||||||||||
| No | 6,513 | 76.1 | 20,793 | 59.8 | <0.0001 | 5,446 | 62.7 | 21,847 | 63.0 | 0.49 |
| Yes | 2,045 | 23.9 | 13,961 | 40.2 | 3,245 | 37.3 | 12,801 | 37.0 | ||
| Family history of breast cancer | ||||||||||
| No | 7,913 | 92.5 | 30,716 | 88.4 | <0.0001 | 7,747 | 89.1 | 30,901 | 89.2 | 0.91 |
| Yes | 645 | 7.5 | 4,038 | 11.6 | 944 | 10.9 | 3,747 | 10.8 | ||
| Family history of other cancer | ||||||||||
| No | 5,326 | 62.2 | 20,787 | 59.8 | <0.0001 | 5,332 | 61.4 | 20,897 | 60.3 | 0.08 |
| Yes | 3,232 | 37.8 | 13,967 | 40.2 | 3,359 | 38.6 | 13,751 | 39.7 | ||
| Multivitamin use | ||||||||||
| No | 5,617 | 65.6 | 21,041 | 60.5 | <0.0001 | 5,348 | 61.5 | 21,322 | 61.5 | >0.99 |
| Yes | 2,941 | 34.4 | 13,713 | 39.5 | 3,342 | 38.5 | 13,326 | 38.5 | ||
| Calcium supplement use | ||||||||||
| No | 5,942 | 69.4 | 19,504 | 56.1 | <0.0001 | 5,089 | 58.6 | 20,355 | 58.8 | 0.74 |
| Yes | 2,616 | 30.6 | 15,250 | 43.9 | 3,602 | 41.4 | 14,293 | 41.2 | ||
| Aspirin use | ||||||||||
| No | 7,149 | 83.5 | 29,035 | 83.5 | 0.98 | 7,240 | 83.3 | 28,939 | 83.5 | 0.62 |
| Yes | 1,409 | 16.5 | 5,719 | 16.5 | 1,451 | 16.7 | 5,709 | 16.5 | ||
| History of hypertension | ||||||||||
| No | 6,387 | 74.6 | 25,676 | 73.9 | 0.15 | 6,290 | 72.4 | 25,617 | 73.9 | 0.003 |
| Yes | 2,171 | 25.4 | 9,078 | 26.1 | 2,400 | 27.6 | 9,031 | 26.1 | ||
| History of high cholesterol | ||||||||||
| No | 7,073 | 82.6 | 26,000 | 74.8 | <0.0001 | 6,570 | 75.6 | 26,449 | 76.3 | 0.15 |
| Yes | 1,485 | 17.4 | 8,754 | 25.2 | 2,121 | 24.4 | 8,199 | 23.7 | ||
| History of cardiovascular disease | ||||||||||
| No | 7,661 | 89.5 | 31,153 | 89.6 | 0.74 | 7,745 | 89.1 | 31,039 | 89.6 | 0.20 |
| Yes | 897 | 10.5 | 3,601 | 10.4 | 946 | 10.9 | 3,609 | 10.4 | ||
| History of rheumatoid arthritis | ||||||||||
| No | 8,070 | 94.3 | 32,884 | 94.6 | 0.24 | 8,221 | 94.6 | 32,764 | 94.6 | 0.91 |
| Yes | 488 | 5.7 | 1,870 | 5.4 | 470 | 5.4 | 1,884 | 5.4 | ||
| History of osteoarthritis | ||||||||||
| No | 6,323 | 73.9 | 24,575 | 70.7 | <0.0001 | 6,138 | 70.6 | 24,707 | 71.3 | 0.21 |
| Yes | 2,235 | 26.1 | 10,179 | 29.3 | 2,553 | 29.4 | 9,942 | 28.7 | ||
| History of osteoporosis | ||||||||||
| No | 8,216 | 96.0 | 32,861 | 94.6 | <0.0001 | 8,213 | 94.5 | 32,855 | 94.8 | 0.23 |
| Yes | 342 | 4.0 | 1,893 | 5.4 | 478 | 5.5 | 1,794 | 5.2 | ||
| History of diabetes | ||||||||||
| No | 8,199 | 95.8 | 33,523 | 96.5 | 0.004 | 8,337 | 95.9 | 33,368 | 96.3 | 0.10 |
| Yes | 359 | 4.2 | 1,231 | 3.5 | 354 | 4.1 | 1,280 | 3.7 | ||
Abbreviations: MET, metabolic equivalent; SD, standard deviation.
Excludes 1,969 women with mammograms for symptoms.
Logistic models predicting use of screening mammography were developed for each 2-year period to capture any varying effects by time. Screening mammography increased over the period of the study, with the odds more than doubling from 1988 to 1998, but many of the above factors remained important predictors (refer to Web Appendix Table 1). (This information is described in a supplementary table that is posted on the Journal’s website (http://aje.oxfordjournals.org/).) For ease of presentation, a model combining all years using generalized estimating equations is displayed in Table 2.
Table 2.
Predictors of Mammography Screening in the Next 2 Years in the Nurses’ Health Study, Combining Years 1988–1998
| Odds Ratio | 95% Confidence Interval | P Value | |
| Time | |||
| 1988 | 0.89 | 0.86, 0.91 | <0.0001 |
| 1990 | 1.00 | NA | NA |
| 1992 | 1.15 | 1.12, 1.19 | <0.0001 |
| 1994 | 1.21 | 1.17, 1.25 | <0.0001 |
| 1996 | 1.54 | 1.48, 1.61 | <0.0001 |
| 1998 | 2.31 | 2.20, 2.42 | <0.0001 |
| Age, years | |||
| 40–44 premenopausal | 0.77 | 0.72, 0.83 | <0.0001 |
| 45–49 | 0.89 | 0.84, 0.94 | <0.0001 |
| 50–54 | 1.00 | NA | NA |
| 55–59 | 0.89 | 0.86, 0.92 | <0.0001 |
| 60–64 postmenopausal | 0.49 | 0.46, 0.53 | <0.0001 |
| 65–69 | 0.47 | 0.43, 0.50 | <0.0001 |
| 70–74 | 0.41 | 0.38, 0.45 | <0.0001 |
| ≥75 | 0.33 | 0.29, 0.37 | <0.0001 |
| Postmenopausala age, years | |||
| 40–49 | 0.62 | 0.57, 0.68 | <0.0001 |
| 50–59 | 0.58 | 0.54, 0.62 | <0.0001 |
| Bilateral oophorectomy | 0.80 | 0.75, 0.85 | <0.0001 |
| Hormone therapy | |||
| Never | 1.00 | NA | NA |
| Current estrogen + progesterone | 3.34 | 3.17, 3.52 | <0.0001 |
| Current estrogen only | 2.64 | 2.49, 2.81 | <0.0001 |
| Current other | 2.89 | 2.72, 3.07 | <0.0001 |
| Past estrogen + progesterone | 2.04 | 1.88, 2.22 | <0.0001 |
| Past estrogen only | 1.48 | 1.38, 1.59 | <0.0001 |
| Past other | 1.54 | 1.45, 1.64 | <0.0001 |
| Parity | |||
| 0–1 | 1.00 | NA | NA |
| 2 | 1.26 | 1.19, 1.34 | <0.0001 |
| ≥3 | 1.21 | 1.14, 1.27 | <0.0001 |
| Body mass index, kg/m2 | |||
| <25 | 1.00 | NA | NA |
| 25–29 | 0.90 | 0.85, 0.97 | 0.0028 |
| ≥30 | 0.77 | 0.72, 0.82 | <0.0001 |
| Body mass index × menopause | |||
| 25–29 × menopause | 1.11 | 1.03, 1.19 | 0.0068 |
| ≥30 × menopause | 1.19 | 1.11, 1.29 | <0.0001 |
| Smoking | |||
| Never | 1.00 | NA | NA |
| Past | 0.98 | 0.94, 1.02 | 0.40 |
| Current | 0.72 | 0.67, 0.77 | <0.0001 |
| No. of packs/day | 0.74 | 0.70, 0.79 | <0.0001 |
| Alcohol use | |||
| None | 1.00 | NA | NA |
| 0–<1 drink/day | 1.19 | 1.15, 1.22 | <0.0001 |
| ≥1 drinks/day | 1.23 | 1.18, 1.29 | <0.0001 |
| Physical activity, MET hours/week | |||
| <10 | 1.00 | NA | NA |
| 10–19 | 1.10 | 1.06, 1.13 | <0.0001 |
| ≥20 | 1.11 | 1.07, 1.14 | <0.0001 |
| Multivitamin use | 1.04 | 1.01, 1.07 | 0.0056 |
| Calcium supplement use | 1.24 | 1.21, 1.28 | <0.0001 |
| Aspirin use | 1.00 | 0.97, 1.03 | 0.88 |
| Benign breast disease | 1.85 | 1.78, 1.92 | <0.0001 |
| Family history of breast cancer | 1.41 | 1.33, 1.49 | <0.0001 |
| Family history of other cancer | 1.10 | 1.06, 1.14 | <0.0001 |
| History of hypertension | 1.17 | 1.13, 1.22 | <0.0001 |
| History of high cholesterol | 1.40 | 1.36, 1.45 | <0.0001 |
| History of cardiovascular disease | 0.88 | 0.84, 0.93 | <0.0001 |
| History of rheumatoid arthritis | 0.95 | 0.89, 1.02 | 0.13 |
| History of osteoarthritis | 1.12 | 1.08, 1.17 | <0.0001 |
| History of osteoporosis | 0.98 | 0.93, 1.04 | 0.57 |
| History of diabetes | 0.96 | 0.89, 1.03 | 0.28 |
Abbreviations: MET, metabolic equivalent; NA, not applicable.
Assumes that all women over the age of 60 years are postmenopausal.
Women currently using hormone therapy had as much as 3 times the odds of having a screening mammogram, with past users also more likely to be screened. Odds were higher for women taking a combination of estrogen plus progesterone than those taking estrogen alone. Among those not using hormone therapy, screening mammography was highest among premenopausal women aged 50–54 years and was lower among women who were either younger or older.
Current cigarette smokers were less likely to be screened, with the effect strengthening with number of cigarettes per day. A woman smoking a pack/day (20 cigarettes) had 0.53 (95% confidence interval (CI): 0.51, 0.56) times the odds of undergoing screening as a nonsmoker. Women who drank alcohol in moderation or exercised regularly were more likely to be screened. Overweight or obese women were less likely to be screened, although this was attenuated among postmenopausal women. Premenopausal women with a body mass index of 30 or higher had an odds ratio of 0.77 (95% CI: 0.72, 0.82) for screening, which increased to 0.92 (95% CI: 0.88, 0.96) if postmenopausal. In addition, women who took multivitamins or, especially, calcium supplements were more likely to be screened.
Women with a history of benign breast disease or with a family history of cancer, especially breast cancer, were more likely to be screened. Other medical conditions were positively associated, possibly because of increased medical attention, including history of hypertension, high cholesterol, and osteoarthritis.
Models were similar across time periods, although there were some significant interactions with time (refer to Web Appendix Table 1). The greatest difference was in the association with current hormone therapy. The odds ratio for screening for a current versus never user of estrogen alone was 2.7 (95% CI: 2.4, 3.0) in 1988 and 6.8 (95% CI: 5.5, 8.3) in 1998. Because of these differences, stabilized weights were created from models estimated for each 2-year time period separately. The median weight was 0.88 (interquartile range, 0.83–0.99), with a minimum of 0.27 and a maximum of 46.5. The weighting removed the associations seen in the crude analyses (Table 1).
Predictors of breast cancer
In the unweighted log-incidence model (Table 3), in a woman of average height and body mass index, the incidence of breast cancer increased by 9.2% per year during premenopausal years, by 3.5% per year after natural menopause, and by 2.5% per year after surgical menopause. These estimates changed very little in the weighted model, to 8.9% per premenopausal year, 3.5% per year after natural menopause, and 2.7% after surgical menopause.
Table 3.
Breast Cancer Incidence Model for 1988–2000 Among Women in the Nurses’ Health Study, Unweighted and Weighted by Inverse Probability of Screening
| Variable | Unweighted | Weighted | ||
| β (SE) | P Value | β (SE) | P Value | |
| Constant | −9.597 (0.362) | <0.0001 | −9.379 (0.367) | <0.0001 |
| Premenopause duration, yearsa | 0.088 (0.009) | <0.0001 | 0.085 (0.009) | <0.0001 |
| Menopause duration, years | ||||
| Natural | 0.034 (0.006) | <0.0001 | 0.034 (0.006) | <0.0001 |
| Bilateral oophorectomy | 0.025 (0.007) | 0.0006 | 0.027 (0.007) | 0.0003 |
| Pregnancy history | ||||
| Age at first birth − age at menarche | 0.0067 (0.0050) | 0.18 | 0.0048 (0.0052) | 0.36 |
| Birth indexb | −0.0030 (0.0007) | <0.0001 | −0.0036 (0.0007) | <0.0001 |
| Benign breast disease | ||||
| BBD, yes versus no | 0.638 (0.601) | 0.29 | 0.203 (0.628) | 0.75 |
| BBD × age at menarche | 0.051 (0.024) | 0.037 | 0.064 (0.026) | 0.014 |
| BBD × premenopause duration | −0.019 (0.012) | 0.10 | −0.014 (0.012) | 0.24 |
| BBD × menopause duration | −0.015 (0.006) | 0.016 | −0.015 (0.007) | 0.027 |
| Hormone therapy | ||||
| Oral estrogen duration | 0.029 (0.008) | 0.0002 | 0.027 (0.008) | 0.0016 |
| Oral estrogen and progesterone duration | 0.062 (0.011) | <0.0001 | 0.062 (0.012) | <0.0001 |
| Other hormone types’ duration | 0.036 (0.011) | 0.0007 | 0.028 (0.011) | 0.01 |
| Current use | 0.073 (0.072) | 0.31 | −0.104 (0.077) | 0.17 |
| Past use | −0.095 (0.074) | 0.20 | −0.151 (0.076) | 0.05 |
| Body mass index, kg/m2 | ||||
| Average BMI during premenopause × premenopause durationc | −0.00089 (0.00024) | 0.0002 | −0.00087 (0.00025) | 0.0006 |
| Average BMI during postmenopause × menopause durationd | 0.0034 (0.0006) | <0.0001 | 0.0034 (0.0006) | <0.0001 |
| Height, inches | ||||
| Height × premenopause durationc | 0.00098 (0.00031) | 0.0015 | 0.00100 (0.00033) | 0.002 |
| Height × menopause durationd | −0.00044 (0.00130) | 0.74 | −0.00039 (0.00133) | 0.77 |
| Alcohol use | ||||
| Cumulative ounces before menopause | 0.00038 (0.00009) | <0.0001 | 0.00040 (0.00009) | <0.0001 |
| Cumulative ounces after menopause | ||||
| With hormone therapy | −0.00087 (0.0004) | 0.030 | −0.00071 (0.00041) | 0.08 |
| Without hormone therapy | −0.00022 (0.00030) | 0.47 | −0.00018 (0.00030) | 0.55 |
| Family history of breast cancer | 0.403 (0.061) | <0.0001 | 0.339 (0.064) | <0.0001 |
Abbreviations: BBD, benign breast disease; BMI, body mass index; SE, standard error.
Premenopause duration = minimum(age, age at menopause) − age at menarche.
Birth index = sum of (minimum(age, age at menopause) − age at ith birth) over all births for parous women; birth index = 0 for nulliparous women.
Including time postmenopausal while taking postmenopausal hormones.
Time postmenopausal while not taking postmenopausal hormones.
Although the one-time estimated adverse association of first pregnancy seen in previous models (15) was not statistically significant in either weighted or unweighted models, parity and age at each birth, as summarized by the birth index, showed a strong inverse relation with breast cancer. Relative to a nulliparous woman with age at menarche of 13 years and age at menopause of 50 years, a woman with a single birth at age 35 had a relative risk of 1.12 (95% CI: 0.89, 1.40) in the unweighted model and 1.06 (95% CI: 0.84, 1.33) in the weighted model. A woman with births at ages 20, 23, 26, and 29 had a relative risk of 0.78 (95% CI: 0.66, 0.91) versus 0.71 (95% CI: 0.61, 0.84) in the 2 models.
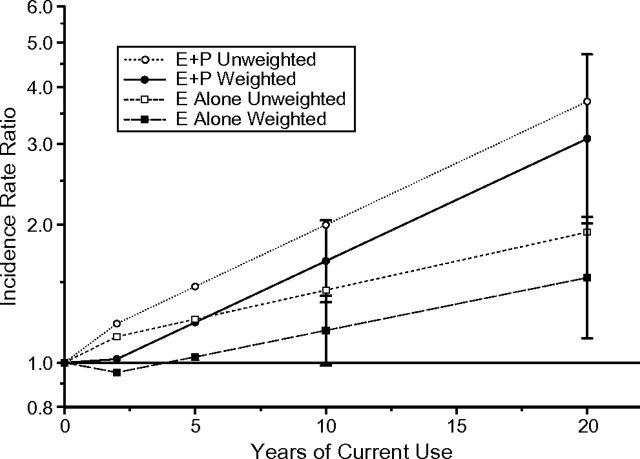
Weighting impacted the estimates associated with hormone therapy use. Figure 2 shows the estimated incidence rate ratio by duration of current use among women who used combined estrogen plus progesterone and estrogen only. The estimated rate ratio associated with 10 years of estrogen plus progesterone use decreased from 2.00 (95% CI: 1.65, 2.42) to 1.67 (95% CI: 1.36, 2.05). That for estrogen use alone for 10 years decreased from 1.44 (95% CI: 1.22, 1.70) to 1.18 (95% CI: 0.99, 1.40), with larger estimated effects with longer duration. The estimated effects of past use showed similar decreases after weighting.
Figure 2.
Estimated effects of current hormone therapy use on breast cancer in unweighted and weighted models among women in the Nurses’ Health Study, 1988–2000. E alone, estrogen alone; E + P, combined estrogen plus progestin.
The weighting also attenuated estimated effects of history of benign breast disease. For a woman who reached menarche at age 13 and menopause at age 50, the relative risk associated with a positive history at age 50 was 1.80 (95% CI: 1.51, 2.14) in the unweighted model and 1.66 (95% CI: 1.39, 1.98) in the weighted model. At age 60, these became 1.54 (95% CI: 1.40, 1.70) versus 1.43 (95% CI: 1.29, 1.59), respectively. The estimated association with family history of breast cancer was lower in the weighted model, changing from a relative risk of 1.50 (95% CI: 1.33, 1.68) to 1.40 (95% CI: 1.24, 1.59). The weighting had less impact on the estimated effects of body mass index, height, and alcohol.
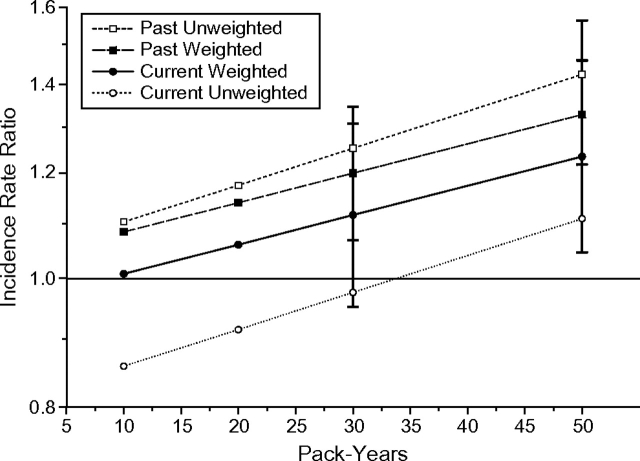
Although not part of the original breast cancer model, the estimated effect of cigarette smoking was examined in both unweighted and weighted models (Figure 3). In the unweighted model, there was a nonsignificant lower estimated risk among current smokers of 10 pack-years (incidence rate ratio (RR) = 0.86, 95% CI: 0.71, 1.04) that was eliminated after weighting (RR = 1.01, 95% CI: 0.84, 1.22). In addition, the unweighted model suggested a 25% higher risk among past smokers of 30 pack-years (RR = 1.25, 95% CI: 1.12, 1.40) that was attenuated to 20% after weighting (RR = 1.20, 95% CI: 1.07, 1.35).
Figure 3.
Estimated effects of current and past smoking on breast cancer in unweighted and weighted models among women in the Nurses’ Health Study, 1988–2000.
Finally, to determine whether screening mammography had a larger impact on risks associated with detection of small rather than larger tumors, we fit separate models for tumor sizes of 2 cm or less and for those more than 2 cm (Table 4). The unweighted and weighted estimated effects of 10 years of current use of combined estrogen plus progesterone were 2.28 versus 1.89 for small tumors and 1.45 and 1.27 for larger tumors. In general, weighting did not seem to have a larger impact on the model for small size tumors.
Table 4.
Weighted and Unweighted Breast Cancer Incidence Model by Tumor Size Among Women in the Nurses’ Health Study, 1988–2000
| Variable | Tumor Size, ≤2 cm | Tumor Size, >2 cm | ||||||
| Unweighted | Weighted | Unweighted | Weighted | |||||
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |
| Constant | −10.569 (0.446) | <0.001 | −10.387 (0.453) | <0.001 | −9.379 (0.694) | <0.001 | −9.127 (0.695) | <0.001 |
| Premenopause duration, yearsa | 0.099 (0.011) | <0.001 | 0.096 (0.012) | <0.001 | 0.058 (0.018) | 0.002 | 0.058 (0.018) | 0.0016 |
| Menopause duration, years | ||||||||
| Natural | 0.040 (0.007) | <0.001 | 0.039 (0.007) | <0.001 | 0.010 (0.011) | 0.35 | 0.012 (0.011) | 0.30 |
| Bilateral oophorectomy | 0.031 (0.009) | <0.001 | 0.032 (0.009) | <0.001 | 0.0029 (0.015) | 0.84 | 0.0063 (0.0147) | 0.67 |
| Pregnancy history | ||||||||
| Age at first birth − age at menarche | 0.0081 (0.0061) | 0.18 | 0.0085 (0.0064) | 0.19 | 0.0061 (0.0097) | 0.53 | −0.0018 (0.0097) | 0.85 |
| Birth index | −0.0023 (0.0008) | 0.008 | −0.0025 (0.0009) | 0.005 | −0.0047 (0.0014) | 0.001 | −0.0064 (0.0015) | <0.001 |
| Benign breast disease | ||||||||
| BBD, yes versus no | 1.102 (0.742) | 0.14 | 0.789 (0.780) | 0.31 | −0.299 (1.146) | 0.79 | −1.114 (1.185) | 0.35 |
| BBD × age at menarche | 0.042 (0.03) | 0.16 | 0.048 (0.032) | 0.14 | 0.055 (0.047) | 0.24 | 0.087 (0.049) | 0.076 |
| BBD × premenopause duration | −0.028 (0.014) | 0.051 | −0.024 (0.015) | 0.11 | 0.0004 (0.023) | 0.98 | 0.0090 (0.0231) | 0.70 |
| BBD × menopause duration | −0.021 (0.008) | 0.008 | −0.020 (0.008) | 0.013 | 0.0069 (0.012) | 0.58 | 0.0091 (0.0126) | 0.47 |
| Hormone therapy | ||||||||
| Oral estrogen duration | 0.030 (0.01) | 0.002 | 0.029 (0.010) | 0.004 | 0.026 (0.017) | 0.12 | 0.019 (0.017) | 0.27 |
| Oral estrogen and progesterone duration | 0.065 (0.014) | <0.001 | 0.065 (0.015) | <0.001 | 0.051 (0.025) | 0.039 | 0.051 (0.026) | 0.049 |
| Other hormone types’ duration | 0.022 (0.014) | 0.11 | 0.016 (0.014) | 0.25 | 0.065 (0.019) | <0.001 | 0.049 (0.021) | 0.017 |
| Current use | 0.177 (0.088) | 0.043 | −0.011 (0.094) | 0.90 | −0.134 (0.144) | 0.35 | −0.271 (0.152) | 0.074 |
| Past use | 0.017 (0.089) | 0.85 | −0.026 (0.092) | 0.78 | −0.302 (0.153) | 0.049 | −0.377 (0.157) | 0.016 |
| Body mass index, kg/m2 | ||||||||
| Average BMI during premenopause × premenopause durationb | −0.0012 (0.0003) | <0.001 | −0.0012 (0.0003) | <0.001 | −0.0002 (0.0004) | 0.63 | −0.00019 (0.00047) | 0.69 |
| Average BMI during postmenopause × menopause durationc | 0.0035 (0.0007) | <0.001 | 0.0037 (0.0007) | <0.001 | 0.0034 (0.0012) | 0.004 | 0.0032 (0.0012) | 0.006 |
| Height, inches | ||||||||
| Height × premenopause durationb | 0.0010 (0.0004) | 0.005 | 0.0011 (0.0004) | 0.006 | 0.0011 (0.0006) | 0.073 | 0.0011 (0.0006) | 0.094 |
| Height × menopause durationc | −0.0008 (0.0016) | 0.61 | −0.00075 (0.00163) | 0.65 | −0.0010 (0.0026) | 0.71 | −0.0013 (0.0027) | 0.63 |
| Alcohol use | ||||||||
| Cumulative ounces before menopause | 0.0004 (0.0001) | <0.001 | 0.00041 (0.00011) | <0.001 | 0.0003 (0.0002) | 0.076 | 0.00042 (0.00017) | 0.013 |
| Cumulative ounces after menopause | ||||||||
| With hormone therapy | −0.0009 (0.0005) | 0.066 | −0.00066 (0.00049) | 0.17 | −0.0005 (0.0008) | 0.53 | −0.00051 (0.00079) | 0.52 |
| Without hormone therapy | −0.0003 (0.0004) | 0.46 | −0.00019 (0.00037) | 0.60 | −0.0003 (0.0006) | 0.65 | −0.00023 (0.00061) | 0.71 |
| Family history of breast cancer | 0.470 (0.072) | <0.001 | 0.416 (0.077) | <0.001 | 0.217 (0.129) | 0.092 | 0.138 (0.136) | 0.31 |
Abbreviations: BBD, benign breast disease; BMI, body mass index; SE, standard error.
Premenopause duration = minimum(age, age at menopause) − age at menarche.
Including time postmenopausal while taking postmenopausal hormones.
Time postmenopausal while not taking postmenopausal hormones.
DISCUSSION
This paper examines factors associated with screening mammography and screening's impact on a breast cancer prediction model in the Nurses’ Health Study, a cohort of largely white female health professionals. The rate of screening rose from 77% to 92% across 2-year periods from 1988 to 2000, which was higher than in representative national samples in 1987–1989 (54%) (24), 1995–1997 (71%) (24), and 1998–2000 (76%) (1), possibly because of greater access to mammography among nurses. Even with such high rates, the lack of screening experienced by roughly 20% of the cohort could potentially distort estimated effects. Age and menopausal status were among the strongest predictors of screening mammography. Women aged 50–54 years were most likely to be screened, with lower rates among both younger and older women, particularly postmenopausal women. A similar decrease with age was also seen among a cohort of Medicare beneficiaries aged 65 or over in 2000, even after adjustment for current health status (7). There, the screening rate was only 39% over 2 years. In a cohort from Ontario studied from 1999 to 2002, 46.5% of the women had a screening mammogram within a 2-year period, a rate that also decreased with age (8). The Hawaii and Los Angeles Multiethnic Cohort also found the highest mammography use among women in their 50’s (5).
As anticipated, a history of benign breast disease or a family history of breast cancer was a strong predictor of mammography use. In addition, women with other medical conditions, such as hypertension, elevated cholesterol, and osteoarthritis, were more likely to undergo mammography, perhaps because of more frequent contact with medical professionals. Those with the more serious diagnosis of cardiovascular disease, however, were less likely to pursue breast cancer screening. The Ontario study also found lower screening rates among diabetics (8), a finding that was not observed here.
We found reduced use of mammography screening among women who were overweight or obese, but only if premenopausal. Results from the 1998 National Health Interview Survey also saw decreased rates with higher body mass index among white women only (25), and data from the 1998 Behavioral Risk Factor Surveillance Survey found reduced use among both obese and underweight women (26). Analyses of more recent Behavioral Risk Factor Surveillance Survey data from 2004, however, found no reduction in use among overweight or obese women, although lower use persisted among underweight women (9). None of these previous reports examined this relation by age or menopausal status.
Among the strongest predictors of screening mammography was hormone therapy, which is not unexpected given prior work suggesting a link between such hormones and increased breast cancer risk (2, 3). The odds of screening mammography were 2–3 times higher among current users of hormone therapy, an increase that grew larger over the years from 1988 to 1998.
How to best control for screening remains subject to debate. Weiss (12) suggested stratified or multivariable analyses adjusting for the effect of screening. Because screening may also play the role of intermediate variable, however, such means of control may not be sufficient (13). Joffe et al. (21) suggest restricting the analysis to those previously screened, which could reduce the degree of confounding but also restrict the generalizablility of predictive models. Others argue that such restriction to screened populations may not be enough if screening is not complete (27). For example, hormone therapy users who undergo screening may be different from those who do not. These authors suggest using sensitivity analysis to develop a range of plausible values for the effects of such exposures. Although Joffe et al. used only subsets in which the particular exposure effect on screening was small, we explored the use of a simple restriction to all those who had a screening mammogram in the previous 2-year period. This restriction led to estimates similar to those from the unweighted model. For example, the estimated rate ratio for 10 years of current use of estrogen plus progesterone was 2.01, and that for estrogen alone was 1.45, similar to the unweighted estimates of 2.00 and 1.44, respectively.
The current analysis first estimates the association of several factors with screening mammography and then uses these to compute weights for a predetermined breast cancer prediction model. This procedure is similar but not identical to marginal structural models using inverse probability of treatment weights (23, 28). Here, the aim is to remove the influence of screening from the estimated effects of other risk predictors, rather than to estimate the effect of screening itself. The computed model estimates the risk of breast cancer in a population of women of whom 80% undergo screening mammography, with such screening evenly distributed by the selected risk factors. The structural model attempts to estimate the direct effects of risk factors on breast cancer apart from their effect on screening (22, 23).
Weighting led to some attenuation of estimated effects in the breast cancer model, such as for history of benign breast disease, family history of breast cancer, and smoking. The largest impact was on the estimated effects of hormone therapy, likely due to its strong impact on screening. The weighted results tend to agree more closely with randomized results from the Women's Health Initiative, a trial of the health effects of hormone therapy. There, women assigned to estrogen plus progesterone experienced a 26% increase in risk of breast cancer compared with women assigned to placebo over an average 5.6 years of follow-up (29, 30), while those with a prior hysterectomy assigned to estrogen alone had a lower risk of breast cancer over an average 7.1 years (31). Because of the lack of full compliance in the Women's Health Initiative, the results are difficult to compare directly, however. More recent analyses of both the combined estrogen-progesterone (32) and estrogen-only (33) data from the Women's Health Initiative examined time from menopause to initiation of hormone therapy and suggested closer agreement between the trial and observational findings.
Limitations of the current analysis include the strong assumption of no unmeasured confounding, which is necessary to determine causality. The Nurses’ Health Study collects a wide range of variables every 2 years of follow-up, including many known risk factors for breast cancer. It is possible that others are either not available or not well controlled. The validity of any causal inference depends on this control.
Detection bias associated with screening must be considered in analyses of any predictors when routine screening examinations are advised. Although randomized trial data are available for some exposures, such as hormone therapy, it is impossible to conduct trials to study the effects of other risk factors, including reproductive factors or medical history. In addition, when examining changing rates of breast cancer incidence (34), researchers must consider screening, as well as its relation to risk factors such as hormone therapy. Causal inference models and sample reweighting, particularly when time-varying data are available, can attempt to address these complex issues.
Supplementary Material
Acknowledgments
Author affiliations: Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Nancy R. Cook); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Bernard A. Rosner, Susan E. Hankinson); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Nancy R. Cook, Susan E. Hankinson, Graham A. Colditz); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Bernard A. Rosner); and Washington University School of Medicine, St. Louis, Missouri (Graham A. Colditz).
This work was supported by grant CA087969 from the National Cancer Institute.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- RR
incidence rate ratio
References
- 1.Use of mammograms among women aged ≥ 40 years—United States, 2000–2005. MMWR Morb Mortal Wkly Rep. 2007;56(3):49–51. [PubMed] [Google Scholar]
- 2.Steinberg KK, Thacker SB, Smith SJ, et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA. 1991;265(15):1985–1990. [PubMed] [Google Scholar]
- 3.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 4.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 5.Edwards QT, Li AX, Pike MC, et al. Ethnic differences in the use of regular mammography: the multiethnic cohort. Breast Cancer Res Treat. 2009;115(1):163–170. doi: 10.1007/s10549-008-0049-7. [DOI] [PubMed] [Google Scholar]
- 6.Schairer C, Byrne C, Keyl PM, et al. Menopausal estrogen and estrogen-progestin replacement therapy and risk of breast cancer. Cancer Causes Control. 1994;5(6):491–500. doi: 10.1007/BF01831376. [DOI] [PubMed] [Google Scholar]
- 7.Bynum JP, Braunstein JB, Sharkey P, et al. The influence of health status, age, and race on screening mammography in elderly women. Arch Intern Med. 2005;165(18):2083–2088. doi: 10.1001/archinte.165.18.2083. [DOI] [PubMed] [Google Scholar]
- 8.Lipscombe LL, Hux JE, Booth GL. Reduced screening mammography among women with diabetes. Arch Intern Med. 2005;165(18):2090–2095. doi: 10.1001/archinte.165.18.2090. [DOI] [PubMed] [Google Scholar]
- 9.Berz D, Sikov W, Colvin G, et al. ‘Weighing in’ on screening mammography. Breast Cancer Res Treat. 2009;114(3):569–574. doi: 10.1007/s10549-008-0037-y. [DOI] [PubMed] [Google Scholar]
- 10.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3(2):143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 12.Weiss NS. Adjusting for screening history in epidemiologic studies of cancer: why, when, and how to do it. Am J Epidemiol. 2003;157(11):957–961. doi: 10.1093/aje/kwg062. [DOI] [PubMed] [Google Scholar]
- 13.Joffe MM. Invited commentary: screening as a nuisance variable in cancer epidemiology: methodological considerations. Am J Epidemiol. 2003;157(11):962–964. doi: 10.1093/aje/kwg063. [DOI] [PubMed] [Google Scholar]
- 14.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 16.Barlow WE, White E, Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98(17):1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 17.Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cary, NC: SAS Institute, Inc; 2007. Sas, version 9.1. [Google Scholar]
- 19.Colditz GA, Rosner BA, Chen WY, et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96(3):218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 21.Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening, and breast cancer: characterization and control of a bias. Epidemiology. 2001;12(4):429–438. doi: 10.1097/00001648-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Blackman DK, Bennett EM, Miller DS. Trends in self-reported use of mammograms (1989–1997) and Papanicolaou tests (1991–1997)—Behavioral Risk Factor Surveillance System. MMWR CDC Surveill Summ. 1999;48(6):1–22. [PubMed] [Google Scholar]
- 25.Wee CC, McCarthy EP, Davis RB, et al. Obesity and breast cancer screening. J Gen Intern Med. 2004;19(4):324–331. doi: 10.1111/j.1525-1497.2004.30354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine KR, Heo M, Allison DB. Body weight and cancer screening among women. J Womens Health Gend Based Med. 2001;10(5):463–470. doi: 10.1089/152460901300233939. [DOI] [PubMed] [Google Scholar]
- 27.Sjölander A, Humphreys K, Palmgren J. On informative detection bias in screening studies. Stat Med. 2008;27(14):2635–2650. doi: 10.1002/sim.3091. [DOI] [PubMed] [Google Scholar]
- 28.Cook NR, Cole SR, Hennekens CH. Use of a marginal structural model to determine the effect of aspirin on cardiovascular mortality in the Physicians’ Health Study. Am J Epidemiol. 2002;155(11):1045–1053. doi: 10.1093/aje/155.11.1045. [DOI] [PubMed] [Google Scholar]
- 29.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 32.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative Clinical Trial and Observational Study. Am J Epidemiol. 2008;167(12):1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prentice RL, Chlebowski RT, Stefanick ML, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167(10):1207–1216. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerlikowske K, Miglioretti DL, Buist DS, et al. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99(17):1335–1339. doi: 10.1093/jnci/djm111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.