Abstract
Although cigarette smoking and alcohol consumption increase risk for head and neck cancers, there have been few attempts to model risks quantitatively and to formally evaluate cancer site-specific risks. The authors pooled data from 15 case-control studies and modeled the excess odds ratio (EOR) to assess risk by total exposure (pack-years and drink-years) and its modification by exposure rate (cigarettes/day and drinks/day). The smoking analysis included 1,761 laryngeal, 2,453 pharyngeal, and 1,990 oral cavity cancers, and the alcohol analysis included 2,551 laryngeal, 3,693 pharyngeal, and 3,116 oval cavity cancers, with over 8,000 controls. Above 15 cigarettes/day, the EOR/pack-year decreased with increasing cigarettes/day, suggesting that greater cigarettes/day for a shorter duration was less deleterious than fewer cigarettes/day for a longer duration. Estimates of EOR/pack-year were homogeneous across sites, while the effects of cigarettes/day varied, indicating that the greater laryngeal cancer risk derived from differential cigarettes/day effects and not pack-years. EOR/drink-year estimates increased through 10 drinks/day, suggesting that greater drinks/day for a shorter duration was more deleterious than fewer drinks/day for a longer duration. Above 10 drinks/day, data were limited. EOR/drink-year estimates varied by site, while drinks/day effects were homogeneous, indicating that the greater pharyngeal/oral cavity cancer risk with alcohol consumption derived from the differential effects of drink-years and not drinks/day.
Keywords: alcohol drinking, risk model, smoking
Cigarette smoking and alcohol consumption increase risk of cancers of the larynx, pharynx, and oral cavity, although the magnitudes of these effects differ by site (1–3). A detailed, quantitative characterization of the source of the differential risks has not been conducted but may help to clarify disease etiology.
Studies have traditionally analyzed odds ratios by cigarettes/day and duration of smoking or by drinks/day and duration of drinking. However, the interpretation of odds ratios for exposure rate and duration is problematic, because odds ratios with an increasing exposure rate at a fixed duration or with an increasing duration at a fixed exposure rate embed the effects of increasing total exposure (4). We evaluate total exposure and exposure rate (pack-years and cigarettes/day or drink-years and drinks/day), which reformulates the analysis in terms of risk with total exposure and the modifying effects of the delivery rate, that is, a comparison of risk for total exposure delivered at higher exposure rates for shorter durations with risk for an equal total exposure delivered at lower exposure rates for longer durations.
Previous analyses of cigarette smoking found that, for exposure rates above 15 cigarettes/day, increasing cigarettes/day reduced the strength of the association between pack-years and disease risk for several smoking-related cancers, including cancers of the lung, bladder, larynx, pharynx, oral cavity, pancreas, liver, and esophagus (4–6). These results are consistent with both biologic processes related to the metabolism of carcinogens and DNA damage repair and to intensity-dependent inhalation, whereby heavier smokers inhale less vigorously, inducing a decline in the strength of the pack-years association. Those analyses included studies of head and neck cancers, but the numbers of cases were limited (5, 6). For alcohol consumption, our analysis represents the first joint evaluation of drink-years and drinks/day and, for both exposures, a formal evaluation of differential risk patterns by cancer site.
We analyze pooled data from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium of molecular epidemiologic studies of head and neck cancer (7). (Refer to http://inhance.iarc.fr/.)
MATERIALS AND METHODS
Study data
Data were derived from 15 case-control studies with detailed information on cigarette smoking and alcohol consumption (version 1.1, September 27, 2007) (7). Compared with version 1.0, version 1.1 omitted the Paris (8) and North Carolina (9) studies because of insufficiently detailed smoking or alcohol information and added studies from New York City (10) and Boston (11) (Table 1).
Table 1.
Numbers of Cases and Controls and Percentages of Smokers and Drinkersa
| Never and Current Cigarette-Only Smokers |
Never and Ever Consumers of Alcohol |
||||||||||||
| Study Location (Reference No.) | No. |
% |
No. |
% |
|||||||||
| Larynx | Pharynx | Oral Cavity | Controls | Neverb | ≥10 Cigarettes/Dayc | Larynx | Pharynx | Oral Cavity | Controls | Neverd | ≤5 Drinks/Daye | ≤10 Drinks/Daye | |
| Total | 1,761 | 2,453 | 1,990 | 10,114 | 59.6 | 83.1 | 2,551 | 3,693 | 3,116 | 15,589 | 27.0 | 79.8 | 93.7 |
| Europe | |||||||||||||
| Milan, Italy (41) | 184 | 51 | 41 | 1,194 | 48.0 | 82.9 | 237 | 61 | 43 | 1,484 | 20.0 | 74.6 | 98.7 |
| Aviano, Italy (42) | 104 | 174 | 65 | 579 | 47.8 | 81.5 | 145 | 215 | 83 | 844 | 6.8 | 45.4 | 80.4 |
| Italy (43) | 286 | 290 | 92 | 1,632 | 58.8 | 74.0 | 430 | 393 | 120 | 2,483 | 9.8 | 79.0 | 96.8 |
| Switzerland (44) | 101 | 201 | 121 | 683 | 60.9 | 93.6 | 123 | 231 | 132 | 846 | 50.1 | 93.1 | 98.6 |
| Central Europe (45) | 272 | 80 | 115 | 557 | 49.4 | 85.1 | 372 | 146 | 183 | 850 | 7.3 | 86.4 | 97.6 |
| North America | |||||||||||||
| New York City, NY (10) | 0 | 303 | 335 | 458 | 53.7 | 93.4 | 0 | 435 | 468 | 793 | 30.9 | 80.8 | 90.1 |
| Seattle, WA (46) | 0 | 115 | 132 | 309 | 62.1 | 88.9 | 0 | 163 | 210 | 598 | 7.5 | 94.4 | 98.0 |
| Iowa (47) | 55 | 96 | 157 | 431 | 65.4 | 92.6 | 91 | 160 | 252 | 751 | 44.3 | 88.0 | 96.9 |
| Tampa, FL (48) | 46 | 37 | 19 | 516 | 66.7 | 93.0 | 61 | 56 | 22 | 879 | 39.8 | 96.2 | 98.9 |
| Los Angeles, CA (49) | 58 | 87 | 35 | 605 | 76.4 | 67.8 | 86 | 167 | 52 | 1,004 | 24.9 | 92.4 | 97.5 |
| Houston, TX (49) | 88 | 227 | 156 | 500 | 75.4 | 85.4 | 153 | 424 | 234 | 862 | 45.8 | 96.4 | 99.6 |
| Boston, MA (11) | 57 | 131 | 80 | 280 | 69.6 | 87.1 | 111 | 291 | 137 | 659 | 10.5 | 88.3 | 95.4 |
| South/Central America | |||||||||||||
| Puerto Rico (50) | 0 | 116 | 51 | 273 | 71.8 | 87.0 | 0 | 192 | 87 | 487 | 22.8 | 67.6 | 87.5 |
| Latin America (22) | 510 | 340 | 278 | 986 | 48.8 | 82.0 | 742 | 440 | 363 | 1,517 | 32.0 | 64.7 | 77.3 |
| International (51) | 0 | 205 | 313 | 1,111 | 67.3 | 80.7 | 0 | 319 | 730 | 1,532 | 55.4 | 82.3 | 94.9 |
Data are from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium of case-control studies of head and neck cancer.
Percentage of never and current cigarette-only smoking control subjects (n = 10,114).
Percentage of current cigarette-only smoking control subjects (n = 4,091).
Percentage of never and ever drinking control subjects (n = 15,589).
Percentage of drinking control subjects (n = 11,375).
Study questionnaires differed but generally included information on the use of cigarettes, cigars, pipes, snuff, and chewing tobacco, although information on noncigarette products was sometimes limited. Although definitions varied by study, never smokers were those who never smoked regularly for more than a brief period of time (7).
Consumption of beer, wine, and liquor varied across studies (7, 12). The usual number of drinks/day was converted into total ethanol/day on the basis of the ethanol content in standard portion sizes in the different countries and then converted to standardized drinks/day on the basis of 15.6 mL of ethanol/drink.
Written informed consent was obtained from all study subjects, and studies were approved by appropriate institutional review boards.
We analyzed laryngeal, pharyngeal, and oral cavity cancers only, omitting cases with overlapping lesions (n = 277) or oral cavity-pharyngeal cancer not otherwise specified (n = 862). We excluded 58 subjects from the New York City (n = 55) and South America (n = 3) studies who reported ≥400 drinks/day.
Statistical models
We followed an approach described previously (4, 13). We first cross-classified total pack-years and cigarettes/day and computed joint odds ratios relative to never smokers. The odds ratios (ORs) increased approximately linearly with pack-years within each cigarettes/day category. For continuous pack-years d, we fitted a linear model within i = 1, …, I categories of cigarettes/day, OR(d) = 1 + γi d, where each slope parameter, γi, represented the excess odds ratio (EOR) per pack-year. The parameters γ1, …, γI characterized risks with pack-years and their variation across categories of cigarettes/day. A natural extension for continuous cigarettes/day, n, was the model,
| (1) |
where β was the EOR/pack-year at g(n) = 1, and g(.) described the variation of the EOR/pack-year with cigarettes/day, with βg(n) representing the slope of a linear relation between disease and pack-years (4). Several formulations for g(.) provided adequate fit; however, we selected g(n) = exp(ϕ1 ln(n) + ϕ2 ln(n)2) for consistency with previous analyses (4, 13).
Software for polytomous regression that incorporated model 1 was not available for estimating risks by cancer site. As an alternative approach, we appended 3 “studies” consisting of each case type (larynx, pharynx, and oral cavity) and all controls into one data set. This permitted testing the homogeneity of effects across “studies” by extending model 1,
| (2) |
where distinct βs parameters and gs(.) functions replaced β and g(.), and where ds equals d and ns equals n within stratum s and zero otherwise. The goal was testing whether differences in site-specific risk resulted from total exposure (different β’s) or exposure rate (different g(.) functions) or both. We compared this approach with a polytomous logistic model for a simple log-linear model for cigarettes/day and pack-years and found that statistical inferences were generally similar.
We used similar models for drink-years and drinks/day.
Models included multiplicative stratum parameters for sex, study population/center (39 levels), age (<40, 40–44, …, 70–74, ≥70 years), and main effects parameters for education (none, did not complete high school, high school graduate, technical school or some college, college graduate). For smoking, we analyzed only never and current cigarette-only smokers to remove complications from risks in former smokers and use of other tobacco products, and we stratified additionally on drink-years (never drinker and quartiles) and adjusted for drinks/day (never drinker and <1.0, 1.0–2.9, 3.0–4.9, ≥5.0). For alcohol consumption, we included all subjects because we could not identify former drinkers in all studies, and we stratified additionally on pack-years (never smoked and quartiles) and adjusted for cigarettes/day (never smoker, <20, 20–29, 30–39, ≥40) and use of other tobacco products.
We used likelihood ratio tests to evaluate nested models. The Epicure computer program was used for analysis (14).
RESULTS
Cigarette smoking
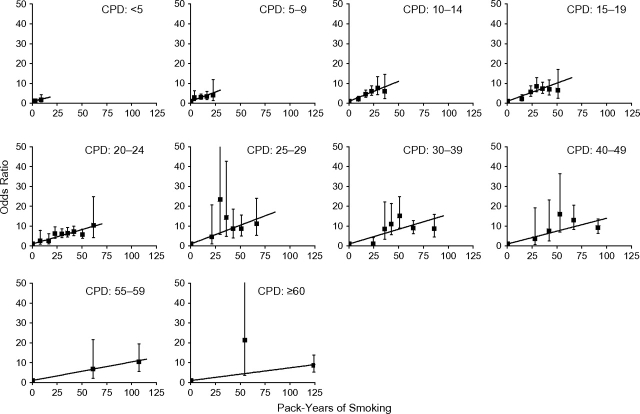
Among never and current cigarette-only smokers, there were 1,761 laryngeal, 2,453 pharyngeal, and 1,990 oral cavity cancer cases. Controls numbered 7,963 for laryngeal and 10,114 for pharyngeal cases and for oral cavity cases (Table 1). Not all studies enrolled laryngeal cancers resulting in fewer controls. We cross-classified pack-years and cigarettes/day, using 10 categories for each, combined adjacent pack-years categories when data were sparse, and computed 46 odds ratios relative to never smokers. For pharyngeal cancer, the odds ratios with 95% confidence intervals increased with pack-years within each cigarettes/day category (Figure 1). Tests of no departure from linearity did not reject for any category, while estimates of slope (γi) varied significantly (P < 0.01). Odds ratio patterns were similar for laryngeal and oral cavity cancers, with odds ratios for laryngeal cancer from 2- to 5-fold the odds ratios for pharyngeal and oral cavity cancers (not shown). For each site, estimates of slope (γi) varied significantly (P < 0.01). Among the 30 tests of no departure from linearity of odds ratios within the cigarettes/day category, tests were rejected for 2 categories of cigarettes/day (for the larynx, P = 0.03 for ≥60 cigarettes/day; for the oral cavity, P < 0.01 for 25.0–29.9 cigarettes/day).
Figure 1.
Odds ratios for pharyngeal cancer by categories of pack-years of cigarette smoking and number of cigarettes smoked per day (CPD), as well as fitted linear odds ratio models in pack-years. Bars, 95% confidence interval. Pooled data from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium were limited to never and current cigarette-only smokers.
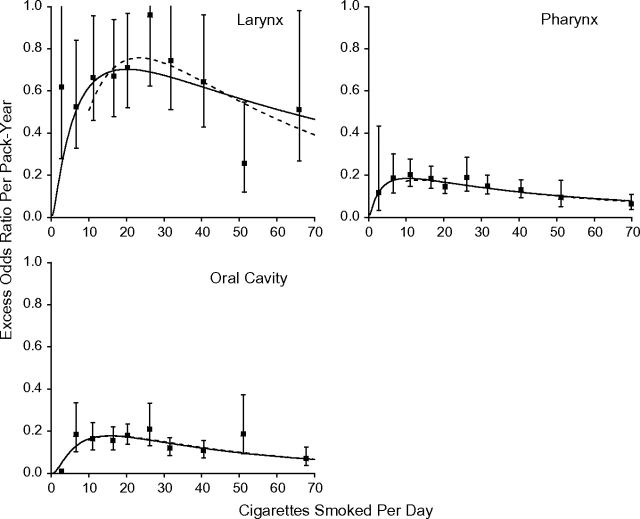
Figure 2 plots EOR/pack-year estimates and 95% confidence intervals by mean cigarettes/day (square symbol). For each site, model 1 (solid line) closely followed the estimates. Variations by cigarettes/day were significant (P = 0.04 for laryngeal and P < 0.01 for pharyngeal and oral cavity cancers when testing ϕ1 = 0 and ϕ2 = 0). At lower cigarettes/day, there was a direct delivery rate effect, whereby the strength of the association with pack-years increased with increasing cigarettes/day, indicating that for equal pack-years smoking more cigarettes/day for a shorter duration was more deleterious than fewer cigarettes/day for a longer duration. Above about 15 cigarettes/day, there was an inverse delivery rate pattern, whereby the strength of the association decreased with increasing cigarettes/day, indicating that for equal pack-years smoking more cigarettes/day for a shorter duration was less deleterious than fewer cigarettes/day for a longer duration. Among less than 10 cigarettes/day smokers, the range for pack-years was limited, increasing uncertainty in estimating effects. The interquartile range was 4.5–13.1 pack-years, compared with an interquartile range of 22.5–54.0 pack-years among all smokers. We fitted model 1 to never and ≥10 cigarettes/day smokers and again observed a good fit (dash line).
Figure 2.
Estimated excess odds ratios per pack-year for cancers of the larynx, pharynx, and oral cavity within categories of cigarettes/day (square symbol) with 95% confidence intervals. Model 1 was fitted to all data (solid line) and to never and ≥10 cigarettes-per-day smokers (dashed line). Pooled data from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium were limited to never and current cigarette-only smokers.
We used model 2 to evaluate homogeneity of risks across sites by comparing deviances when β replaced 3 βs parameters and/or ϕ1 and ϕ2 replaced 6 intensity parameters ϕ1, s and ϕ2, s. For all data and for never and ≥10 cigarettes/day smokers, risks differed significantly by site (P < 0.01) (Table 2). In the restricted data, the fit did not degrade after replacing the βs parameters with β while adjusting for site by cigarettes/day (P = 0.71) or after replacing the ϕ1, s and ϕ2, s parameters with ϕ1 and ϕ2 while adjusting for site by pack-years (P = 0.17). However, changes in deviance (0.7 vs. 6.4, respectively) suggested that differences by site resulted from differential cigarettes/day effects, while pack-year effects (βs) were homogeneous.
Table 2.
Parameter Estimates by Cancer Site From Fitting Modelsa to Never Smokers and Current Cigarette-Only Smokersb
| Data/Site | Cases, no. | Controls, no. | βs | ϕ1, s | ϕ2, s | Effect Modification of Smoking by Cancer Site |
||
| Site byc | Deviance (No.)d | P Valuee | ||||||
| All data | ||||||||
| Larynx | 1,761 | 7,963 | 0.0642 | 1.594 | −0.265 | Both | (9) | |
| Pharynx | 2,453 | 10,114 | 0.0496 | 1.120 | −0.238 | Cigarettes/day | 2.1 (7) | 0.35 |
| Oral | 1,990 | 10,114 | 0.0067 | 2.392 | −0.436 | Pack-years | 6.7 (5) | 0.17 |
| None | 104.9 (3) | <0.01 | ||||||
| Restricted dataf | ||||||||
| Larynx | 1,662 | 7,378 | 0.0032 | 3.469 | −0.550 | Both | (9) | |
| Pharynx | 2,323 | 9,422 | 0.0233 | 1.573 | −0.306 | Cigarettes/day | 0.7 (7) | 0.71 |
| Oral | 1,894 | 9,422 | 0.0045 | 2.623 | −0.468 | Pack-years | 6.4 (5) | 0.17 |
| None | 103.7 (3) | <0.01 | ||||||
Odds ratio = 1 + Σs βs d gs (n), where gs (n) = exp(ϕ1, s ln(n) + ϕ2, s ln(n)2); d is total pack-years of exposure; n is the number of cigarettes smoked per day; β, ϕ1, and ϕ2 are unknown parameters; and subscript “s” denotes separate parameters for site-specific cancers (refer to text). Models are adjusted for study/center, age, sex, education, drink-years, drinks/day, and indicators of cancer site and its controls.
Data are from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium of case-control studies of head and neck cancer.
Denotes effect modification of smoking by cancer site, including the following: “both,” the modification of both pack-years and cigarettes/day by site, βs d gs (n); “cigarettes/day,” modification of cigarettes smoked per day by site, β d gs (n); “pack-years,” modification of pack-years by site, βs d g(n); and “none,” no effect modification, β d g(n).
Change in deviance relative to model with pack-years and cigarettes/day variation by site. The number of smoking-related parameters is shown in the parentheses.
P value for the test of model fit relative to the full interaction model.
Never smokers and current cigarette-only smokers consuming ≥10 cigarettes/day.
Comparing only pharyngeal and oral cavity cases and controls, we found that smoking-related risk patterns were homogeneous (P = 0.92). Comparing the laryngeal “study” with the combined “study” of pharyngeal/oral cavity cases using one control group, we found no degradation in model fit after β replaced 2 βs parameters (P = 0.43) but a nearly significant change in fit after ϕ1 and ϕ2 replaced 4 ϕ1, s and ϕ2, s parameters (P = 0.08). These post hoc comparisons supported effect modification by cigarettes/day as the primary determinant of site-specific differences.
The patterns in Figure 2 were generally consistent for the individual studies. For ≥10 cigarettes/day, the EOR/pack-year estimates decreased with increasing cigarettes/day in each study except the Central Europe and Switzerland studies, where there was no consistent variation with cigarettes/day (not shown).
Alcohol consumption
For alcohol consumption, we analyzed 2,551 laryngeal, 3,693 pharyngeal, and 3,116 oral cavity cancer cases. Controls numbered 12,179 for laryngeal cancer and 15,589 for pharyngeal cancer and oral cavity cancer. The drinks/day distributions were skewed (Table 1). Therefore, we analyzed all subjects and 2 subgroups, never drinkers and 10 drinks/day or less drinkers (95.4% of controls) and never drinkers and 5 drinks/day or less (85.3% of controls), to limit the influence of high drinks/day values.
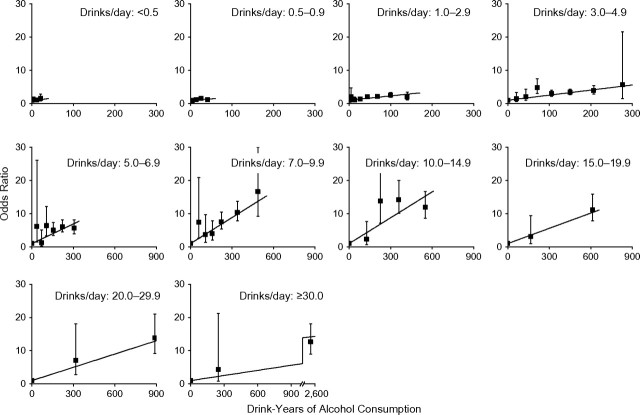
We computed odds ratios relative to never drinkers for a cross-classification of drink-years and drinks/day. For pharyngeal cancer, the odds ratios generally increased with drink-years within the drinks/day category (Figure 3). Results were similar for laryngeal and oral cavity cases (not shown). Trends varied across drinks/day categories (P < 0.001). Among the 30 tests of no departures from linearity of odds ratios within the drinks/day category, 4 tests rejected linearity (for pharyngeal cancer, P < 0.01 for 5.0–6.9 drinks/day and P = 0.01 for 20.0–29.9 drinks/day; for oral cavity cancer, P = 0.01 for 1.0–2.9 drinks/day and for ≥30 drinks/day). Odds ratios for pharyngeal and oral cavity cancer were similar and nearly 2 times the odds ratios for laryngeal cancer.
Figure 3.
Odds ratios for pharyngeal cancer by categories of drink-years and number of drinks/day, as well as fitted linear odds ratio models in drink-years (drinks/day × years of consumption). Bars, 95% confidence interval. Pooled data from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium were for all pharyngeal cancer cases and controls.
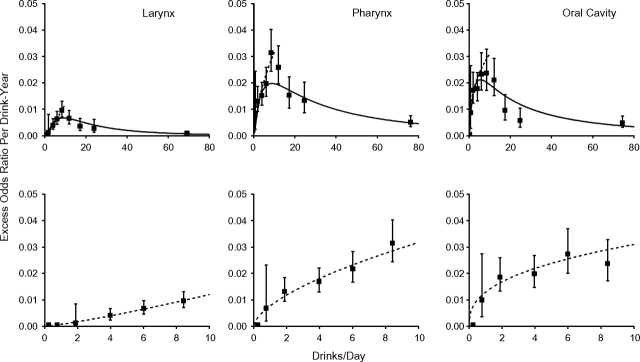
Figure 4 shows estimates of EOR/drink-year by mean drinks/day (square symbol) for all data (upper panels) and for 10 drinks/day or less (lower panels). EOR/drink-year estimates increased through 10–15 drinks/day, before decreasing. We fitted model 1 to all data with linear and quadratic terms in ln(drinks/day) (solid line) and to the restricted data with a linear term in ln(drinks/day) (dashed line), because an additional quadratic term did not improve fit. Parameter estimates are given in Table 3. For 10 drinks/day or less, there was a direct delivery rate effect, whereby the strength of the disease association with total drink-years increased with increasing drinks/day, indicating that, for equal drink-years, greater drinks/day for a shorter duration was more deleterious than fewer drinks/day for a longer duration. Above 10 drinks/day, high drinks/day values influenced model fit.
Figure 4.
Estimated excess odds ratios per drink-year for cancers of the larynx, pharynx, and oral cavity within categories of drinks/day (square symbol) with 95% confidence intervals. Model 1 was fitted to all data (solid line) (upper panels) and to subjects consuming ≤10 drinks/day (dashed line). Pooled data from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium were for all pharyngeal cancer cases and controls.
Table 3.
| Data/Site | Cases, no. | Controls, no. | βs | ϕ1, s | ϕ2, s | Effect Modification by Cancer Site |
||
| Site byc | Deviance (No.)d | P Valuee | ||||||
| All data | ||||||||
| Larynx | 2,551 | 12,179 | 0.00046 | 2.416 | −0.546 | Both | – (9) | |
| Pharynx | 3,693 | 15,589 | 0.00478 | 1.307 | −0.301 | Drinks/day | 25.2 (7) | <0.01 |
| Oral | 3,116 | 15,589 | 0.01009 | 0.870 | −0.255 | Drink-years | 14.7 (5) | 0.01 |
| None | 86.9 (3) | <0.01 | ||||||
| Never and ≤10-drinks/day drinkers | ||||||||
| Larynx | 2,159 | 11,612 | 0.00075 | 1.205 | –f | Both | – (6) | |
| Pharynx | 2,957 | 14,874 | 0.00776 | 0.611 | –f | Drinks/day | 33.8 (4) | <0.01 |
| Oral | 2,656 | 14,874 | 0.01299 | 0.377 | –f | Drink-years | 11.0 (4) | <0.01 |
| None | 74.8 (2) | <0.01 | ||||||
| Never and ≤5-drinks/day drinkers | ||||||||
| Larynx | 1,559 | 10,265 | 0.00076 | 1.185 | –f | Both | – (6) | |
| Pharynx | 2,200 | 13,296 | 0.00803 | 0.597 | –f | Drinks/day | 16.0 (4) | <0.01 |
| Oral | 2,191 | 13,296 | 0.01447 | 0.330 | –f | Drink-years | 2.2 (4) | 0.33 |
| None | 58.6 (2) | <0.01 | ||||||
Odds ratio = 1 + βs d gs (c), where gs (c) = exp(ϕ1, s ln(c) + ϕ2, s ln(c)2); d is total drink-years of exposure; c is the number of drinks/day; β, ϕ1, and ϕ2 are unknown parameters; and subscript “s” denotes separate parameters for site-specific cancers (refer to text). Models were adjusted for study/center, age, sex, education, pack-years, cigarettes/day, and indicators of cancer site and its controls.
Data are from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium of case-control studies of head and neck cancer.
Denotes effect modification of drinking by cancer site, including “both,” modification of both drink-years and drinks/day by site, βs d gs (c); “drinks/day,” the modification of drinks/day by site, β d gs (c); “drink-years,” the modification of drink-years by site, βs d g(c); and “none,” no effect modification, β d g(c).
Change in deviance relative to model with drink-years and drinks/day variation by site. The number of alcohol-related parameters is shown in parentheses.
P value for the test of model fit relative to the full interaction model.
–, parameter ϕ2 was not statistically significant and was omitted from the model.
We used the appended data set of 3 “studies” to evaluate differences by cancer site. Among subjects consuming 10 drinks/day or less, model 2 degraded significantly after β replaced the 3 βs parameters with adjustment for the interaction of site by drinks/day (P < 0.01) and after ϕ1 replaced the 3 ϕs, 1 parameters with adjustment for site by pack-years (P < 0.01) (Table 3), although the deviance change was much smaller for the latter (33.8 vs. 11.0). For 5 drinks/day or less, model fit degraded significantly when β replaced βs (P < 0.01) but did not degrade significantly when ϕ1 replaced ϕ1, s (P = 0.33), suggesting that site-specific differences in risk derived from variation in risk with drink-years, while the effects of drinks/day were homogeneous.
Comparing pharyngeal and oral cavity cases and controls only, the effects of drink-years and of drinks/day were homogenous across the 2 sites (P = 0.21).
For individual studies, the risk patterns for drink-years and drinks/day exhibited greater variability than those for smoking. However, for never and 5 drinks/day or less drinkers, we found an increasing drinks/day effect for all studies, except the Houston, New York, and Latin American studies.
DISCUSSION
We observed distinct delivery rate patterns for the risk of head and neck cancer by cigarette smoking and by alcohol consumption, and we confirmed prior study results showing that smoking was more strongly associated with laryngeal cancer and that alcohol consumption was more strongly associated with pharyngeal and oral cavity cancers (1–3). Results suggested that the greater laryngeal cancer risk with smoking derived from the differential effects of cigarettes/day and not pack-years, while the greater pharyngeal and oral cavity cancer risk with alcohol consumption derived from the differential effects of total drink-years and not drinks/day.
Our analysis is the first to characterize the alcohol consumption rate controlling for total alcohol exposure. For subjects consuming 10 drinks/day or less, which included 95% of controls, the strength of the disease association with total exposure (drink-years) increased with the increasing exposure rate (drinks/day), suggesting that alcohol-related causal mechanisms are not exposure-rate limited at or below 10 drinks/day. Above 10 drinks/day, the strength of the association between drink-years and head and neck cancers decreased with increasing drinks/day; however, interpretation was problematic because of the relatively few drinkers of more than 10 drinks/day and few studies contributing information, resulting in increased heterogeneity among studies.
Ethanol may act as a carcinogenic initiator or as a promoter that enhances permeability of cells to other environmental carcinogens, notably tobacco smoke (1, 2, 15, 16). Ethanol is oxidized to acetaldehyde primarily though the enzymatic activity of alcohol dehydrogenase and, to a lesser extent, cytochrome P450 enzymes, including CYP2E1, especially in chronic drinkers. Acetaldehyde is metabolized by aldehyde dehydrogenase to acetate (17). The metabolism of ethanol by alcohol dehydrogenase and aldehyde dehydrogenase occurs primarily in the liver and, to a lesser extent, the stomach (15, 17). Acetaldehyde is classified as a possible human carcinogen (group 2B) (18). The effects of acetaldehyde may thus explain the increased cancer risk with alcohol consumption, which has been observed for oral cavity, oropharyngeal, laryngeal, esophagus, liver, colon/rectum, and breast cancers (15). However, the increased risk for oral cavity and pharyngeal cancers compared with laryngeal cancer, which was observed in our analysis and noted by others (3, 7, 15, 19–21), suggests that site-specific factors in addition to acetaldehyde must also play a role. These factors may include increased production of acetaldehyde from oral bacterial flora concomitant with increased alcohol intake or poor dentition that may act directly through the release of proinflammatory cytokines (22) or may enhance the effects of bacterial flora (23, 24).
Unlike the smoking analysis of never and current smokers, the drinking analysis did not limit data by drinking status, because information on drinking status was not available for 4 studies (Milan, Aviano, Central Europe, and New York studies). If we omitted these 4 studies and restricted analyses to never and current drinkers, patterns of EOR/drink-year and drinks/day were similar, while the inference in Table 3 was clearer. For example, model fit degraded significantly when β replaced βs (P = 0.01 for ≤10 drinks/day and P = 0.06 for ≤5 drinks/day), but it did not degrade significantly when ϕ1 replaced ϕ1, s (P = 0.37 for ≤10 drinks/day and P = 0.53 for ≤5 drinks/day). These results indicated that site-specific differences in risk derived from variation in risk with drink-years, while drinks/day effects were homogeneous, suggesting the same relative impact of drinks/day for laryngeal, pharyngeal, and oral cavity cancers.
Alcohol-dependent recall bias, with heavier drinkers underestimating drinks/day, may have occurred (25). Such bias could induce overestimation of the association between disease and drink-years that increased with drinks/day. Analyses have reported correlations of 0.6–0.7 for prospectively and retrospectively collected estimates of alcohol intake and for absolute differences in consumption of 1 g of ethanol per day or less or less than one-tenth of a can of beer or glass of wine (26–28). In our data, the EOR/drink-year increased smoothly through 10 drinks/day, with a 1.3-fold (oral cavity), 1.5-fold (pharynx), and 2.3-fold (larynx) increase in the fitted EOR/drink-year at 10 drinks/day relative to 5 drinks/day (Table 3). This suggests that recall bias would be insufficient to produce the observed magnitude of effects for increasing drinks/day.
For cigarette smoking, there was an inverse delivery rate or “reduced potency” effect above about 15 cigarettes/day, whereby for equal pack-years smoking more cigarettes/day for a shorter duration was less deleterious than smoking fewer cigarettes/day for a longer duration. This pattern has been observed for a variety of smoking-related cancers, including cancers of the esophagus, lung, kidney, bladder, pancreas, and liver (4–6), and it suggests a broader smoking-related phenomenon.
The inverse exposure rate pattern for cigarettes/day likely reflects both biologic effects and intensity-dependent inhalation characteristics. The inverse exposure rate pattern is consistent with various biologic processes linked to carcinogens in cigarette smoke, including increased DNA repair (29–32), saturation of activation pathways (33–35), and increased induction of detoxification enzymes (36). The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent carcinogen, which can be characterized by its urinary metabolites. In data from 4 clinical studies, the ratio of NNK metabolites to urinary cotinine declined with increasing cotinine, suggesting reduced NNK uptake per unit of cotinine with increasing cotinine (37), a pattern consistent with inverse exposure rate effects. The inverse exposure rate pattern may also reflect heavier smokers inhaling less vigorously, leading to lower carcinogenic exposure per cigarette, as suggested by a study of 190 smokers which reported increased cotinine and nicotine levels with increased intensity and a marginally significant (P = 0.08) decline in “nicotine boost,” that is, an increase in blood plasma nicotine per cigarette (38). Nonetheless, evidence suggests that inhalation characteristics do not fully explain the inverse exposure rate pattern. In a lung cancer case-control study, inhalation was unrelated to cigarettes/day within pack-year categories, suggesting that inhalation does not confound pack-years-adjusted cigarettes/day patterns (4), while a sensitivity analysis, based on the relation between urinary cotinine and cigarettes/day, indicated that inhalation characteristics do not fully account for the exposure rate pattern (39). Finally, although inhalation characteristics may have contributed to an inverse exposure rate pattern for lung cancer, we would expect depth and frequency of inhalation to have a lesser impact on modifying the delivered dose for risk of head and neck cancers. However, a literature search failed to find any analyses of head and neck cancer risk in relation to cigarette inhalation patterns.
Consistent with several studies, this study found that smoking-related risks were higher for cancer of the larynx compared with cancer of the pharynx and oral cavity (1, 2, 7, 19, 20). Our modeling suggested that differences in risk resulted from the differential effects of cigarettes/day, while pack-year effects were homogeneous, indicative of a heightened responsiveness of the larynx to changes in cigarettes/day. However, this may be a chance finding because the variation was not statistically significant (P = 0.17).
The risk patterns for smoking were generally consistent across individual studies, which agrees with other smoking-related analyses (5, 6). Results for drink-years and drinks/day, while broadly consistent, exhibited greater heterogeneity among studies, particularly at higher drinks/day. This agrees with a meta-analysis that reported greater heterogeneity in alcohol-related risks compared with smoking risks (20). Increased heterogeneity may be due to population differences in the amount, type (beer, wine, liquor, and so on), and formulation (straight or mixed drink for liquor) of the alcohol products consumed. Reports have linked ethanol concentration to increased risk of head and neck cancer (3, 21, 40), although an analysis of data from the International Head and Neck Cancer Epidemiology Consortium did not find marked differences in risk by type of drink (12).
In summary, we observed an inverse exposure rate effect for cigarette smoking above 15 cigarettes/day, whereby the strength of the association between head and neck cancer and pack-years decreased with cigarettes/day, and a direct exposure rate effect for drinks/day ≤10 drinks/day, whereby the strength of the association between head and neck cancer and total drink-years increased with drinks/day. Smoking risks were greater for the larynx than for the pharynx and oral cavity, while alcohol risks were greater for the pharynx and oral cavity. We found suggestive evidence that greater smoking-related risk of laryngeal cancer was derived primarily from the differential effects of cigarettes/day, while the effect of pack-years was similar by site, and that the greater alcohol-related risk for pharyngeal and oral cavity cancers was derived from a greater effect of total drink-years, while the modification of drink-years–related risk by drinks/day was similar for each site.
Acknowledgments
Author affiliations: National Cancer Institute, Bethesda, Maryland (Jay H. Lubin, Mark Purdue, Debbie Winn, Richard B. Hayes); Center for Environmental Health and Technology, Brown University, Providence, Rhode Island (Karl T. Kelsey); School of Public Health, University of California, Los Angeles, California (Zuo-Feng Zhang); the University of Texas–M. D. Anderson Cancer Center, Houston, Texas (Qingyi Wei, Erich M. Sturgis); Aviano Cancer Centre, Aviano, Italy (Renato Talamini); Institute of Occupational Medicine, Lodz, Poland (Neonilia Szeszenia-Dabrowska); College of Public Health, University of Iowa, Iowa City, Iowa (Elaine Smith); Cancer Research Centre, Moscow, Russia (Oxana Shangina); Fred Hutchinson Cancer Research Center, Seattle, Washington (Stephen M. Schwartz, Chu Chen); National Institute of Environmental Health, Budapest, Hungary (Peter Rudnai); Universidade de Sao Paulo, Sao Paulo, Brazil (Jose Eluf Neto, Victor Wünsch-Filho); College of Medicine, The Pennsylvania State University, Hershey, Pennsylvania (Joshua Muscat, Philip Lazarus); Departments of Epidemiology and Environmental Health Sciences, School of Public Health, and Comprehensive Cancer Center, University of Michigan, Ann Arbor, Michigan (Hal Morgenstern); Universidade Federal de Pelotas, Pelotas, Brazil (Ana Menezes); Institute of Oncology Angel H. Roffo, University of Buenos Aires, Buenos Aires, Argentina (Elena Matos); University of Medicine and Pharmacy “Carol Davila,” Bucharest, Romania (Ioan Nicolae Mates); the M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Department of Cancer Epidemiology and Prevention, Warsaw, Poland (Jolanta Lissowska); Institut de médecine sociale et preventive, Université de Lausanne, Lausanne, Switzerland (Fabio Levi); Istituto di Ricerche Farmacologiche Mario Negri and University of Milan, Milan, Italy (Carlo La Vecchia); Escola Nacional de Saude Publica, Fundaçao Oswaldo Cruz, Rio de Janeiro, Brazil (Sergio Koifman); Instituto de Investigación Epidemiológica, San José, Costa Rica (Rolando Herrero); International Agency for Research on Cancer, Lyon, France (Silvia Franceschi, Maria Paula Curado, Paul Brennan, Paolo Boffetta, Mia Hashibe); Institute of Oncology and Radiobiology, Havana, Cuba (Leticia Fernandez); Specialized State Health Institute, Banská Bystrica, Slovakia (Eleonora Fabianova); Hospital de Clinicas de Porto Alegre, Porto Alegre, Brazil (Alexander W. Daudt); Aviano Cancer Centre, Aviano, Italy (Luigino Dal Maso); and Institut Català d'Oncologia (ICO), IDIBELL, CIBER-ESP, L'Hospitalet de Llobregat, Barcelona, Spain (Xavier Castellsague).
This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute, Department of Health and Human Services.
Conflict of interest: none declared.
Glossary
Abbreviations
- EOR
excess odds ratio
- NNK
nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- OR
odds ratio
References
- 1.Mayne AT, Morse DE, Winn DM. Cancers of the oral cavity and pharynx. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press, Inc; 2006. pp. 674–696. [Google Scholar]
- 2.Olshan AF. Cancer of the larynx. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press, Inc; 2006. pp. 627–637. [Google Scholar]
- 3.Bagnardi V, Blangiardo M, La Vecchia C, et al. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85(11):1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15(3):517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 5.Lubin JH, Alavanja MC, Caporaso N, et al. Cigarette smoking and cancer: modeling total exposure and intensity. Am J Epidemiol. 2007;166(4):479–489. doi: 10.1093/aje/kwm089. [DOI] [PubMed] [Google Scholar]
- 6.Lubin JH, Virtamo J, Weinstein SJ, et al. Cigarette smoking and cancer: intensity patterns in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finnish men. Am J Epidemiol. 2008;167(8):970–975. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
- 7.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 8.Benhamou S, Tuimala J, Bouchardy C, et al. DNA repair gene XRCC2 and XRCC3 polymorphisms and susceptibility to cancers of the upper aerodigestive tract. Int J Cancer. 2004;112(5):901–904. doi: 10.1002/ijc.20474. [DOI] [PubMed] [Google Scholar]
- 9.Olshan AF, Weissler MC, Watson MA, et al. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(2):185–191. [PubMed] [Google Scholar]
- 10.Muscat JE, Richie JP, Jr, Thompson S, et al. Gender differences in smoking and risk for oral cancer. Cancer Res. 1996;56(22):5192–5197. [PubMed] [Google Scholar]
- 11.Peters ES, McClean MD, Marsit CJ, et al. Glutathione S-transferase polymorphisms and the synergy of alcohol and tobacco in oral, pharyngeal, and laryngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2196–2202. doi: 10.1158/1055-9965.EPI-06-0503. [DOI] [PubMed] [Google Scholar]
- 12.Purdue MP, Hashibe M, Berthiller J, et al. Type of alcoholic beverage and risk of head and neck cancer—a pooled analysis within the INHANCE Consortium. Am J Epidemiol. 2009;169(2):132–142. doi: 10.1093/aje/kwn306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubin JH, Kogevinas M, Silverman D, et al. Evidence for an intensity dependent interaction of NAT2 acetylation genotype and cigarette smoking in the Spanish Bladder Cancer Study. Int J Epidemiol. 2007;36(1):236–241. doi: 10.1093/ije/dym043. [DOI] [PubMed] [Google Scholar]
- 14.Preston DL, Lubin JH, Pierce DA, et al. Epicure User's Guide. Seattle, WA: HiroSoft International Corporation; 2006. [Google Scholar]
- 15.Marshall JR, Freudenheim J. Alcohol. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press, Inc; 2006. pp. 243–258. [Google Scholar]
- 16.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30(1):38–47. [PMC free article] [PubMed] [Google Scholar]
- 17.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29(4):245–254. [PMC free article] [PubMed] [Google Scholar]
- 18.International Agency for Research on Cancer. Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Lyon, France: IARC Press; 1999. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol 71. [PMC free article] [PubMed] [Google Scholar]
- 19.Hashibe M, McKay JD, Curado MP, et al. Multiple ADH genes are associated with upper aerodigestive cancers. Nat Genet. 2008;40(6):707–709. doi: 10.1038/ng.151. [DOI] [PubMed] [Google Scholar]
- 20.Zeka A, Gore R, Kriebel D. Effects of alcohol and tobacco on aerodigestive cancer risks: a meta-regression analysis. Cancer Causes Control. 2003;14(9):897–906. doi: 10.1023/b:caco.0000003854.34221.a8. [DOI] [PubMed] [Google Scholar]
- 21.Corrao G, Bagnardi V, Zambon A, et al. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38(5):613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Guha N, Boffetta P, Wünsch Filho V, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166(10):1159–1173. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 23.Pöschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39(3):155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 24.Homann N, Tillonen J, Meurman JH, et al. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21(4):663–668. doi: 10.1093/carcin/21.4.663. [DOI] [PubMed] [Google Scholar]
- 25.Poikolainen K. Underestimation of recalled alcohol intake in relation to actual consumption. Br J Addict. 1985;80(2):215–216. doi: 10.1111/j.1360-0443.1985.tb03276.x. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Stampfer MJ, Colditz GA, et al. Recall and selection bias in reporting past alcohol-consumption among breast-cancer cases. Cancer Causes Control. 1993;4(5):441–448. doi: 10.1007/BF00050863. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Serdula MK, Byers T, et al. Reliability of alcohol intake as recalled from 10 years in the past. Am J Epidemiol. 1996;143(2):177–186. doi: 10.1093/oxfordjournals.aje.a008727. [DOI] [PubMed] [Google Scholar]
- 28.Stockwell T, Donath S, Cooper-Stanbury M, et al. Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction. 2004;99(8):1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 29.Wei QY, Cheng L, Amos CI, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 30.Spitz MR, Wei QY, Dong Q, et al. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12(8):689–698. [PubMed] [Google Scholar]
- 31.Shen H, Spitz MR, Qiao YW, et al. Smoking, DNA repair capacity and risk of nonsmall cell lung cancer. Int J Cancer. 2003;107(1):84–88. doi: 10.1002/ijc.11346. [DOI] [PubMed] [Google Scholar]
- 32.Hung RJ, Brennan P, Canzian F, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer Inst. 2005;97(8):567–576. doi: 10.1093/jnci/dji101. [DOI] [PubMed] [Google Scholar]
- 33.Lewtas J, Walsh D, Williams R, et al. Air pollution exposure DNA adduct dosimetry in humans and rodents: evidence for non-linearity at high doses. Mutat Res. 1997;378(1-2):51–63. doi: 10.1016/s0027-5107(97)00097-3. [DOI] [PubMed] [Google Scholar]
- 34.Lutz WK. Dose-response relationships in chemical carcinogenesis: superposition of different mechanisms of action, resulting in linear-nonlinear curves, practical thresholds, J-shapes. Mutat Res. 1998;405(2):117–124. doi: 10.1016/s0027-5107(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 35.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23(12):1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 36.Gu J, Liang D, Wang Y, et al. Effects of N-acetyl transferase 1 and 2 polymorphisms on bladder cancer risk in Caucasians. Mutat Res. 2005;581(1-2):97–104. doi: 10.1016/j.mrgentox.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Lubin JH, Caporaso N, Hatsukami DK, et al. The association of a tobacco-specific biomarker and cigarette consumption and its dependence on host characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1852–1857. doi: 10.1158/1055-9965.EPI-07-0018. [DOI] [PubMed] [Google Scholar]
- 38.Patterson F, Benowitz N, Shields P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12(5):468–471. [PubMed] [Google Scholar]
- 39.Lubin JH, Caporaso N, Wichmann HE, et al. Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiology. 2007;18(5):639–648. doi: 10.1097/EDE.0b013e31812717fe. [DOI] [PubMed] [Google Scholar]
- 40.Polesel J, Dal Maso L, Bagnardi V, et al. Estimating dose-response relationship between ethanol and risk of cancer using regression spline models. Int J Cancer. 2005;114(5):836–841. doi: 10.1002/ijc.20756. [DOI] [PubMed] [Google Scholar]
- 41.Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50(20):6502–6507. [PubMed] [Google Scholar]
- 42.Barón AE, Franceschi S, Barra S, et al. A comparison of the joint effects of alcohol and smoking on the risk of cancer across sites in the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2(6):519–523. [PubMed] [Google Scholar]
- 43.Bosetti C, Gallus S, Trichopoulou A, et al. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003;12(10):1091–1094. [PubMed] [Google Scholar]
- 44.Levi F, Pasche C, La Vecchia C, et al. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77(5):705–709. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 45.Hashibe M, Boffetta P, Zaridze D, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2006;15(4):696–703. doi: 10.1158/1055-9965.EPI-05-0710. [DOI] [PubMed] [Google Scholar]
- 46.Rosenblatt KA, Daling JR, Chen C, et al. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64(11):4049–4054. doi: 10.1158/0008-5472.CAN-03-3425. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Ritchie JM, Smith EM, et al. Alcohol dehydrogenase 3 and risk of squamous cell carcinomas of the head and neck. Cancer Epidemiol Biomarkers Prev. 2005;14(3):626–632. doi: 10.1158/1055-9965.EPI-04-0343. [DOI] [PubMed] [Google Scholar]
- 48.Elahi A, Zheng Z, Park J, et al. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002;23(7):1229–1234. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- 49.Cui Y, Morgenstern H, Greenland S, et al. Polymorphism of xeroderma pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer. 2006;118(3):714–720. doi: 10.1002/ijc.21413. [DOI] [PubMed] [Google Scholar]
- 50.Hayes RB, Bravo-Otero E, Kleinman DV, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10(1):27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 51.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]