Abstract
The caliber of the retinal vessels has been shown to be associated with stroke events. However, the consistency and magnitude of association, and the changes in predicted risk independent of traditional risk factors, are unclear. To determine the association between retinal vessel caliber and the risk of stroke events, the investigators combined individual data from 20,798 people, who were free of stroke at baseline, in 6 cohort studies identified from a search of the Medline (National Library of Medicine, Bethesda, Maryland) and EMBASE (Elsevier B.V., Amsterdam, the Netherlands) databases. During follow-up of 5–12 years, 945 (4.5%) incident stroke events were recorded. Wider retinal venular caliber predicted stroke (pooled hazard ratio = 1.15, 95% confidence interval: 1.05, 1.25 per 20-μm increase in caliber), but the caliber of retinal arterioles was not associated with stroke (pooled hazard ratio = 1.00, 95% confidence interval: 0.92, 1.08). There was weak evidence of heterogeneity in the hazard ratio for retinal venular caliber, which may be attributable to differences in follow-up strategies across studies. Inclusion of retinal venular caliber in prediction models containing traditional stroke risk factors reassigned 10.1% of people at intermediate risk into different, mostly lower, risk categories.
Keywords: cohort studies, meta-analysis, retinal vessels, risk, stroke
Each year in the United States, 1 in 18 deaths result from stroke, and approximately 610,000 people will experience their first stroke (1). Many of these stroke events are preventable; however, current methods to identify people at high risk of stroke are limited (2). The retinal and cerebral microcirculations share embryologic, anatomic, and physiologic similarities (3–6), and subtle variation in retinal blood vessel caliber, as measured from retinal photography, has been shown to be independently associated with increased risk of stroke (7–9). These findings have raised the possibility that retinal photography could provide additional information that may be of use in predicting the risk of stroke in asymptomatic people.
Results reported thus far have not been consistent. One study found that a smaller arterio-venular ratio, which may reflect narrower arterioles or wider venules, is associated with incident stroke (9), whereas others report that only wider retinal venules predict stroke (8, 10). Another study suggests that both of these retinal vascular measures (narrower arterioles and wider venules) predict stroke mortality, but only for those younger than 70 years of age (11). A recent meta-analysis using published estimates of different studies reported associations between only wider retinal venular caliber and stroke, with no associations for narrower retinal arteriolar caliber (12). The issue of which microcirculatory changes are related to stroke, and in which subgroups, is important, because these findings may provide insights into the etiology of stroke. Finally, the extent to which retinal vascular imaging changes stroke risk classification when used in conjunction with traditional stroke risk factors is unknown.
To provide robust evidence to address these questions, we conducted a systematic review and an individual-participant meta-analysis to determine the associations of retinal vessel caliber with stroke risk while adjusting for traditional stroke risk factors. We specifically examined whether an individual's classification of stroke risk as low, intermediate, or high was altered by measurement of retinal vessel caliber in conjunction with traditional risk factors (13).
MATERIALS AND METHODS
Data extraction
We reviewed the literature to identify all studies conducted in general populations that measured retinal vessel caliber and documented stroke events. A Medline (National Library of Medicine, Bethesda, Maryland) and EMBASE (Elsevier B.V., Amsterdam, the Netherlands) database search was conducted of all studies published between 1950 and July 21, 2009.
The Medline search terms we used were (exp Retinal Diseases/, (retina or retinal).tw., retinopathy.tw., Arteriolar narrowing.tw., Arterio-venous nicking.tw., Arteriovenous nicking.tw., venular dilatation.tw., venular dilation.tw., arterio-venular ratio.tw.) and (Cardiovascular Diseases/,exp Heart Diseases/,exp Vascular Diseases/, cardiovascular.tw., stroke.tw., mortality.tw.) and (incidence/, exp Mortality/, exp epidemiologic studies/, prognos$.tw., Prognosis/, predict$.mp.,course.tw., (score or scoring or scored).tw., observ$.mp., risk:.mp., between group:.tw.) and (Photography/, Photomicrography/, photo$.tw., image$.tw.). We then searched the selected papers to identify studies that met the inclusion criteria: they were carried out in general populations, had measured retinal vascular caliber from either 35 mm photographic film or digital photographs using computer-assisted methods, and had recorded stroke events.
We contacted the principal or lead investigators of the chosen studies and obtained individual-participant data from each of the studies to enable investigation of heterogeneity in published results and, if appropriate, to calculate pooled estimates of the associations between retinal vascular caliber and stroke risk. Study investigators who agreed to participate in this collaborative project were then requested to provide original recorded data on the following variables: individual retinal vessel caliber measurements, fatal and nonfatal stroke events and time to these events, baseline measurements of the traditional risk factors (age, sex, systolic blood pressure, serum total cholesterol, high density lipoprotein cholesterol, current smoking status, use of blood-pressure-lowering medications, prevalent coronary heart disease), the presence of diabetes, and previous stroke.
We excluded people with diabetes from this meta-analysis. Current guidelines for primary stroke prevention already recommend tight control of blood pressure and lipid levels among this group (2), so measurement of these additional risk factors may be of limited use (14).
Statistical analysis
The standard deviation for the means of arteriolar and venular caliber was approximately 20 μm. We estimated hazard ratios associated with a 20-μm decrease in arteriolar caliber and a 20-μm increase in venular caliber, each adjusted for the other retinal vessel caliber and adjusted for the traditional risk factors. These were estimated separately for each study by using a proportional hazards model. Data were then combined for all studies, and a stratified proportional hazards model was used to test for interaction between the study stratification variable and retinal vessel caliber variables, as well as the traditional risk factors. Doing so tests heterogeneity across studies in associations with retinal vessel caliber. When no heterogeneity was present, we obtained a pooled hazard ratio adjusted for the traditional risk factors. The stratified proportional hazards model allows the baseline hazard function to differ between the studies but assumes that the effect of the retinal vessel caliber and the other variables is fixed.
Fatal events in all studies were identified from searches of death registers supplemented by contact with relatives and/or local medical providers in all studies (11, 15–18). For nonfatal stroke outcomes, the Australian Diabetes, Obesity and Lifestyle (AusDiab) study; Beaver Dam Eye Study (BDES); and Blue Mountains Eye Study (BMES) identified nonfatal stroke events among only those who returned for subsequent study visits (19). These participants were asked whether they had had a stroke event. In BMES and AusDiab, this question was then verified from participants’ medical records, but not in BDES. In AusDiab, 82% of participants returned for the follow-up examination (20); in BMES and BDES, 75% returned for the 5-year follow-up visit (19, 21). The remaining 3 studies—the Atherosclerosis Risk in Communities (ARIC) study, Cardiovascular Health Study, and the Rotterdam Study—identified nonfatal events by using a process of continuous monitoring, which included regular telephone interviews and contacts with general practitioners and local hospitals (15, 17, 18). In all of the studies except BDES, stroke events were validated by clinician review of available medical records. Transient ischemic attack was not included in the definition of stroke for this meta-analysis.
In each study, the appropriate functional form of each of the continuous variables in the models was assessed by using fractional polynomials, and the proportional hazards assumption was tested by using plots of the Schoenfeld residuals and by testing for the effect of adding time-dependent covariates (22).
We conducted a number of sensitivity analyses to assess the robustness of the results to different methods of analysis. We repeated the main analysis by 1) standardizing the retinal vessel caliber measurements by dividing them by the study-specific standard deviations to allow for different means and standard deviations of the retinal vessel caliber measurements in the different studies (23) and 2) estimating the pooled hazard ratio by using a 2-stage process. First, the hazard ratios for the retinal caliber were estimated separately for each study; second, these estimates were pooled by using a random-effects model (23).
Reclassification of participants when adding retinal caliber to the risk prediction model
Among people without prevalent coronary heart disease, we calculated the difference between the 5-year risk of incident stroke predicted by the model that included both retinal vessel caliber and the traditional risk factors and the risk of stroke predicted by the model that included only the traditional risk factors (24). This difference was then plotted against the risk of stroke predicted by the model that included only the traditional risk factors. In this plot, we indicated those participants who would change risk categories, as suggested by Nambi et al. (13) (low: <2% risk, intermediate: 2%–5% risk, and high: >5% risk). We also plotted the percentage of people who change risk categories, from the risk level predicted by the traditional risk factors to the new risk level after incorporating retinal vessel caliber into the model, against the risk predicted by the traditional risk factors. In this plot, we overlaid the percentage of people in the pooled population for each risk level predicted by the traditional risk factors.
RESULTS
Characteristics of the studies identified
Our initial search strategy found 3,952 papers. Twenty-five studies were then identified that had performed retinal assessments or vessel caliber measurements and had followed participants over time to monitor stroke events. Eighteen of these studies recorded only the presence of retinopathy and not retinal vessel caliber or they were conducted exclusively among people with diabetes or recorded only fatal events (25–42). This exclusion process resulted in 7 studies that met our inclusion criteria. One study, the Multi-Ethnic Study of Atherosclerosis (MESA), did not have sufficient outcome data available at the time of the analysis (43). Investigators from all 6 of the other studies agreed to provide data for the individual-participant meta-analysis. These 6 studies were ARIC (9), the Cardiovascular Health Study (8), the AusDiab study (44), BMES (19), BDES (45), and the Rotterdam Study (10).
Table 1 shows the characteristics of 20,798 participants without diabetes from each of the 6 studies included in the analysis. Measurement of retinal vessel caliber was similar in each study, with some slight variations (44, 46–50). Briefly, for participants of each study, retinal photographs (film or digital) were taken for a single eye or both eyes, centered on the optic disc and macula. BDES and BMES used the Zeis FF3 camera and 30° fields (11), ARIC and the Cardiovascular Health Study used the Canon CR6-45NM camera with 45° fields (46, 49), AusDiab used the Canon CR45UAF camera with 45° fields (44), and the Rotterdam Study used the Topcon TRC-SS2 camera with 20° fields (10). In BDES, BMES, AusDiab, and the Rotterdam Study, stereoscopic images were taken; in ARIC and the Cardiovascular Health Study, single images were taken. In the AusDiab study, a digital image was captured, whereas in the other studies the color slide or transparency was digitized by using a scanner. BDES, BMES, and the Rotterdam Study all took retinal photographs following pharmacologic mydriasis, whereas ARIC, AusDiab, and the Cardiovascular Health Study took nonmydriatic photographs (44, 46–50). The Rotterdam Study used the photograph with the best image quality from either eye to measure retinal vessel caliber; in all other studies, a prespecified eye (usually the right eye) was used for the measurement and analysis of retinal vessel caliber, which was replaced with the measurement from the other eye (usually the left eye) only if the image of the first eye was not usable (44, 46–50). In a random subsample of 100 participants, the Rotterdam Study found no difference in summary measures for the right and left eyes (10).
Table 1.
Characteristics of Study Participants Included in the Meta-Analysisa
| Study (Reference No.) | No. of Participants Included in the Stroke Meta-Analysis | No. of Stroke Events | Median Follow-up (IQR), years | Arteriolar Caliber,b μm | Venular Caliber,b μm | Age, years | Systolic Blood Pressure, mm Hg | Serum Total Cholesterol, mmol/L | Serum HDL Cholesterol, mmol/L | Using Blood-pressure-lowering Medication | Current Smoker |
Prevalent Coronary Heart Disease |
|||
| Mean (Standard Deviation) |
No. | % | No. | % | No. | % | |||||||||
| ARIC (9) | 9,083 | 226 | 9.0 (1.4) | 162 (17) | 192 (16) | 60 (6) | 123 (18) | 5.4 (1.0) | 1.4 (0.5) | 2,389 | 26 | 1,617 | 18 | 700 | 8 |
| AusDiab (44) | 1,002 | 4 | 5.0 (0.1) | 176 (24) | 207 (23) | 56 (13) | 134 (19) | 5.6 (1.0) | 1.4 (0.4) | 196 | 20 | 106 | 11 | 36 | 4 |
| BDES (45) | 3,047 | 138 | 12.1 (5.0) | 202 (20) | 229 (20) | 59 (10) | 130 (19) | 6.0 (1.1) | 1.4 (0.4) | 905 | 30 | 635 | 21 | 158 | 5 |
| BMES (19) | 1,842 | 33 | 5.0 (0.7) | 193 (20) | 225 (20) | 64 (8.5) | 145 (20) | 6.0 (1.0) | 1.4 (0.4) | 516 | 28 | 222 | 12 | 132 | 7 |
| CHS (8) | 1,285 | 102 | 5.8 (0.8) | 165 (19) | 189 (18) | 79 (4) | 131 (20) | 5.3 (1.0) | 1.4 (0.4) | 681 | 53 | 84 | 6 | 169 | 13 |
| Rotterdam (10) | 4,539 | 442 | 12.0 (3.2) | 182 (18) | 219 (18) | 67 (8) | 138 (22) | 6.6 (1.2) | 1.4 (0.4) | 1,256 | 28 | 1,051 | 23 | 564 | 12 |
Abbreviations: ARIC; Atherosclerosis Risk in Communities; AusDiab, Australian Diabetes, Obesity and Lifestyle; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; CHS, Cardiovascular Health Study; HDL, high density lipoprotein; IQR, interquartile range.
Excluding people with diabetes or previous stroke.
Values reported are the central retinal equivalent. Calculated by using the Parr-Hubbard formula (46).
Optic disc photographs were then viewed by trained graders masked to participant characteristics. Graders measured the diameters of all arterioles and venules coursing through a zone surrounding the optic disc, one-half to one disc diameter away from the optic disc margin, using a computer-assisted software program. The measurement module was custom programmed in Khoros (public domain image processing software from the University of New Mexico, Albuquerque) and utilized the green channel of the digital image to enhance contrast of the retinal vessels against the retinal pigment epithelium. The ARIC, BDES, and BMES studies used an earlier version of this software to measure retinal vessel caliber, whereas AusDiab, the Cardiovascular Health Study, and the Rotterdam Study used a later version of the same software. Both versions are available on request from the authors or from the Wisconsin Fundus Photograph Reading Center, University of Wisconsin-Madison. The individual mean retinal vessel calibers provided by each study were summarized for the present meta-analysis by using the Parr-Hubbard formula (46).
Participants in all studies completed baseline questionnaires on previous medical history, and traditional stroke risk factors were measured. ARIC, Cardiovascular Health Study, and Rotterdam Study participants underwent a more extensive clinical examination than participants in the other studies (17, 18, 51). All studies used standard methods to measure the traditional cerebrovascular disease risk factors of systolic blood pressure, smoking status, serum total cholesterol, and high density lipoprotein cholesterol. The only differences between studies were that BMES recorded systolic blood pressure taken from one measurement at the baseline visit, whereas all other studies used the average of at least 2 measurements taken at the baseline visit (52). In addition, the Rotterdam Study and BDES measured nonfasting cholesterol and high density lipoprotein cholesterol levels; all other studies measured fasting values (11, 50).
Assessment of proportional hazards assumption
No evidence of a nonlinear relation was found in any of the studies between any covariates and the log-hazard function, so all covariates were fitted as linear in the proportional hazards model. The proportional hazards assumption was satisfied in all data sets.
Assessment of heterogeneity of hazard ratios between studies
Table 2 provides hazard ratios for the stroke events outcomes among people without diabetes in each study, adjusted for the traditional risk factors. The hazard ratios for arteriolar caliber were not significant in any of the studies. Four of the 6 hazard ratios for venular caliber were greater than 1, with 2 of the studies showing a significant association. The 2 studies with hazard ratios of less than 1 had the smallest number of events and also recorded nonfatal stroke events among only those who returned for a subsequent visit at 5 years. There was weak evidence that the effect of retinal venular caliber was heterogeneous between studies (P = 0.06). Excluding the AusDiab and BMES studies removed any evidence of heterogeneity. There was evidence that the effect of systolic blood pressure on stroke risk varied between studies (P = 0.01). However, accounting for this interaction had no effect on the hazard ratios for retinal vessel caliber. There was no evidence that the effect of the other traditional risk factors on risk of stroke varied between studies.
Table 2.
Relation of Retinal Vascular Caliber and Incident Stroke, Adjusted for Traditional Risk Factorsa
| Study | No. of Participantsb Included in Stroke-events Meta-Analysis | No. of Stroke Events | Retinal Arteriolar Caliberc |
Retinal Venular Caliberd |
||
| HR | 95% CI | HR | 95% CI | |||
| ARIC | 9,083 | 226 | 0.92 | 0.76, 1.12 | 1.18 | 0.97, 1.43 |
| AusDiab | 1,002 | 4 | 1.17 | 0.48, 2.84 | 0.80 | 0.27, 2.36 |
| BDES | 3,047 | 138 | 0.98 | 0.80, 1.22 | 1.04 | 0.84, 1.28 |
| BMES | 1,842 | 33 | 1.02 | 0.68, 1.54 | 0.74 | 0.48, 1.13 |
| CHS | 1,285 | 102 | 1.06 | 0.82, 1.36 | 1.37 | 1.05, 1.81 |
| Rotterdam | 4,539 | 442 | 1.00 | 0.88, 1.34 | 1.16 | 1.03, 1.31 |
| Pooled cohort | 20,798 | 945 | 1.00 | 0.92, 1.08 | 1.15 | 1.05, 1.25 |
| P value for test of heterogeneity of study-specific hazard ratios | 0.70 | 0.06 | ||||
Abbreviations: ARIC; Atherosclerosis Risk in Communities; AusDiab, Australian Diabetes, Obesity and Lifestyle; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; CHS, Cardiovascular Health Study; CI, confidence interval; HR, hazard ratio.
The traditional risk factors are age, systolic blood pressure, serum cholesterol, serum high density lipoprotein cholesterol, current smoking status, current use of antihypertensive medication, and prevalent coronary heart disease. The hazard ratios were also adjusted for the other retinal vessel caliber.
Excluding people with diabetes or previous stroke.
Per 20-μm decrease in arteriolar caliber.
Per 20-μm increase in venular caliber.
Pooled hazard ratios for stroke
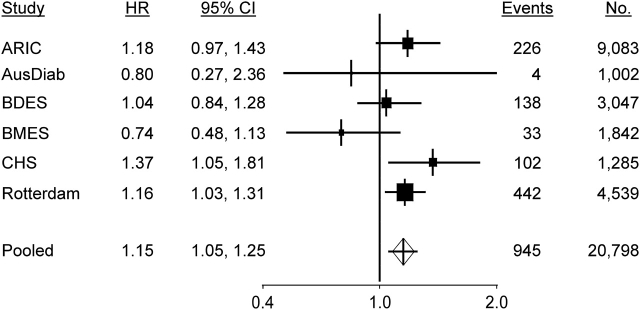
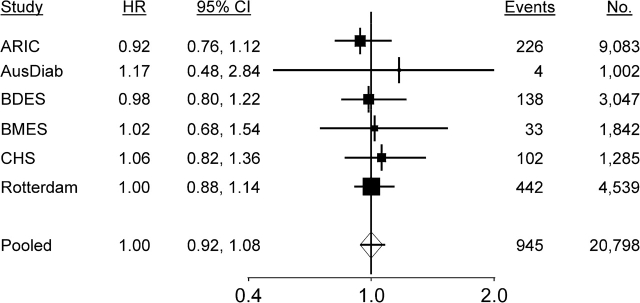
We found evidence that wider venular caliber was associated with an increased risk of stroke (hazard ratio = 1.15, 95% confidence interval: 1.05, 1.25 per 20-μm increase in caliber), and there was no evidence of an association between arteriolar caliber and risk of stroke (hazard ratio = 1.00, 95% confidence interval: 0.92, 1.08 per 20-μm decrease in caliber) (Table 2). Study-specific and pooled hazard ratios are summarized in Figures 1 and 2.
Figure 1.
Forest plots of the hazard ratio (HR) for stroke events per 20-μm increase in retinal venular caliber adjusted for age, systolic blood pressure, use of antihypertensives, total cholesterol, high density lipoprotein cholesterol, current smoking status, prevalent coronary heart disease, and retinal arteriolar caliber. The sizes of the black squares are proportional to the inverse of the variance of the estimates. ARIC, Atherosclerosis Risk in Communities; AusDiab, Australian Diabetes, Obesity and Lifestyle; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; CHS, Cardiovascular Health Study; CI, confidence interval.
Figure 2.
Forest plots of the hazard ratio (HR) for stroke events per 20-μm decrease in retinal arteriolar caliber adjusted for age, systolic blood pressure, use of antihypertensives, total cholesterol, high density lipoprotein cholesterol, current smoking status, prevalent coronary heart disease, and retinal venular caliber. The sizes of the black squares are proportional to the inverse of the variance of the estimates. ARIC, Atherosclerosis Risk in Communities; AusDiab, Australian Diabetes, Obesity and Lifestyle; BDES, Beaver Dam Eye Study; BMES, Blue Mountains Eye Study; CHS, Cardiovascular Health Study; CI, confidence interval.
Table 3 shows the pooled hazard ratios for retinal venular caliber and stroke in subgroups stratified by age, sex, and hypertension status. We found no evidence that the effect of retinal vessel caliber differed by sex and hypertension status. There was weak evidence that the effect of the retinal calibers differed by age (P = 0.07).
Table 3.
Relation of Retinal Venular Caliber and Incident Stroke in Subgroups of the Pooled Population, Adjusted for Traditional Risk Factorsa
| No. at Riskb | No. of Stroke Events | Retinal Arteriolar Caliberc |
Retinal Venular Caliberd |
|||
| HR | 95% CI | HR | 95% CI | |||
| Age, years | ||||||
| <60 | 8,543 | 131 | 1.10 | 0.86, 1.40 | 1.36 | 1.07, 1.73 |
| 60–69 | 7,860 | 372 | 0.94 | 0.82, 1.08 | 1.09 | 0.94, 1.25 |
| 70–79 | 3,432 | 314 | 1.06 | 0.92, 1.23 | 1.20 | 1.04, 1.38 |
| ≥80 | 963 | 128 | 0.78 | 0.62, 0.98 | 0.91 | 0.73, 1.15 |
| Male | 8,797 | 426 | 1.01 | 0.89, 1.15 | 1.18 | 1.04, 1.35 |
| Female | 12,001 | 519 | 0.98 | 0.87, 1.10 | 1.11 | 0.99, 1.24 |
| Hypertension | 9,677 | 667 | 0.96 | 0.87, 1.06 | 1.10 | 0.99, 1.22 |
| No hypertension | 11,121 | 278 | 1.06 | 0.90, 1.25 | 1.23 | 1.05, 1.45 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Stratified by study and adjusted for age, systolic blood pressure, use of antihypertensive medication, total cholesterol, high density lipoprotein cholesterol, current smoking status, and prevalent coronary heart disease. The hazard ratios were also adjusted for the other retinal vessel caliber.
Excluding people with diabetes or previous stroke.
Per 20-μm decrease in central retinal arteriolar equivalent.
Per 20-μm increase in central retinal venular equivalent.
Findings were similar when the retinal caliber measurements were standardized by using the study-specific standard deviations and also when the study-specific hazard ratios were combined by using a random-effects model.
Reclassification of participants when adding retinal caliber to the risk prediction model
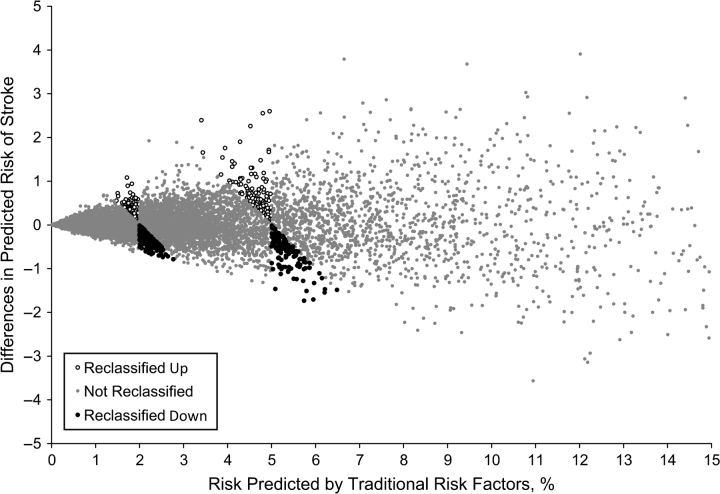
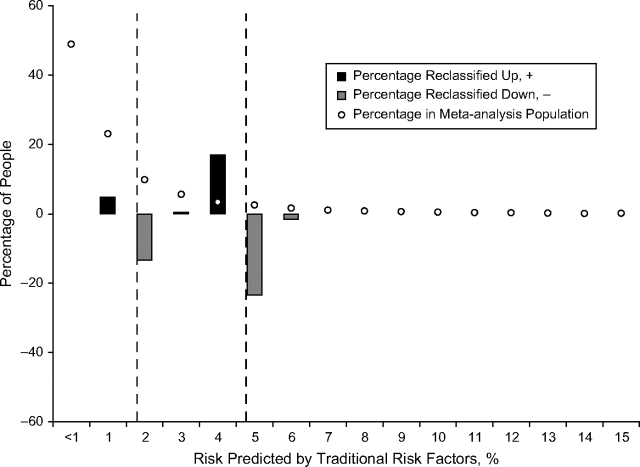
Figure 3 shows that, for people who changed risk categories when retinal vessel caliber was added to the prediction model, their risk predicted by the traditional risk factors tended to be close to a boundary where risk categories change (2% and 5%). Figure 4 displays the percentage who changed risk category for each level of risk predicted by the traditional risk factors. The largest amount of reclassification occurred in the intermediate risk category, where 10.1% were reclassified. Among the 3,600 participants at intermediate risk, 250 (6.9%) were reclassified down and 115 (3.2%) were reclassified up.
Figure 3.
Difference in the 5-year risk of stroke predicted with and without retinal vessel caliber plotted against risk predicted by using only traditional risk factors.
Figure 4.
Percentage of people reclassified after inclusion of retinal vessel caliber by risk predicted by traditional risk factors. Vertical, dashed lines separate predicted risks into low (<2% risk), intermediate (2%–5% risk), and high (>5% risk) (13).
DISCUSSION
In this individual-participant-level meta-analysis of 20,798 participants without diabetes, we showed that retinal venular caliber predicts an increased risk of incident stroke, independent of traditional stroke risk factors. Addition of information on retinal venular caliber reclassified 1 in 10 persons at intermediate risk into different risk categories. In contrast, retinal arteriolar caliber was not associated with stroke. There was weak evidence of heterogeneity in the hazard ratio for retinal venular caliber, which may be attributable to differences in follow-up strategies across studies. Summarized results were consistent among subgroups stratified by age, sex, and the presence of hypertension.
Our results confirm the recent observation from some studies, and a recent meta-analysis, that wider retinal venular caliber, rather than narrower retinal arteriolar caliber, is related to risk of stroke. This association supports increasing evidence that venules may play a key role in pathogenesis of stroke more than merely reflecting passive conductance vessels, and it highlights the importance of viewing the venular microvasculature as a dynamic component responsive to changes in circulatory flow. The mechanisms linking wider retinal venules with increased risk of stroke are unclear but may be related to concurrent circulatory phenomena such as altered vessel wall stresses, cerebral hypoxia (53), or venous insufficiency in both the retina and brain (54). It is also possible that retinal venular caliber is a more precise measure than arteriolar caliber of retinal microvascular flow. It has been shown that wider retinal venules are associated with carotid artery disease (50) and reduced arterial wall compliance, another marker of stroke risk that influences blood pressure and hemodynamics (55). Other investigators have suggested that wider retinal venular caliber is related to systemic markers of inflammation (43, 50, 56) and may be an indicator of impaired cerebral oxygen perfusion (56), which is consistent with clinical observations that ocular ischemic syndrome from carotid occlusion leads to mural hypoxia and retinal venular dilation (57). Our findings thus support the continuing research in a relatively unexplored area—the study of venular pathophysiology in both the retina and brain—that may provide further insights into the etiology of microvascular stroke (54, 58).
Estimation of stroke risk using traditional risk factors is currently recommended in the primary prevention of stroke (2), and it has recently been proposed that the predicted risks be stratified and that those persons at intermediate risk may benefit from further testing to help guide treatment (13). Adding retinal vessel caliber measurements to prediction models containing only traditional risk factors reassigned 10.1% of persons at intermediate risk. Most of those reassigned were moved into lower risk categories. At the individual level, the absolute change in predicted risks for individuals who changed risk categories was small and would be unlikely to influence treatment strategies. At the population level, whether reassigning 10.1% of those at intermediate risk would result in a cost-effective improvement in outcomes has yet to be determined. Further work on the costs of implementing retinal vessel imaging and whether preventive therapies significantly improve outcomes is required before this reassignment can be recommended.
Stroke is a heterogenous disorder with multiple etiologies (59). Some forms of stroke, such as lacunar stroke and deep cerebral hemorrhages, are believed to be primarily due to cerebral small vessel disease, and studying the retinal vascular changes in these subtypes may provide valuable clues to their pathophysiology. Our meta-analysis did not examine the relation of retinal vascular caliber to specific stroke subtypes because few of the studies had these data available. A large, multicenter cohort study is currently under way to examine the relation of retinal vascular calibers to specific stroke subtypes (e.g., ischemic, hemorrhagic, lacunar) (60).
The strengths of this meta-analysis are that we identified and analyzed all known population studies to date that have measured retinal vessel calibers and stroke outcomes, resulting in a large sample of 20,798 individuals and 945 stroke events. We were also able to include unpublished data from some studies (ARIC and AusDiab) of the relation between individual retinal vessel calibers and incident stroke. Because measurement of retinal vessel caliber using retinal photography is a relatively new technique, we were able to collaborate with all of the researchers worldwide who have reported using this technology in cohort studies that have recorded stroke events. We thus think that publication bias is highly unlikely to be present in our review and meta-analysis. We were able to standardize inclusion criteria for study subjects, the method of analysis of retinal vessel caliber, covariates adjusted for, and definitions for stroke events because we had access to individual-participant data records. This strategy provides more accurate risk estimates than meta-analyses do, which rely on published estimates (12).
This meta-analysis has a number of limitations. Error in measurement of the retinal vessel calibers and the traditional risk factors was not taken into account and may have resulted in overestimation or underestimation of the true association between retinal vessel caliber and stroke (61). These errors may differ between the studies because of the different photographic procedures and software used. In addition, in our study, we summarized retinal vessel caliber using the Parr-Hubbard formula because all 6 studies provided these data (46). A revised formula is available; however, use of this formula has been shown not to affect the estimated associations between retinal vessel caliber and cardiovascular disease outcomes (62). Finally, there was some variability in the collection of data on stroke outcomes across studies. Three of the studies (AusDiab, BDES, and BMES) recorded nonfatal events among only those who returned for a subsequent visit and responded positively to a question on stroke events. These 3 studies had the lowest hazard ratios. Removal of these studies from our analysis removed any evidence of heterogeneity and increased the pooled hazard ratio. People in poorer health, and so at greater risk of stroke, may not have returned for the follow-up visit. It is possible that the less stringent assessment of stroke events in these 3 studies reduced the ability of these studies to detect an association.
In summary, our meta-analysis utilizing individual data records on 20,798 middle- to older-aged individuals without diabetes confirmed that wider retinal venular caliber is independently associated with an increased risk of stroke events. No association with narrower retinal arteriolar caliber was observed. Our findings suggest that if retinal vessel caliber assessments are added to the traditional risk factors, 1 in 10 people without diabetes at intermediate risk would be reclassified from their current risk categories. The applicability and clinical utility of using retinal imaging for stroke risk prediction requires further study, but our results highlight the importance of examining venular widening in understanding pathophysiologic associations of the retinal and cerebral microcirculations.
Acknowledgments
Author affiliations: Screening and Test Evaluation Program, School of Public Health, University of Sydney, Sydney, New South Wales, Australia (Kevin McGeechan, Petra Macaskill, Les Irwig); Centre for Vision Research, Department of Ophthalmology, Westmead Millennium Institute, University of Sydney, Sydney, New South Wales, Australia (Gerald Liew, Jie Jin Wang, Paul Mitchell); Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin (Ronald Klein, Barbara E. K. Klein); Centre for Eye Research Australia, University of Melbourne, Melbourne, Victoria, Australia (Jie Jin Wang, Tien Y. Wong); Department of Epidemiology and Biostatistics, Erasmus Medical Center, Rotterdam, the Netherlands (Johannes R. Vingerling, Jacqueline C. M. Witteman, Monique M. B. Breteler); Department of Ophthalmology, Erasmus Medical Center, Rotterdam, the Netherlands (Johannes R. Vingerling, Paulus T. V. M. de Jong); Department of Ophthalmology, Academic Medical Center, Amsterdam, the Netherlands (Paulus T. V. M. de Jong); Department of Ophthalmogenetics, The Netherlands Institute for Neuroscience, Royal Netherlands Academy of Arts and Sciences, Amsterdam, the Netherlands (Paulus T. V. M. de Jong); Baker IDI Heart and Diabetes Institute, Melbourne, Victoria, Australia (Jonathan Shaw, Paul Zimmet); and Singapore Eye Research Institute, Yong Loo Lin School of Medicine, National University of Singapore, Singapore (Tien Y. Wong).
This work was supported by the Australian National Health and Medical Research Council (program grants 402764 and 358395). The research reported in this article was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurological Disorders and Stroke. The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
The authors thank the staff of the ARIC study for their important contributions.
Kevin McGeechan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ruth Mitchell provided advice on the construction of the Medline and EMBASE search strategies.
A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Conflict of interest: none declared.
Glossary
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- AusDiab
Australian Diabetes, Obesity and Lifestyle
- BDES
Beaver Dam Eye Study
- BMES
Blue Mountains Eye Study
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [electronic article] Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(6):1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 3.Baker ML, Hand PJ, Wang JJ, et al. Retinal signs and stroke: revisiting the link between the eye and brain. Stroke. 2008;39(4):1371–1379. doi: 10.1161/STROKEAHA.107.496091. [DOI] [PubMed] [Google Scholar]
- 4.Kwa VI, van der Sande JJ, Stam J, et al. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59(10):1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 2004;3(3):179–183. doi: 10.1016/s1474-4422(04)00682-9. [DOI] [PubMed] [Google Scholar]
- 6.Patton N, Aslam T, Macgillivray T, et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikram MK, De Jong FJ, Van Dijk EJ, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129(pt 1):182–188. doi: 10.1093/brain/awh688. [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med. 2006;166(21):2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 10.Ikram MK, de Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66(9):1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 11.Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28(16):1984–1992. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- 12.Doubal FN, Hokke PE, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry. 2009;80(2):158–165. doi: 10.1136/jnnp.2008.153460. [DOI] [PubMed] [Google Scholar]
- 13.Nambi V, Hoogeveen RC, Chambless L, et al. Lipoprotein-associated phospholipase A2 and high-sensitivity C-reactive protein improve the stratification of ischemic stroke risk in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(2):376–381. doi: 10.1161/STROKEAHA.107.513259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program (U.S.) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final Report. Bethesda, MD: National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health; 2002. [Google Scholar]
- 15.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 16.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116(2):151–157. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Hofman A, Grobbee DE, de Jong PT, et al. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7(4):403–422. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P, Wang JJ, Wong TY, et al. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65(7):1005–1009. doi: 10.1212/01.wnl.0000179177.15900.ca. [DOI] [PubMed] [Google Scholar]
- 20.Barr EL, Tonkin AM, Welborn TA, et al. Validity of self-reported cardiovascular disease events in comparison to medical record adjudication and a statewide hospital morbidity database: the AusDiab study. Intern Med J. 2009;39(1):49–53. doi: 10.1111/j.1445-5994.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 21.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care. 2002;25(10):1790–1794. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 22.Sauerbrei W, Meier-Hirmer C, Benner A, et al. Multivariable regression model building by using fractional polynomials: description of SAS, STATA and R programs. Comput Stat Data Anal. 2006;50(12):3464–3485. [Google Scholar]
- 23.Fibrinogen Studies Collaboration. Danesh J, Lewington S, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 24.McGeechan K, Macaskill P, Irwig L, et al. Assessing new biomarkers and predictive models for use in clinical practice: a clinician's guide. Arch Intern Med. 2008;168(21):2304–2310. doi: 10.1001/archinte.168.21.2304. [DOI] [PubMed] [Google Scholar]
- 25.van Hecke MV, Dekker JM, Nijpels G, et al. Retinopathy is associated with cardiovascular and all-cause mortality in both diabetic and nondiabetic subjects: the Hoorn Study. Diabetes Care. 2003;26(10):2958. doi: 10.2337/diacare.26.10.2958. [DOI] [PubMed] [Google Scholar]
- 26.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med. 2004;164(17):1917–1924. doi: 10.1001/archinte.164.17.1917. [DOI] [PubMed] [Google Scholar]
- 27.van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28(6):1383–1389. doi: 10.2337/diacare.28.6.1383. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Stratton IM, Aldington SJ, et al. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122(11):1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 29.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looker HC, Krakoff J, Knowler WC, et al. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in Pima Indians. Diabetes Care. 2003;26(2):320–326. doi: 10.2337/diacare.26.2.320. [DOI] [PubMed] [Google Scholar]
- 31.Voutilainen-Kaunisto RM, Teräsvirta ME, Uusitupa MI, et al. Occurrence and predictors of retinopathy and visual acuity in Type 2 diabetic patients and control subjects. 10-year follow-up from the diagnosis. J Diabetes Complications. 2001;15(1):24–33. doi: 10.1016/s1056-8727(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 32.McCarty DJ, Fu CL, Harper CA, et al. Five-year incidence of diabetic retinopathy in the Melbourne Visual Impairment Project. Clin Experiment Ophthalmol. 2003;31(5):397–402. doi: 10.1046/j.1442-9071.2003.00685.x. [DOI] [PubMed] [Google Scholar]
- 33.Leske MC, Wu SY, Hyman L, et al. Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology. 1999;106(10):1893–1899. doi: 10.1016/s0161-6420(99)90398-6. [DOI] [PubMed] [Google Scholar]
- 34.Ling R, Ramsewak V, Taylor D, et al. Longitudinal study of a cohort of people with diabetes screened by the Exeter Diabetic Retinopathy Screening Programme. Eye. 2002;16(2):140–145. doi: 10.1038/sj.eye.6700081. [DOI] [PubMed] [Google Scholar]
- 35.Agardh CD, Agardh E, Torffvit O. The prognostic value of albuminuria for the development of cardiovascular disease and retinopathy: a 5-year follow-up of 451 patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 1996;32(1-2):35–44. doi: 10.1016/0168-8227(96)01218-1. [DOI] [PubMed] [Google Scholar]
- 36.Yanko L, Goldbourt U, Michaelson IC, et al. Prevalence and 15-year incidence of retinopathy and associated characteristics in middle-aged and elderly diabetic men. Br J Ophthalmol. 1983;67(11):759–765. doi: 10.1136/bjo.67.11.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy MS, Peng B, Roy A. Risk factors for coronary disease and stroke in previously hospitalized African-Americans with Type 1 diabetes: a 6-year follow-up. Diabet Med. 2007;24(12):1361–1368. doi: 10.1111/j.1464-5491.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Wang YX, Xie XW, et al. Retinopathy and mortality. The Beijing Eye Study. Graefes Arch Clin Exp Ophthalmol. 2008;246(6):923–925. doi: 10.1007/s00417-008-0773-z. [DOI] [PubMed] [Google Scholar]
- 39.Qiu C, Cotch MF, Sigurdsson S, et al. Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility-Reykjavik study. Diabetes. 2008;57(6):1645–1650. doi: 10.2337/db07-1455. [DOI] [PubMed] [Google Scholar]
- 40.Stolk RP, Vingerling JR, Cruickshank JK, et al. Rationale and design of the AdRem study: evaluating the effects of blood pressure lowering and intensive glucose control on vascular retinal disorders in patients with type 2 diabetes mellitus. Contemp Clin Trials. 2007;28(1):6–17. doi: 10.1016/j.cct.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Tillin T, Evans RM, Witt NW, et al. Ethnic differences in retinal microvascular structure. Diabetologia. 2008;51(9):1719–1722. doi: 10.1007/s00125-008-1096-7. [DOI] [PubMed] [Google Scholar]
- 42.Miller RG, Prince CT, Klein R, et al. Retinal vessel diameter and the incidence of coronary artery disease in type 1 diabetes. Am J Ophthalmol. 2009;147(4):653–660. doi: 10.1016/j.ajo.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tikellis G, Wang JJ, Tapp R, et al. The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia. 2007;50(11):2263–2271. doi: 10.1007/s00125-007-0822-x. [DOI] [PubMed] [Google Scholar]
- 45.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 46.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 47.Wong TY, Knudtson MD, Klein R, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 48.Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44(7):2900–2904. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- 49.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the Cardiovascular Health Study. Ophthalmology. 2003;110(4):658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 50.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 51.Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 52.Wang JJ, Liew G, Wong TY, et al. Retinal vascular calibre and the risk of coronary heart disease-related death. Heart. 2006;92(11):1583–1587. doi: 10.1136/hrt.2006.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun C, Wang JJ, Mackey DA, et al. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54(1):74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 suppl):S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 55.Cheung N, Islam FM, Jacobs DR, Jr, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Ann Neurol. 2007;62(6):618–624. doi: 10.1002/ana.21236. [DOI] [PubMed] [Google Scholar]
- 56.de Jong FJ, Ikram MK, Witteman JC, et al. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol. 2007;61(5):491–495. doi: 10.1002/ana.21129. [DOI] [PubMed] [Google Scholar]
- 57.Klijn CJ, Kappelle LJ, van Schooneveld MJ, et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke. 2002;33(3):695–701. doi: 10.1161/hs0302.104619. [DOI] [PubMed] [Google Scholar]
- 58.Liew G, Wang JJ, Mitchell P, et al. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1(2):156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 59.Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 60.Lindley RI. Retinal microvascular signs: a key to understanding the underlying pathophysiology of different stroke subtypes? Int J Stroke. 2008;3(4):297–305. doi: 10.1111/j.1747-4949.2008.00215.x. [DOI] [PubMed] [Google Scholar]
- 61.Bennett DA. Review of analytical methods for prospective cohort studies using time to event data: single studies and implications for meta-analysis. Stat Methods Med Res. 2003;12(4):297–319. doi: 10.1191/0962280203sm319ra. [DOI] [PubMed] [Google Scholar]
- 62.Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]