Abstract
Preterm delivery (PTD) is a complex trait with a significant familial component. However, no specific inheritance patterns have been established. The authors examined the contribution of PTDs in both the woman's family and her partner's family to her risk of PTD. The authors linked birth information from Danish national registers with pedigree information from the Danish Family Relations Database for 1,107,124 live singleton deliveries occurring from 1978 to 2004. Risk ratios were estimated comparing women with and without various PTD histories. Women with previous PTDs were at greatly increased risk of recurrent PTD (risk ratio = 5.6, 95% confidence interval: 5.5, 5.8); however, their PTD risk was unaffected by a partner's history of preterm children with other women. PTDs to a woman's mother, full sisters, or maternal half-sisters also increased her PTD risk (risk ratio = 1.6, 95% confidence interval: 1.5, 1.6), whereas PTDs in her paternal half-sisters, the female partners of her male relatives, or members of her partner's family did not affect her PTD risk. Inheritance patterns were similar for all gestational ages from very early through late PTD. The substantial portion of PTD risk explained by effects passed through the female line suggests a role for either imprinting or mitochondrial inheritance.
Keywords: cohort studies, family, genetics, inheritance patterns, premature birth
Preterm delivery (PTD) and its sequelae are responsible for 3 million deaths worldwide annually and, with low birth weight, contribute to the loss of over 100 million disability-adjusted life-years (1). Although PTD is etiologically heterogeneous, genetic factors are widely thought to account for a significant proportion of PTDs (2). Twin studies have shown that up to 40% of PTDs may have a genetic component (3, 4), and heritability studies have suggested that gestational length has heritable maternal and fetal components (5, 6). The strongest established risk factor for PTD is a previous PTD (7), but women who themselves were born preterm or whose sisters delivered prematurely have also been shown to be at increased risk of delivering preterm (8–11).
Despite recognition of a familial component to PTD, no specific mode of inheritance has been posited for PTD attributable to genetic factors, and no common genetic variants have yet been confirmed as contributing to PTD. However, previous studies do offer some intriguing clues to likely mechanisms. In a study from 1974, the sisters of women who delivered preterm appeared to be almost twice as likely as sisters-in-law to themselves deliver prematurely (12). In addition, Wilcox et al. (11) found that being born preterm increased a man's risk of fathering a preterm child very little, if at all. Furthermore, Basso et al. (13) found that, whereas switching female partners reduced a man's risk of having a subsequent preterm child, switching male partners did not affect a woman's recurrence risk (14).
With the help of a unique database that allowed us to construct pedigrees for the entire Danish population, we systematically examined the contribution of PTDs in both a woman's family and her male partner's family to her risk of PTD, and we evaluated the contributions of the maternal and paternal lines to familial clustering of PTD.
MATERIALS AND METHODS
Data sources
Since April 1, 1968, the Danish Civil Registration System has registered demographic and vital status information on all persons residing in Denmark, aided by the unique personal identification number assigned to each Danish resident (15). The personal identification number permits tracking of individuals in the Danish population over time and accurate linkage of individual-level information from Denmark's nationwide population-based registers, including the Medical Birth Register and the Danish Family Relations Database. The Medical Birth Register has recorded information on all livebirths and stillbirths in Denmark since 1973 and is considered close to complete (16). Gestational length, based on the date of the last menstrual period and corrected for ultrasound measurements where available, has been registered since 1978. The Danish Family Relations Database is based on kinship information in the Civil Registration System and permits identification of all relatives registered in the Civil Registration System for anyone with a personal identification number.
Study cohorts
By linking data from the Civil Registration System, Medical Birth Register, and Danish Family Relations Database, we assembled a data set on which all subsequent analyses were based. First, we identified all women who delivered live singletons with known gestational length between January 2, 1978, and December 31, 2004, and determined the identity of each baby's father. For each parent, we then identified parents, siblings, and half-siblings and determined which of these family members also had children with known gestational length born in Denmark in the period 1978–2004.
We defined a PTD as a delivery resulting in a living child occurring before 37 completed weeks’ gestation. For each delivery, we assessed whether the mother had 1 or more of 4 possible histories of PTD as follows. 1) A woman had a personal history of PTD if she had had 1 or more previous PTDs. 2) A positive partner personal history was assigned if a woman's male partner had previously had 1 or more preterm children with another woman. 3) A woman had a family history of PTD if 1 or more of her female relatives or her male relatives’ partners had previously had a PTD. 4) A woman had a partner family history of PTD if 1 or more of her male partner's relatives had previously had a preterm child. For each of the 4 PTD histories, a study cohort was created that included all women where such a history was possible. The effect of a given PTD history was then evaluated among the women within the relevant study cohort. Thus, for example, to be included in the family history cohort, a woman had to have at least 1 relative who had previously delivered a child registered with known gestational length. Twin and triplet deliveries were ignored when defining PTD histories, as the mechanisms governing the initiation of labor in singleton and multiple gestations may differ.
Statistical analyses
Using log-linear binomial regression, we estimated risk ratios comparing the risks of PTD in women with various PTD histories with those of women with no such histories. All risk ratios were adjusted for the calendar period. In additional analyses, we included interaction terms with sex to assess whether the overall effect of any history of PTD varied by the sex of the offspring.
We also stratified our analyses by the number of relatives with livebirths after gestations of known length that could be identified (≤3 vs. >3). However, the results were similar in the 2 strata. This suggests that any bias produced by differences between women in exposure opportunity was negligible and that it was acceptable to analyze data from the 2 strata together.
Women were allowed to contribute multiple deliveries to each cohort. The potential effect of correlated outcomes within the same woman was evaluated in additional analyses by estimating risk ratios with 95% confidence intervals according to family history of PTD using generalized estimating equations. The use of generalized estimating equations had a negligible effect on the risk ratios and confidence intervals, suggesting little effect of correlated outcomes within the same woman.
We also evaluated whether the effect of a family history of PTD on a woman's risk of PTD might be influenced by a positive personal history of PTD, a known strong risk factor for subsequent PTDs, by including a personal history of PTD and a history of PTD among a woman's female relatives in a model together. Because the personal and family history effects appeared to be unaffected by adjustment for one another, we opted not to adjust our family history results for personal history.
In groups where an effect of PTD history (personal or family) was observed, we examined the effect in closer detail by gestational length. For the gestational length intervals 20–29 weeks, 30–32 weeks, 33–35 weeks, and 36 weeks, we estimated the risk ratios associated with delivery in these intervals according to lowest gestational age among 1) a woman's own previous deliveries or 2) previous deliveries to relevant relatives. The risk ratios for a given interval were estimated among women at risk, that is, among women with a gestational length in the interval or in a later interval.
Ethics approval
This study was approved by the Danish Data Protection Agency.
RESULTS
Of the 1,799,325 live singleton deliveries that occurred in Denmark in the period 1978–2004, 1,487,159 (82.7%) had gestational length recorded in the Medical Birth Register. Over the period, the proportion of deliveries with known gestational length increased from 59.7% in 1978, 79.4% in the 1980s, 87.3% in the 1990s, to 97.4% in 2004; 1,107,124 of these deliveries were included in at least 1 analysis, and 51,223 (4.6%) were preterm. There were 163,346 deliveries to women with some type of history of PTD; 12,891 (7.9%) of these were PTDs. Overall, women with any history (personal, partner, family, or partner's family) of PTD were 92% more likely (95% confidence interval (CI): 89, 96) than women with no history of PTD to deliver prematurely; this result did not depend on the sex of the coming child (data not shown).
Women with a personal history of PTD (≥1 previous PTDs) were 5.6 times (95% CI: 5.5, 5.8) more likely than women who had never delivered prematurely to have another preterm child. The effect of a personal history of PTD was substantial in both women delivering children fathered by the same man as their other children and women who had changed partners between children (Table 1). In contrast, there was little effect of personal history of PTD in men who had changed partners between children (risk ratio (RR) = 1.1, 95% CI: 1.0, 1.2).
Table 1.
A Woman's Risk of Delivering Prematurely, by Personal History and Partner's Personal History of Preterm Delivery, Denmark, 1978–2004a
| History of PTD | Risk of PTD |
Risk Ratiob | 95% Confidence Interval | |||||
| No History of PTD |
History of PTD |
|||||||
| % | No. of PTDs | Total No. of Deliveries | % | No. of PTDs | Total No. of Deliveries | |||
| Personal historyc | ||||||||
| Same male partnerd | 2.9 | 15,897 | 547,508 | 17 | 5,170 | 30,107 | 5.9 | 5.7, 6.1 |
| Different male partner | 4.8 | 4,423 | 91,371 | 20 | 1,499 | 7,552 | 4.1 | 3.9, 4.3 |
| Partner's personal historye | ||||||||
| Same female partnerd | 2.9 | 15,897 | 547,508 | 17 | 5,170 | 30,107 | 5.9 | 5.7, 6.1 |
| Different female partner | 5.9 | 5,167 | 87,732 | 6.7 | 440 | 6,591 | 1.1 | 1.0, 1.2 |
Abbreviation: PTD, preterm delivery.
“Preterm delivery” was defined as a delivery resulting in a living child occurring at or before 36 completed weeks’ gestation.
Risk ratio comparing the risk of delivering prematurely in women with a history of PTD in the specified category with the risk in women with no such history.
A woman had a personal history of PTD if she had ≥1 previous PTDs.
The same numbers and risk ratio appear in both places because those women who kept the same male partner from one delivery to the next are also those whose male partner had not switched women between children.
A woman had a partner personal history of PTD if her male partner had previously had ≥1 preterm children with another woman.
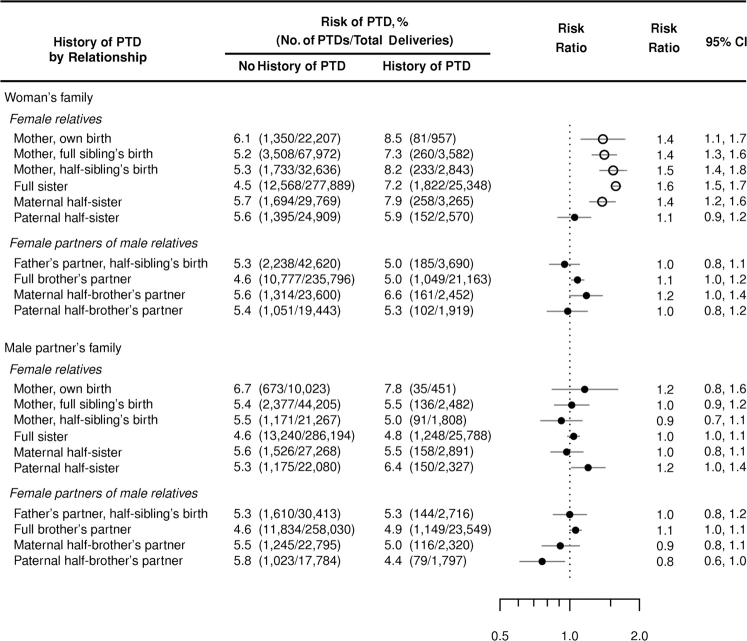
Figure 1 shows risks and risk ratios for delivering prematurely by history of PTD in specific members of a woman's own family (family history) and her male partner's family (partner family history). Women whose mothers delivered prematurely were at increased risk of having a preterm child, compared with women whose mothers had no history of PTD, regardless of whether it was the women themselves, their full sibling(s), or their maternal half-sibling(s) who had been born preterm (RR range, 1.4–1.5) (the ratio of the RR for the woman herself being delivered preterm to the RR for her siblings being delivered preterm was 0.92, 95% CI: 0.72, 1.17). Women whose full sisters or maternal half-sisters had preterm children were also at increased risk of PTD, with risk ratios of similar magnitude (RRs = 1.6 and 1.4, respectively). Overall, women with a history of PTD among their mothers, full sisters, or maternal half-sisters were 55% more likely (95% CI: 49, 61) to deliver prematurely than women with no such history.
Figure 1.
A woman's risk of preterm delivery (PTD), by history of PTD in her own family and in her male partner's family, Denmark, 1978–2004. Female members of the woman's family and her male partner's family who delivered live children before the woman herself in the period 1978–2004 after gestations of known length were included in the analyses. A history of PTD was defined as having 1 or more female family members in the category in question with children born at or before 36 completed weeks’ gestation and before the birth of the woman's child. The risk ratios compare the risk of PTD in women with a history of PTD (≥1 PTD) in the specified female family members with the risk in women with no such family history. The open circles identify risk ratios related to a history of PTD in female relatives on the woman's maternal side. CI, confidence interval.
In contrast, a history of PTD among a woman's paternal half-sisters or the female partners of her male relatives did not affect her risk of PTD (Figure 1). The risk ratios given PTD history in maternal versus paternal half-sisters were significantly different (P = 0.007), the ratio of risk ratios being 1.33 (95% CI: 1.08, 1.63). Furthermore, a woman's risk of PTD was, in general, unaffected by PTDs among her male partner's female relatives or the female partners of his male relatives (partner family history) (Figure 1). The effects of a history of PTD among a woman's male and female siblings and among her male partner's siblings did not depend on the sex of the coming child (data not shown).
Table 2 examines the relation between a (personal or family) history of PTD in given gestational intervals and her risk of delivering preterm at similar and dissimilar time points. A woman whose lowest gestational age among her own previous deliveries was in any of the 4 intervals had an increased risk of PTD in all 4 intervals when compared with a woman who had previously delivered at term but not earlier. Risk ratios linking a history of early preterm birth with the risk of similar subsequent preterm births were the largest, ranging from 10 to 18 for women with PTD histories in weeks 30–32 or 20–29.
Table 2.
A Woman's Risk Ratio of Delivering a Preterm Child in a Given Gestational Interval, According to the Lowest Gestational Age Among Her Own Previous Deliveries and Among Deliveries to Her Female Relatives, Denmark, 1978–2004
| Lowest Previous Gestational Age, weeksa | Gestational Interval |
|||||||
| 20–29 Weeks |
30–32 Weeks |
33–35 Weeks |
36 Weeks |
|||||
| Risk Ratio | 95% Confidence Interval | Risk Ratio | 95% Confidence Interval | Risk Ratio | 95% Confidence Interval | Risk Ratio | 95% Confidence Interval | |
| Among own previous deliveries | ||||||||
| 20–29 | 18 | 16, 22 | 12 | 10, 14 | 7.5 | 6.7, 8.3 | 4.6 | 4.0, 5.3 |
| 30–32 | 10 | 8.4, 12 | 12 | 10, 14 | 8.8 | 8.0, 9.6 | 5.2 | 4.6, 5.8 |
| 33–35 | 5.0 | 4.3, 5.8 | 6.2 | 5.6, 7.0 | 7.0 | 6.6, 7.4 | 5.4 | 5.0, 5.7 |
| 36 | 3.3 | 2.7, 4.0 | 3.2 | 2.8, 3.7 | 4.3 | 4.0, 4.6 | 4.4 | 4.1, 4.7 |
| 37–41 (referent) | 1 | 1 | 1 | 1 | ||||
| Among female relatives' deliveriesb | ||||||||
| 20–29 | 2.0 | 1.3, 3.0 | 1.6 | 1.1, 2.3 | 1.8 | 1.5, 2.2 | 1.2 | 0.9, 1.5 |
| 30–32 | 1.7 | 1.2, 2.5 | 1.7 | 1.3, 2.3 | 1.7 | 1.4, 1.9 | 1.6 | 1.3, 1.8 |
| 33–35 | 1.7 | 1.4, 2.1 | 1.5 | 1.3, 1.8 | 1.7 | 1.6, 1.9 | 1.5 | 1.3, 1.6 |
| 36 | 1.5 | 1.2, 1.9 | 1.4 | 1.1, 1.6 | 1.5 | 1.4, 1.7 | 1.4 | 1.3, 1.6 |
| 37–41 (referent) | 1 | 1 | 1 | 1 | ||||
Estimates for week 42 or later are not shown.
Woman's mother, full sisters, and maternal half-sisters.
Women whose mothers, full sisters, or maternal half-sisters had a history of preterm birth in any of the 4 gestational intervals had greater risks of PTD in all 4 intervals compared with women whose mothers, full sisters, or maternal half-sisters had delivered at term but not earlier (Table 2). The risk ratios were, however, much more homogeneous across the table than the personal history risk ratios.
DISCUSSION
The substantial role that PTD plays in infant and maternal mortality makes identifying causes that might lead to improved prevention of PTD a priority (17). This cohort study, which was based on more than 25 years of data on the entire Danish population, confirms that there is a heritable aspect to PTD, as evidenced by associations between a history of PTD in various relatives and a woman's risk of PTD. More importantly, our study strongly indicates that 1) insofar as heritable factors underlie PTD, they exert their effects through the mother, while paternally derived heritable factors play little role, and 2) heritable factors passed through the female line account for a substantial portion of PTD risk.
When a woman had 1 or more previous PTDs, her risk of delivering prematurely in subsequent pregnancies remained high whether or not she had changed partners. Conversely, there was a minimal effect of personal history of PTD in men who had changed partners between children. Additionally, the risk of PTD in a man's female partner was completely unaffected by a history of PTD among any of his family members, female or male. Taken together, these findings indicate that the paternal contribution to the fetal genotype influences the risk of PTD little, if at all, and it is either the maternal genotype or the maternal contribution to the fetal genotype that affects the risk of PTD.
Previous PTDs and a history of PTD among certain female family members increased a woman's risk of PTD, in both the aggregate and for specific gestational intervals. PTDs to her mother (regardless of whether it was the woman herself or her full or half-siblings who were born preterm), full sisters, and maternal half-sisters increased a woman's risk of PTD by 30%–60%. However, PTDs to her paternal half-sisters and to the partners of her male relatives did not affect her risk of delivering prematurely. These results suggest that genetic variants passed on to a woman from her mother have important effects on PTD risk and reinforce that the paternal contribution to PTD is limited (otherwise, we would expect to see an increased risk of PTD in women whose male relatives had fathered preterm children).
Complex traits are generally thought to be influenced by many genetic and environmental factors. We expect this to be the case for preterm delivery too. We note, however, that our findings are difficult to reconcile entirely with traditional polygenic inheritance, where alleles derived from both parents are expressed at each locus. Under such a scenario, we would expect to see an increase in risk linked to PTDs in paternal half-sisters (as well as in maternal half-sisters). On the other hand, variation in either imprinted genes or mitochondrial genes is compatible with the pattern of associations that we observed. Imprinting (epigenetic modification of DNA and chromatin) results in suppression of alleles from 1 parent and expression of the corresponding alleles from the other parent. Numerous studies have shown that imprinting plays an important role in feto-placental development (18–20). In our study, expression of only maternal alleles in genes governing the initiation of delivery could conceivably produce risk patterns such as those we observed. Alternatively, variation in mitochondrial genes could also produce maternal lineage effects consistent with our findings, because the mitochondrial genome is cytoplasmic and therefore transmitted by mothers (only) to all of their children.
Theoretically, variation in maternal genes could affect risk of PTD through effects on either maternal or fetal triggers of parturition (21). In the case of imprinted genes and polygenic inheritance, our results suggest no substantial role of fetal genotype in PTD risk. Under typical polygenic inheritance, if the fetal genotype were important, we would expect to see PTD risk also transmitted through fathers. Under an imprinting scenario, if the fetal genotype were important, we would expect a woman's risk of PTD to be greater if she herself was born preterm than if her siblings were delivered prematurely. We did not see such a trend in our data; the ratio between these risk ratios was 0.92 (95% CI: 0.72, 1.17). In contrast, our results are consistent with both fetal and maternal genotype effects under a mitochondrial inheritance scenario. Interestingly, data from assisted reproductive technology conceptions show that the rates of PTD are similar whether women use their own eggs or donor eggs (which retain mitochondria from the donor) (22), suggesting that the maternal genotype, and not the fetal genotype, is important in PTD. Ultimately, data from more extended pedigrees and comprehensive molecular genetic studies would be needed to fully characterize the genetic architecture of PTD risk.
When dividing PTDs into specific gestational intervals, we found, consistent with previous studies (23–26), that recurrence risks were greatest for women with a personal history of early PTD. We note, however, that the high risk ratio given a personal or family history of early PTD cannot alone account for the aggregated results (PTD in weeks 20–36). For example, women with a personal history of late PTD (week 36) were 3.3 times more likely to have a very early PTD (weeks 20–29) than women who had previously delivered in weeks 37–41 but not earlier. Thus, while mechanisms contributing to PTD may be of varying importance at different time points of gestation, the patterns of inheritance presented here are apparent in all 4 gestational intervals from very early to late preterm delivery.
Although our study results are specific to the Danish population, it seems likely that etiologies for PTD will be shared, although in different proportions, across populations. For example, as in our study, a recent study from Norway found that, compared with mothers themselves born at term, mothers born prematurely had an increased risk (RR = 54%, 95% CI: 42, 67) of PTD; this risk increased further when only early preterm births (births at <35 weeks) were considered (11). Fathers born prematurely had only a small increased risk (RR = 12%, 95% CI: 1, 25) of having a preterm firstborn, and this increase in risk disappeared with restriction to early preterm births. Similarly, a recent extended twin study from the Netherlands on the heritability of birth timing found a significant contribution of maternal genes but no paternal genetic effect (27).
The great strength of the present study lay in our ability to examine the effects of all aspects of personal and family history of PTD on a woman's risk of PTD, as well as to examine the individual effects of PTDs to specific family members in both her own family and her male partner's family. To our knowledge, this is the most comprehensive population-based analysis of familial patterns of preterm delivery. The necessary data were furnished by a unique set of resources. First, the Danish Family Relations Database made it feasible to construct multigenerational pedigrees for an entire country bioinformatically. Second, the Civil Registration System and the Medical Birth Register gave us information on births and gestational length, avoiding recall bias.
One possible drawback of the population-based approach taken here is that data on environmental risk factors, such as intrauterine infection, smoking, socioeconomic status, and maternal nutritional status, were not available and were therefore not accounted for in the analyses. Such risk factors can influence the risk of PTD (28–31), and they could conceivably be passed from mothers to their daughters but less so to their sons, resulting in similar inheritance patterns as those we observe. One example of a nongenetic scenario concerns vertical transmission of genital tract microflora from mother to daughter. The genital tract microbiome is associated with preterm birth (32), so such transmission could give patterns of PTD risk similar to those we observed.
The observed clustering of PTDs could also potentially be due to clustering of menstrual cycle length within families. Under this scenario, families with a tendency for short menstrual length would appear to have gestations of shorter length than they actually have, if gestational length estimates are based on the last menstrual period. To evaluate this, we performed simulations based on the distribution of menstrual cycle length (33) and the distribution of gestational length in Denmark from the Danish National Board of Health. Even with the extreme assumptions that family members have exactly the same length of menstrual cycle and that all gestational length estimates are based on the last menstrual period (in reality many have been corrected for ultrasound measurements), the simulations showed that a spurious increase in risk of PTD of no more than a few percent would be observed among individuals with family history of PTD (data not shown). Thus, clustering of menstrual cycle length within families can not explain the observed clustering of PTD.
We confined the analyses to live singleton births, but it was beyond the scope of this paper to categorize PTDs in finer detail. We note, though, that several different mechanisms can lead to PTD, and that strategies for classifying PTD are a matter of continued discussion. The traditional distinction between spontaneous and medically indicated PTDs may not be optimal, since conditions motivating medical intervention often share mechanisms also leading to spontaneous PTD (34). Furthermore, spontaneous PTD has been associated with increased risks of both recurrent spontaneous PTD and medically indicated PTD, and vice versa (35). A recent study of very early PTD found empirical support for an alternative, biology-based classification scheme with 2 broad groups. One group was associated with intrauterine inflammation, while the other was associated with abnormal placentation (36). PTDs in either group could be genetically mediated. It remains, however, to be investigated how well this classification scheme works for less extreme PTDs. Furthermore, application of this method would necessitate careful chart review and would not be feasible for studies based on health register data (37). Such fine level characterization would be much more feasible in, for example, genome scans with a few thousand cases and controls.
In conclusion, we showed that, in addition to a history of PTD in a woman's own previous deliveries, a history of PTD in her mother, full sister, or maternal half-sister also significantly increases a woman's risk of PTD. In contrast, PTDs in her paternal half-sister, the female partners of her male relatives, or any member of her partner's family do not affect her risk of PTD. We propose that variants in imprinted genes, mitochondrial genes, or other unrecognized maternal effects could play a role in producing these patterns and suggest that the search for genetic variants involved in PTD should pay special attention to such genes in the maternal genome (e.g., by having a lower threshold for including such variants in the follow-up stages of a genome scan).
Acknowledgments
Author affiliations: Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark (Heather A. Boyd, Gry Poulsen, Jan Wohlfahrt, Bjarke Feenstra, Mads Melbye); and Department of Pediatrics, University of Iowa, Iowa City, Iowa (Jeffrey C. Murray).
This study was partially supported by grants from the National Institutes of Health (1U01 HG-004423-01 and 1R01 HD-52953), the March of Dimes Birth Defects Foundation (21-FY06-575), and the Danish Medical Research Council (271-07-0324).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- PTD
preterm delivery
- RR
risk ratio
References
- 1.Lawn JE, Cousens S, Zupan J, et al. 4 million neonatal deaths: when? where? why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107(3):375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 4.Treloar SA, Macones GA, Mitchell LE, et al. Genetic influences on premature parturition in an Australian twin sample. Twin Res. 2000;3(2):80–82. doi: 10.1375/136905200320565526. [DOI] [PubMed] [Google Scholar]
- 5.Lie RT, Wilcox AJ, Skjaerven R. Maternal and paternal influences on length of pregnancy. Obstet Gynecol. 2006;107(4):880–885. doi: 10.1097/01.AOG.0000206797.52832.36. [DOI] [PubMed] [Google Scholar]
- 6.Lunde A, Melve KK, Gjessing HK, et al. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165(7):734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 7.DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med. 2007;25(1):40–51. doi: 10.1055/s-2006-956774. [DOI] [PubMed] [Google Scholar]
- 8.Porter TF, Fraser AM, Hunter CY, et al. The risk of preterm birth across generations. Obstet Gynecol. 1997;90(1):63–67. doi: 10.1016/S0029-7844(97)00215-9. [DOI] [PubMed] [Google Scholar]
- 9.Selling KE, Carstensen J, Finnström O, et al. Intergenerational effects of preterm birth and reduced intrauterine growth: a population-based study of Swedish mother-offspring pairs. BJOG. 2006;113(4):430–440. doi: 10.1111/j.1471-0528.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 10.Winkvist A, Mogren I, Högberg U. Familial patterns in birth characteristics: impact on individual and population risks. Int J Epidemiol. 1998;27(2):248–254. doi: 10.1093/ije/27.2.248. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol. 2008;167(4):474–479. doi: 10.1093/aje/kwm319. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone F, Inglis L. Familial trends in low birth weight. Br Med J. 1974;3(5932):659–661. doi: 10.1136/bmj.3.5932.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basso O, Olsen J, Christensen K. Low birthweight and prematurity in relation to paternal factors: a study of recurrence. Int J Epidemiol. 1999;28(4):695–700. doi: 10.1093/ije/28.4.695. [DOI] [PubMed] [Google Scholar]
- 14.Basso O, Olsen J, Christensen K. Study of environmental, social, and paternal factors in preterm delivery using sibs and half sibs. A population-based study in Denmark. J Epidemiol Community Health. 1999;53(1):20–23. doi: 10.1136/jech.53.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen CB, Gøtzsche H, Møller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 16.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320–323. [PubMed] [Google Scholar]
- 17.Lawn JE, Cousens SN, Darmstadt GL, et al. 1 year after The Lancet Neonatal Survival Series—was the call for action heard? Lancet. 2006;367(9521):1541–1547. doi: 10.1016/S0140-6736(06)68587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore T. Genetic conflict, genomic imprinting and establishment of the epigenotype in relation to growth. Reproduction. 2001;122(2):185–193. doi: 10.1530/rep.0.1220185. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins JF, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet. 2003;4(5):359–368. doi: 10.1038/nrg1062. [DOI] [PubMed] [Google Scholar]
- 20.Fowden AL, Sibley C, Reik W, et al. Imprinted genes, placental development and fetal growth. Horm Res. 2006;65(suppl 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- 21.Smith R. Parturition. N Engl J Med. 2007;356(3):271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 22.Wright VC, Chang J, Jeng G, et al. Assisted reproductive technology surveillance—United States, 2004. MMWR Surveill Summ. 2007;56(6):1–22. [PubMed] [Google Scholar]
- 23.Kristensen J, Langhoff-Roos J, Kristensen FB. Implications of idiopathic preterm delivery for previous and subsequent pregnancies. Obstet Gynecol. 1995;86(5):800–804. doi: 10.1016/0029-7844(95)00275-V. [DOI] [PubMed] [Google Scholar]
- 24.Melve KK, Skjaerven R, Gjessing HK, et al. Recurrence of gestational age in sibships: implications for perinatal mortality. Am J Epidemiol. 1999;150(7):756–762. doi: 10.1093/oxfordjournals.aje.a010078. [DOI] [PubMed] [Google Scholar]
- 25.Adams MM, Elam-Evans LD, Wilson HG, et al. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–1596. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 26.McManemy J, Cooke E, Amon E, et al. Recurrence risk for preterm delivery. Am J Obstet Gynecol. 2007;196(6):576.e1–576.e6. doi: 10.1016/j.ajog.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 27.Kistka ZA, DeFranco EA, Ligthart L, et al. Heritability of parturition timing: an extended twin design analysis. Am J Obstet Gynecol. 2008;199(1):43.e1–43.e5. doi: 10.1016/j.ajog.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 29.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(suppl 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 30.Smith LK, Draper ES, Manktelow BN, et al. Socioeconomic inequalities in very preterm birth rates. Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F11–F14. doi: 10.1136/adc.2005.090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luke B. The evidence linking maternal nutrition and prematurity. J Perinat Med. 2005;33(6):500–505. doi: 10.1515/JPM.2005.088. [DOI] [PubMed] [Google Scholar]
- 32.Guaschino S, De Seta F, Piccoli M, et al. Aetiology of preterm labour: bacterial vaginosis. BJOG. 2006;113(suppl 3):46–51. doi: 10.1111/j.1471-0528.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- 33.Jukic AM, Weinberg CR, Wilcox AJ, et al. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008;167(1):25–33. doi: 10.1093/aje/kwm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savitz DA, Dole N, Herring AH, et al. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19(2):97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 35.Ananth CV, Getahun D, Peltier MR, et al. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195(3):643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 36.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savitz DA. Invited commentary: disaggregating preterm birth to determine etiology. Am J Epidemiol. 2008;168(9):990–992. doi: 10.1093/aje/kwn193. [DOI] [PubMed] [Google Scholar]