Abstract
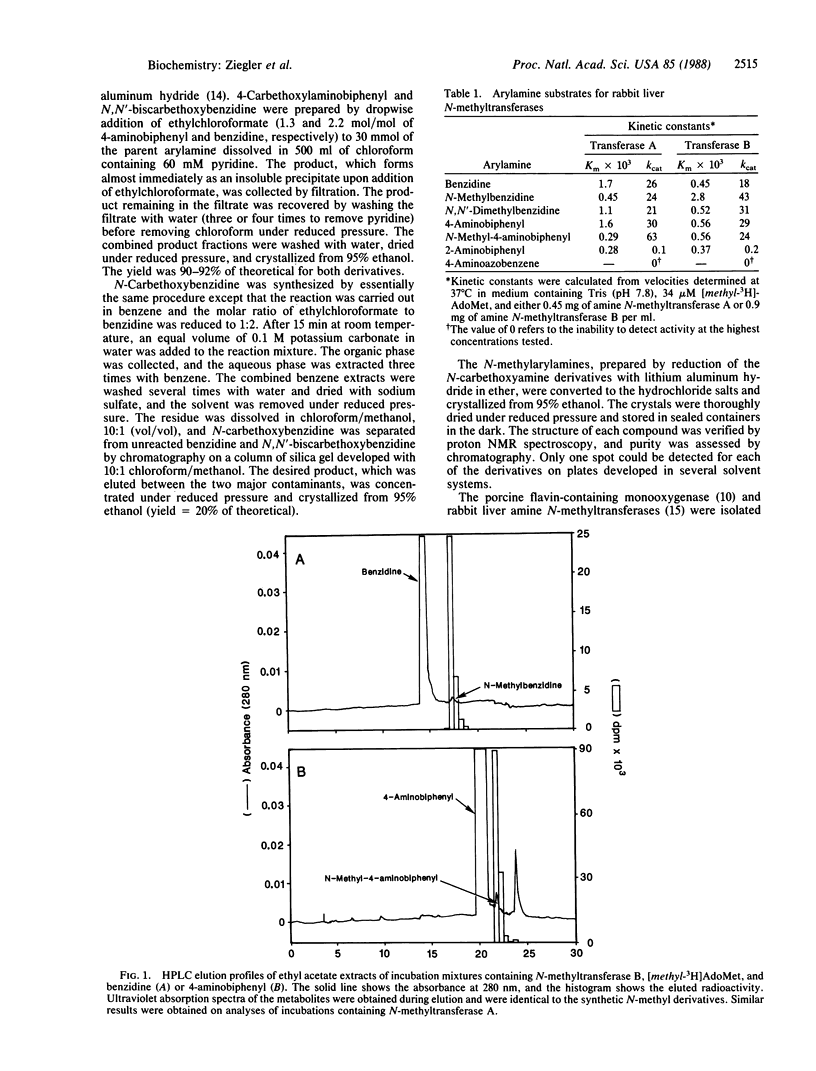
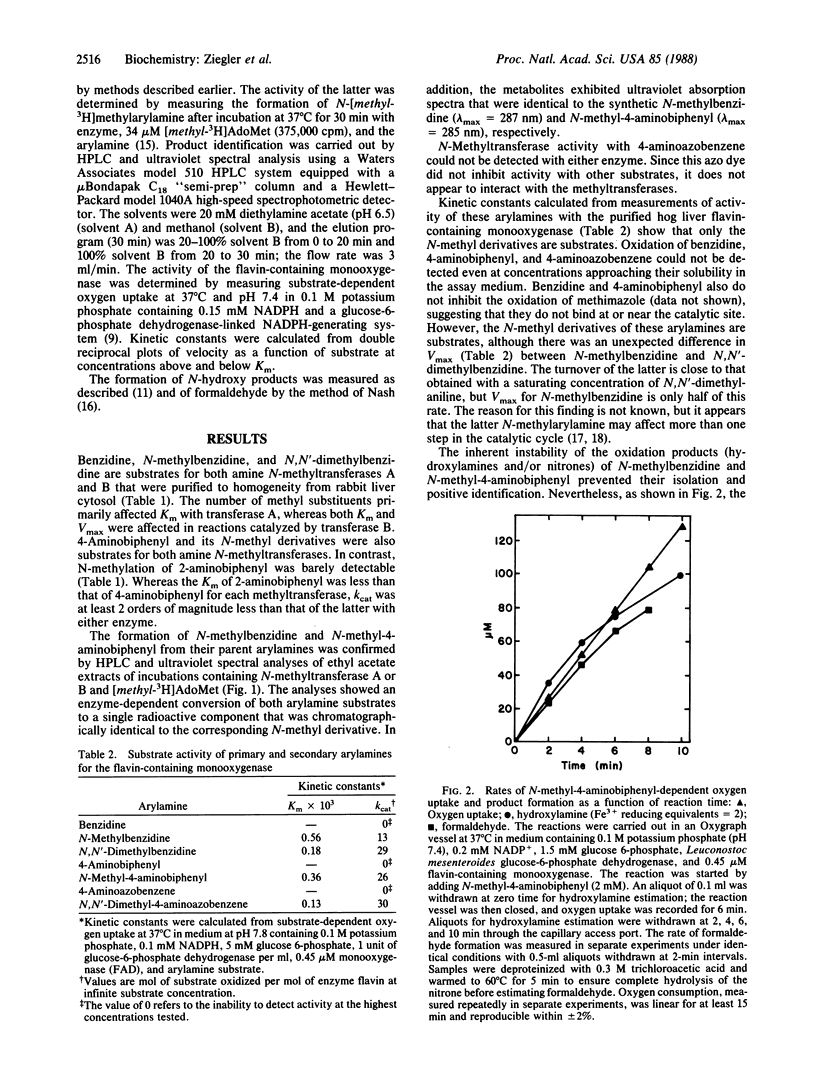
Two amine N-methyltransferases isolated from rabbit liver catalyze S-adenosylmethionine-dependent N-methylation of benzidine and 4-aminobiphenyl but not of 4-aminoazobenzene or 2-aminobiphenyl. The enzymatic reaction products were analyzed and found to be identical to synthetic N-methylbenzidine and N-methyl-4-aminobiphenyl. N-Methylation may be a critical step in the metabolic activation of primary arylamines because N-methylarylamines, unlike primary arylamines, are readily N-oxygenated by the NADPH- and oxygen-dependent microsomal flavin-containing monooxygenase. Kinetic studies carried out with the purified porcine liver monooxygenase demonstrate that, while activity with primary arylamines could not be detected, N-methyl derivatives of benzidine, 4-aminoazobenzene, and 4-aminobiphenyl are substrates. Products formed from N-methyl-4-aminobiphenyl had the properties of the hydroxylamine and/or nitrone in that the enzyme- and time-dependent incubation product(s) reduced Fe3+ to Fe2+, and formaldehyde was formed during the course of the reaction. These data suggest that N-methyl-4-aminobiphenyl is oxidized to N-hydroxy-N-methyl-4-aminobiphenyl, which can undergo further oxidation to a nitrone that hydrolyzes to formaldehyde and N-hydroxy-4-aminobiphenyl.
Full text
PDF