Abstract
Discovering and understanding genetic risk factors for melanoma and their interactions with phenotype, sun exposure, and other risk factors could lead to new strategies for melanoma control. This paper describes the Australian Melanoma Family Study, which uses a multicenter, population-based, case-control-family design. From 2001 to 2005, the authors recruited 1,164 probands including 629 cases with histopathologically confirmed, first-primary cutaneous melanoma diagnosed before age 40 years, 240 population-based controls frequency matched for age, and 295 spouse/friend controls. Information on lifetime sun exposure, phenotype, and residence history was collected for probands and nearly 4,000 living relatives. More than 3,000 subjects donated a blood sample. Proxy-reported information was collected for childhood sun exposure and deceased relatives. Important features of this study include the population-based, family-based design; a focus on early onset disease; probands from 3 major cities differing substantially in solar ultraviolet exposure and melanoma incidence; a population at high risk because of high ultraviolet exposure and susceptible pigmentation phenotypes; population-based, spouse/friend, and sibling controls; systematic recruitment of relatives of case and control probands; self and parent reports of childhood sun exposure; and objective clinical skin examinations. The authors discuss methodological and analytical issues related to the study design and conduct, as well as the potentially novel insights the study can deliver.
Keywords: case-control studies, environmental exposure, family, genetic predisposition to disease, melanoma, risk factors
Most early epidemiologic work on genetics and major diseases has been based on multiple-case families, with ad hoc opportunistic sampling generally representing carriers of high-risk mutations. The population-based, case-control-family design was conceived to minimize ascertainment bias and to provide estimates of risk with potentially wider clinical and population health relevance (1). The Australian Melanoma Family Study takes advantage of this design to investigate genetic, phenotypic, and environmental influences and their interactions on melanoma risk.
Cutaneous melanoma incidence is increasing worldwide in predominantly European populations (2). In Australia, which has the world's highest incidence of melanoma, age-standardized incidence and mortality rates have increased over the past decade by 14% and 4.4%, respectively (3). For young Australian adults aged 15–44 years, melanoma is the most common malignancy and one of the leading causes of cancer death (4).
There is evidence that melanoma might develop through multiple pathways (5–7) and that interactions among genetic, phenotypic, and environmental factors modify risk (8–10). Although progress has been made in recent years (8), the genetic contribution to the majority of melanomas remains largely unexplained. Further discovery and characterization of genetic risk factors, along with an understanding of how these factors interact, could lead to improved understanding of the mechanisms of melanoma development and stimulate new strategies for melanoma prevention, screening, and treatment.
Here, we describe the objectives, design, population, and methods of the Australian Melanoma Family Study. We outline important features of its design that offer advantages over previous studies of melanoma and discuss the potentially novel insights it can deliver.
MATERIALS AND METHODS
Aims and objectives
The primary aim of the Australian Melanoma Family Study is to investigate the influence of genetic, phenotypic, and environmental factors and their interactions on melanoma risk, particularly before age 40 years. Research questions of interest include the following: characterizing risk associated with both rare “high-risk” genetic mutations and common “low-moderate risk” genetic variants, which might be sufficiently common to account for a substantial proportion of disease in the population (8); timing of vulnerable periods of sun exposure in early life; effect of sun exposure for genetically susceptible individuals; and whether modifiable risk factors for melanoma differ between carriers and noncarriers of genetic variants or by family history of melanoma. Many other issues are also addressable by using the resources of the study, especially as the complex genetic architecture of the disease is unraveled. It has already contributed in this way (11).
Study design and population-based sampling
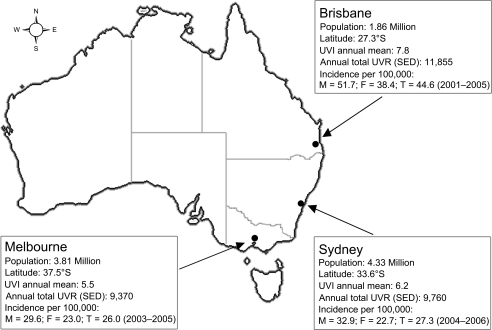
We used a population-based, case-control-family study design (1, 12). Proband recruitment was locally conducted in Sydney, Melbourne, and Brisbane, the 3 largest urban populations in Australia comprising about 50% of the Australian population (13) and accounting for a similar proportion of melanoma incidence (3). These cities differ substantially in latitude, solar ultraviolet (UV) radiation exposure, and melanoma incidence (Figure 1), but they have similar ethnic and demographic composition (14). Ascertainment was based on population-based, case and control probands residing in these cities’ greater metropolitan areas at the time of diagnosis or selection, who could complete an interview in English. Additional ascertainment of spouse/friend controls and sibling controls was generated from these population-based probands.
Figure 1.
Map of Australia showing the location of major cities Brisbane, Sydney, and Melbourne, their population, latitude, solar ultraviolet (UV) radiation estimates (23), and melanoma incidence rates age-standardized to Segi's 1960 world population, obtained from population-based cancer registries, Australian Melanoma Family Study, 2001–2005. F, female; M, male; SED, standard erythemal dose; T, total; UVI, UV index; UVR, UV radiation. UVI is a measure of UVR intensity; it is the maximum biologically effective solar UV radiation for the day and is averaged over 10 or 30 minutes (23); 1 SED is equivalent to an erythemally effective radiant exposure of 100 J/m2 and is a measure of the cumulative ambient solar UVR (23).
Population-based case probands.
Eligible case probands were men and women diagnosed between July 1, 2000, and December 31, 2002, with a histopathologically confirmed first-primary invasive cutaneous melanoma (qualifying melanoma), who were aged from 18 to 39 years at diagnosis. Cases with a previous history of in situ melanoma (n = 5) remained eligible. They were identified through population-based state cancer registries, where notification of cancer is mandatory, and registration is considered to be virtually complete. The cancer registries mailed information about the study to eligible cases after seeking permission from their physician (in Melbourne, after notifying their physician). In Brisbane, the initial approach included a signed letter from their physician and a consent form. In Sydney and Melbourne, consent was sought by the researchers after the case had agreed to have his/her contact details released to the study team. New address details were sought for any returned mail, and nonrespondents were followed up with a reminder letter and telephone calls.
Population-based control probands.
Population-based control probands were selected from the electoral roll (registration to vote is compulsory for adult Australian citizens) and were frequency matched to cases by age (within 5 years) and sex using proportional random sampling. They were eligible if they were aged from 18 to 39 years at the time of approach and had no history of melanoma including in situ melanoma.
Spouse/friend control probands.
Eligible spouse/friend controls were a spouse, partner, or friend nominated by a case proband as a potential control subject. Spouse/friend controls were eligible if they were at least 18 years of age and had no history of melanoma including in situ melanoma; there were no other age, sex, or residency restrictions.
Relatives of case and control probands, including sibling controls for case probands.
Predefined sets of relatives were invited to participate in the study after permission was obtained from the proband or any other appropriate participating relative. All adult first- and second-degree relatives of cases and population-based controls were eligible to participate, that is, parents, siblings (including half-siblings), offspring, grandparents, aunts, and uncles. For spouse/friend controls, first-degree relatives and half-siblings were eligible, but not second-degree relatives. An unaffected sibling of each case who was the closest in age to and, if possible, the same sex as the case was identified as a “sibling control.”
Extended families.
We used the Cannings-Thompson sequential ascertainment scheme (15) to extend the selection of other relatives from case and population-based control families. According to this scheme, any additional diagnosis of melanoma (either in situ or invasive) for any eligible relative subsequently made eligible all first-degree relatives of the newly identified melanoma case and, should any newly eligible relative have had a melanoma, his/her first-degree relatives became eligible, and so on.
Ethics approvals and informed consent
Approval for the study was obtained from the ethics committees of the 3 coordinating centers and the cancer registries. All participants provided written, informed consent.
Data collection
Data were collected predominantly between January 2001 and December 2005. Recruitment and interviews were managed by the local study center, and study materials and procedures were standardized across centers. Data entry from questionnaires was performed centrally.
Family pedigree and family cancer history.
A summary pedigree was constructed for each family by using a standard pedigree questionnaire. For each ascertained family member, data were collected on gender, date of birth, country of birth, ethnicity, vital status, date of death if applicable, and age at diagnosis and site of known previous cancer diagnoses.
Questionnaires.
Consenting probands and relatives completed a lifetime calendar in which they indicated, for each year, their place of residence, place of work or study, number of days spent at work or study each week in both warmer and cooler months, and holiday location. Participants also reported their skin, eye color, natural hair color at age 18, usual tanning and sunburn response to prolonged or repeated exposure of skin to sunlight, use of sunlamps and sunbeds, the density of moles covering their body (based on a 4-level picture scale), and the degree of freckling on the face in childhood and adulthood (based on a 6-level picture scale), and they gave a count of the number of all moles on their back (using picture guides to define the area and describe moles).
A trained interviewer administered structured questionnaires to each participant by telephone. To improve recall, a summary of the completed residence calendar was sent to participants to refer to during the 45-minute interview, in which they were asked to recall their sun exposure at 10, 15, 20, 30, and 40 years of age, and for participants older than age 40 years at interview, also for the most recent decade year of age up to age 70. Questions elicited the frequency of sunscreen use and strength of sun protection factor used, frequency of sunburn and blistering, and time spent outdoors between 9 AM and 5 PM separately for weekdays, weekends, and holidays in both warmer and cooler months. Probands were also asked about the frequency of sun exposure and sunscreen use at the specific anatomic site of the melanoma; for these questions, control probands were assigned a specific site, frequency matched to the expected site distribution in the cases. Participants were also asked about any outdoor beach, swimming pool, or water sport activities between 9 AM and 5 PM on at least 10 days in any year since leaving school, including the years, frequency, duration, and season. Demographic information, ethnicity, and details of previous cancer diagnoses including other skin cancers were also collected. Estimates of total lifetime, age-specific, and average annual sun-related exposures were calculated by established methods (16). Estimates of ambient UV irradiation exposure were obtained by combining information on annual place of residence with an objective measure of cloud-adjusted, monthly mean ambient erythemal UV radiation (kJ/m2) at each residential location, derived from satellite observations (16, 17).
Proxy questionnaire data.
For probands and sibling controls, data on childhood sun exposure at ages 3, 5, 10, and 15 years were collected by proxy interview with a parent (usually the mother). For deceased or noncompetent eligible relatives, information was obtained on demographic information, ethnicity, and details of previous cancer diagnoses through a proxy interview with any suitable next of kin.
Skin examinations.
Skin examinations were conducted for probands and sibling controls at dermatology clinics in Brisbane, Sydney, and Melbourne by dermatologic trainees. All examiners received training on the study protocol, which was based on international guidelines for identifying and recording nevi (18). Thirty body sites were examined, and separate counts were made for melanocytic nevi of 2–5 mm and >5 mm, raised nevi of ≥2 mm, and clinically atypical nevi of ≥2 mm. The number of solar lentigines on the upper back was recorded by using a 6-level picture scale. Reflected skin color, a correlate of melanin content (19), was recorded (mean of 6 readings) from both the outer and inner part of the subject's left upper arm by use of a hand-held reflectance spectrophotometer calibrated before each session. Height and weight were measured, and natural hair color at age 18 and eye color were recorded by using standard wig hair swatches and eye photographs.
Diagnosis of melanoma and other cancers.
Participants reported their own and relatives’ histories of melanoma and other cancers. Verification of reported cancers was sought from cancer registries, hospital and pathology records, treating clinicians, general practitioners, and death certificates, when participant or next-of-kin (if participant deceased) consent had been obtained. Verification of reported first squamous and basal cell carcinomas was sought for probands only. All histopathology reports were reviewed centrally by the study team, and sections stained with hematoxylin and eosin were requested for digitized imaging and pathology review.
Blood collection.
Blood was requested from all probands and their first-degree relatives, from second-degree relatives if they or any of their offspring had been diagnosed with melanoma, and from parents of any person diagnosed with melanoma. A 20-mL blood sample was collected in ethylenediaminetetraacetic acid (EDTA) tubes by local pathology services and transported to a central laboratory in Sydney within 48 hours of collection. White blood cells were separated on a Ficoll gradient, and plasma was obtained by centrifugation and stored at −70°C. Guthrie spots were obtained from 1 mL of blood. The remaining blood was used for DNA extraction. Buccal swabs were collected from participants who did not wish to give blood.
Follow-up
All participants will be tracked through the National Death Index and state cancer registries’ records to prospectively update vital status and cancer incidence and mortality.
Statistical analysis
An overview of the analytical approaches, advantages, and limitations of the population-based, case-control-family design is provided in Table 1. In analysis of these data, sensitivity analyses will be performed to examine the associations using different control groups and after excluding the 5 cases with previous in situ melanoma.
Table 1.
Overview of Analytical Approaches, Advantages, and Limitations of the Population-based, Case-Control-Family Design
| Population-based, Case-Control-Family Design | |||
| Analytical Comparison | Types of Analysis and Uses | Advantages | Limitations |
| Traditional case-control comparisons of case probands with population-based controls and spouse/friend controls | For examining environmental factors, family history, and common genetic factors | More extensive and greater validity of family history data compared with traditional case-control studies, because information is gathered directly from relatives where possible | Poor participation of population-based controls could lead to selection bias and biased estimates, at least for environmental exposures. (This is becoming a major limitation of population-based, case-control studies in general.) |
| Logistic regression | In the current context, parents’ reports of childhood sun exposure can be compared with probands’ reports as a measure of reliability. | ||
| Spouse controls have typically high participation. | |||
| Can contribute to genome-wide association studies | |||
| Case-control comparisons between case probands and sibling controls | For examining rare and common genetic factors, environmental factors, and gene-environment interactions | Sibling controls avoid confounding due to population stratification and/or unmeasured familial risk factors, and they are often well matched for other potential confounders. | Some cases do not have any siblings. |
| Correlated exposures between the proband and sibling should be taken into account, e.g., by using conditional logistic regression or other methods, such as generalized estimating equations. | Sibling controls have relatively high participation. | For some exposures, overmatching within the family for both genetic and shared environmental factors could result in reduced power per subject (1). | |
| A statistically efficient method for studying gene-environment interactions (54) | |||
| Comparisons involving probands and relatives | For examining rare and common genetic factors, environmental factors, and gene-environment interactions | Increases statistical power for estimating genetic associations by making use of data on all family members | Can be resource intensive to recruit relatives |
| Modified segregation analysis (55, 56) can be used to estimate the associations of melanoma with known (measured) and unmeasured genes, as well as environmental and phenotypic factors, allowing for other familial causes of disease (both measured and unmeasured), using polygenic (57) or regressive logistic models (55). | Allows examination of both rare and common genetic factors, environmental factors, and their interaction | Recruitment of relatives of controls might be more difficult than that of relatives of cases. | |
| Prospective and retrospective cohort approaches can be used to estimate the risk of melanoma for different relative groups, including the incidence of disease in relatives of cases or mutation carriers, and comparison of risks between those for relatives of cases and those for relatives of controls (58, 59) | Enables estimation of age-specific cumulative risks and hazard ratios for melanoma associated with family history or with being a carrier of a genetic mutation | Older-onset diseases might result in smaller family sizes; however, in this study, all case probands were aged <40 years at diagnosis. | |
| The associations with environmental and phenotypic factors can be estimated separately for genetically susceptible subgroups and for noncarriers. | |||
| Familial relative risks can be estimated for different relative groups. | |||
| Permits simple adjustment for ascertainment that maximizes use of family data and gives unbiased estimates with direct inference to the population, in contrast to the multiple-case, family-based study design (1, 12) | |||
| Easier to maintain prospective follow-up with family-based recruitment, leading to high cohort retention rates | |||
To provide some indication of the validity of the study design, we report here some results for associations of melanoma with known risk factors (hair color, ability to tan, mole count, family history) using the different control groups, adjusted for potential confounders. Odds ratios and 95% confidence intervals were estimated by using unconditional logistic regression for population-based controls and spouse/friend controls and by using conditional logistic regression matched by family for sibling controls.
RESULTS
Participation of probands
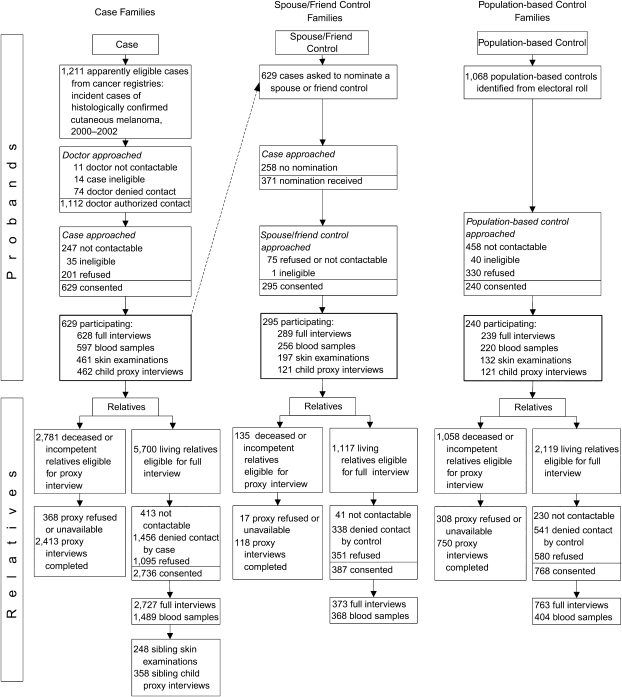
A total of 629 cases, 240 population controls, and 295 spouse/friend controls participated (Figure 2). The main reasons for nonparticipation by eligible cases were inability to contact (21%) and refusal (17%); participation was 54% when calculated as a proportion of those eligible (Brisbane, 64%; Melbourne, 57%; Sydney, 43%) and 76% as a proportion of those contactable. The median interval between diagnosis of melanoma and interview for cases was 10.0 months (25th–75th centile, 6.8–14.1 months). By the end of the data collection period, 14 (2.2%) cases had died.
Figure 2.
Flow diagram showing ascertainment of case, spouse/friend control, and population-based control families, Australian Melanoma Family Study, 2001–2005.
Participation by population controls was 23% when calculated as a proportion of all apparently eligible controls selected (Brisbane, 34%; Melbourne, 29%; Sydney, 12%). Almost half (43%) of selected population-based controls could not be contacted. Participation as a proportion of those contactable was 42%. A spouse or friend was nominated as a potential control subject by 59% of cases; participation was 80% of those nominated and 47% as a proportion of all participating cases (Melbourne, 61%; Sydney, 42%; Brisbane, 37%). Most participating cases and population-based controls were female (61% and 63%, respectively); 49% of spouse/friend controls were female.
Participation of relatives
Case, population-based control, and spouse/friend control probands were similarly likely to permit contact with known, living relatives within the ascertainment scheme: 73%, 71%, and 69% of relatives, respectively. A sibling control was identified for 91% of cases, and 43% of them had a skin examination and 64% had a child proxy questionnaire. Fifty-two percent of eligible relatives with a full or proxy questionnaire and 59% of relatives with a blood sample were female.
Table 2 shows the total numbers of questionnaires, blood samples, and skin examinations that were collected by degree of relatedness to the probands and by study center. There were, on average, 8.2 full or proxy relative interviews completed and 2.4 relatives’ blood samples collected per case family, 6.3 and 1.7 per population-based control family, and 1.7 and 1.2 per spouse/friend control family.
Table 2.
Participation of Individuals in Case, Population-based Control, and Spouse/Friend Control Families, by Study Center and Degree of Relatedness, Australian Melanoma Family Study, 2001–2005a
| Brisbane |
Sydney |
Melbourne |
Total |
|||||||||||||
| Case | Population-based Control | Spouse/ Friend Control | Total | Case | Population-based Control | Spouse/ Friend Control | Total | Case | Population-based Control | Spouse/ Friend Control | Total | Case | Population-based Control | Spouse/ Friend Control | Total | |
| Probands | ||||||||||||||||
| Full questionnaire | 234 | 149 | 85 | 468 | 168 | 57 | 66 | 291 | 226 | 33 | 138 | 397 | 628 | 239 | 289 | 1,156 |
| Blood | 211 | 137 | 75 | 423 | 165 | 54 | 59 | 278 | 221 | 29 | 122 | 372 | 597 | 220 | 256 | 1,073 |
| Skin examination | 129 | 72 | 49 | 250 | 142 | 36 | 38 | 216 | 190 | 24 | 110 | 324 | 461 | 132 | 197 | 790 |
| Child proxy | 145 | 79 | 39 | 263 | 132 | 26 | 32 | 190 | 185 | 16 | 50 | 251 | 462 | 121 | 121 | 704 |
| First-degree relative | ||||||||||||||||
| Full questionnaire | 511 | 294 | 152 | 957 | 434 | 61 | 71 | 566 | 612 | 58 | 150 | 820 | 1,557 | 413 | 373 | 2,343 |
| Proxy questionnaire | 42 | 30 | 34 | 106 | 40 | 7 | 17 | 64 | 53 | 7 | 67 | 127 | 135 | 44 | 118 | 297 |
| Blood | 463 | 271 | 149 | 883 | 398 | 63 | 97 | 558 | 548 | 52 | 122 | 722 | 1,409 | 386 | 368 | 2,163 |
| Skin examination | 71 | 0 | 0 | 71 | 74 | 2 | 0 | 76 | 103 | 6 | 0 | 109 | 248 | 8 | 0 | 256 |
| Child proxy | 109 | 0 | 0 | 109 | 103 | 8 | 0 | 111 | 146 | 10 | 0 | 156 | 358 | 18 | 0 | 376 |
| Second- or higher-degree relative | ||||||||||||||||
| Full questionnaire | 469 | 318 | 0 | 787 | 265 | 17 | 0 | 282 | 436 | 15 | 0 | 451 | 1,170 | 350 | 0 | 1,520 |
| Proxy questionnaire | 634 | 407 | 0 | 1,041 | 644 | 171 | 0 | 815 | 1,000 | 128 | 0 | 1,128 | 2,278 | 706 | 0 | 2,984 |
| Blood | 31 | 13 | 0 | 44 | 21 | 2 | 0 | 23 | 28 | 3 | 0 | 31 | 80 | 18 | 0 | 98 |
| Total | ||||||||||||||||
| Full questionnaire | 1,214 | 761 | 237 | 2,212 | 867 | 135 | 137 | 1,139 | 1,274 | 106 | 288 | 1,668 | 3,355 | 1,002 | 662 | 5,019 |
| Proxy questionnaire | 676 | 437 | 34 | 1,147 | 684 | 178 | 17 | 879 | 1,053 | 135 | 67 | 1,255 | 2,413 | 750 | 118 | 3,281 |
| Bloodb | 705 | 421 | 224 | 1,350 | 584 | 119 | 156 | 859 | 797 | 84 | 244 | 1,125 | 2,086 | 624 | 624 | 3,334 |
| Skin examination | 200 | 72 | 49 | 321 | 216 | 38 | 38 | 292 | 293 | 30 | 110 | 433 | 709 | 140 | 197 | 1,046 |
| Child proxy | 254 | 79 | 39 | 372 | 235 | 34 | 32 | 301 | 331 | 26 | 50 | 407 | 820 | 139 | 121 | 1,080 |
The data represent the number in each category.
Includes 28 participants with buccal swabs instead of blood.
Characteristics of probands
Cases’ tumor characteristics were generally similar to all those for the same region, period, and age group as reported by the population-based cancer registries (Table 3). Some differences included a higher proportion of female cases in Melbourne and a slightly lower socioeconomic status of cases and differences in histology in Sydney and Melbourne that are probably due to differences between the study and cancer registries in coding pathology reports. Compared with population data, a higher proportion of controls were born in Australia and had a university degree (Table 4). Control probands from Sydney had a relatively higher socioeconomic status than the general population, but in each region cases and controls had a very similar mean socioeconomic status index.
Table 3.
Characteristics of Case Probands and Their Qualifying (First) Primary Invasive Melanoma by Study Center, and Comparison With All Melanomas Identified in the Same Region and for the Same Age Group at the Population-based Cancer Registries, Australian Melanoma Family Study, 2001–2005a
| Brisbane |
Sydney |
Melbourne |
Total | |||||||||||
| AMFS |
Registry, %b | P Valuec | AMFS |
Registry, %b | P Valuec | AMFS |
Registry, %b | P Valuec | AMFS |
|||||
| No. | % | No. | % | No. | % | No. | % | |||||||
| Total, cases | 234 | 169 | 226 | 629 | ||||||||||
| Sex | ||||||||||||||
| Male | 99 | 42.3 | 45.8 | 71 | 42.0 | 48.3 | 73 | 32.3 | 40.5 | 243 | 38.6 | |||
| Female | 135 | 57.7 | 54.2 | 0.45 | 98 | 58.0 | 51.7 | 0.16 | 153 | 67.7 | 59.5 | 0.04 | 386 | 61.4 |
| Age at diagnosis, years | ||||||||||||||
| Median | 33.1 | 32 | 32.7 | 32 | 33.3 | 32 | 32.9 | |||||||
| 18–24 | 40 | 17.1 | 13.4 | 13 | 7.7 | 11.8 | 25 | 11.1 | 14.8 | 78 | 12.4 | |||
| 25–29 | 41 | 17.5 | 24.4 | 33 | 19.5 | 19.8 | 49 | 21.7 | 20.0 | 123 | 19.6 | |||
| 30–34 | 67 | 28.6 | 21.8 | 60 | 35.5 | 33.0 | 64 | 28.3 | 29.2 | 191 | 30.4 | |||
| 35–39 | 86 | 36.8 | 40.3 | 0.10 | 63 | 37.3 | 35.4 | 0.51 | 88 | 38.9 | 36.0 | 0.53 | 237 | 37.7 |
| Country of birth | ||||||||||||||
| Australia | 208 | 88.9 | 85.9 | 137 | 81.5 | 84.5 | 198 | 87.6 | 89.2 | 543 | 86.5 | |||
| New Zealand | 9 | 3.8 | 6.3 | 8 | 4.8 | 2.5 | 4 | 1.8 | 1.5 | 21 | 3.3 | |||
| United Kingdom, Ireland | 9 | 3.8 | 5.5 | 12 | 7.1 | 4.7 | 10 | 4.4 | 4.4 | 31 | 4.9 | |||
| Other | 8 | 3.4 | 2.3 | 0.58 | 11 | 6.5 | 8.2 | 0.35 | 14 | 6.2 | 4.9 | 0.94 | 33 | 5.3 |
| Unknown | 0 | 1 | 0 | 1 | ||||||||||
| Breslow thickness, mm | ||||||||||||||
| Median | 0.50 | 0.50 | 0.65 | 0.60 | 0.60 | 0.55 | ||||||||
| 0.01–1.00 | 195 | 83.3 | 86.2 | 125 | 74.4 | 75.3 | 185 | 82.6 | 80.1 | 505 | 80.7 | |||
| 1.01–2.00 | 31 | 13.2 | 10.3 | 29 | 17.3 | 15.3 | 23 | 10.3 | 10.9 | 83 | 13.3 | |||
| 2.01–4.00 | 6 | 2.6 | 3.4 | 6 | 3.6 | 6.2 | 10 | 4.5 | 5.6 | 22 | 3.5 | |||
| >4.00 | 2 | 0.9 | 0.1 | 0.67 | 8 | 4.8 | 3.1 | 0.42 | 6 | 2.7 | 3.4 | 0.86 | 16 | 2.6 |
| Unspecified | 0 | 1 | 2 | 3 | ||||||||||
| Anatomic site | ||||||||||||||
| Face, ears | 15 | 6.5 | 4.3 | 12 | 7.1 | 6.6 | 16 | 7.1 | 6.7 | 43 | 6.9 | |||
| Neck, scalp | 12 | 5.2 | 6.5 | 15 | 8.9 | 6.6 | 15 | 6.7 | 5.4 | 42 | 6.7 | |||
| Trunk | 92 | 39.8 | 37.9 | 52 | 30.8 | 37.8 | 72 | 32.1 | 35.6 | 216 | 34.6 | |||
| Upper limb including shoulder | 55 | 23.8 | 19.8 | 32 | 18.9 | 20.7 | 47 | 21.0 | 20.8 | 134 | 21.5 | |||
| Lower limb including hip | 57 | 24.7 | 31.5 | 0.38 | 58 | 34.3 | 28.3 | 0.35 | 74 | 33.0 | 31.5 | 0.90 | 189 | 30.3 |
| Unspecified | 3 | 0 | 2 | 5 | ||||||||||
| Histology | ||||||||||||||
| Superficial spreading | 163 | 69.7 | 75.2 | 74 | 43.8 | 54.2 | 123 | 54.4 | 0.0 | 360 | 57.2 | |||
| Not otherwise specified | 52 | 22.2 | 18.9 | 87 | 51.5 | 35.6 | 84 | 37.2 | 92.6d | 223 | 35.5 | |||
| Nodular | 14 | 6.0 | 4.2 | 3 | 1.8 | 6.9 | 12 | 5.3 | 0.0 | 29 | 4.6 | |||
| Other subtypese | 5 | 2.1 | 1.7 | 0.56 | 5 | 3.0 | 3.3 | 0.001 | 7 | 3.1 | 7.4 | <0.001 | 17 | 2.7 |
| Index of relative socioeconomic disadvantage, quintilesf | ||||||||||||||
| 1 (most disadvantage) | 12 | 5.2 | 6.3 | 7 | 4.3 | 11.4 | 22 | 10.1 | 41 | 6.7 | ||||
| 2 | 53 | 23.1 | 16.0 | 23 | 14.3 | 7.5 | 56 | 25.7 | 132 | 21.7 | ||||
| 3 | 78 | 34.1 | 18.9 | 29 | 18.0 | 7.3 | 53 | 24.3 | 160 | 26.3 | ||||
| 4 | 44 | 19.2 | 27.7 | 48 | 29.8 | 33.0 | 41 | 18.8 | 133 | 21.9 | ||||
| 5 (least disadvantage) | 42 | 18.3 | 31.0 | <0.001 | 54 | 33.5 | 40.7 | <0.001 | 46 | 21.1 | 142 | 23.4 | ||
Abbreviations: AMFS, Australian Melanoma Family Study; SEIFA, Socioeconomic Indexes for Areas.
Missing or unknown data are excluded from percentage calculations.
These data were obtained from the population-based cancer registries and represent all subjects from the Brisbane, Sydney, and Melbourne metropolitan regions, respectively, who were diagnosed with primary invasive melanoma during the study accrual period (from July 1, 2000, to December 31, 2002) and in the same age group (aged 18–39 years at the time of diagnosis).
χ2 test for association comparing the distribution of melanoma risk factors between AMFS subjects and individuals identified by the cancer registry and excluding missing/unknown data. P values are 2 sided.
During this period, the Victorian cancer registry did not code the histology of all melanoma subtypes, so, apart from some of the rarer morphologies, most melanomas including “superficial spreading” and most “nodular” were coded to “not otherwise specified.”
Other histologic subtypes (total number of AMFS cases) include the following: malignant melanoma, regressing (n = 9); lentigo maligna melanoma (n = 3); acral lentiginous melanoma (n = 3); and desmoplastic melanoma (n = 2).
Based on 2001 SEIFA data published by the Australian Bureau of Statistics (www.abs.gov.au), by use of state-wide quintiles. The Index ranks areas on the level of social and economic well-being based on area of residence. SEIFA data were not available from the Victorian cancer registry for Melbourne.
Table 4.
Characteristics of Control Probands and Comparison With Population Data, Australian Melanoma Family Study, 2001–2005
| Population Controls | Spouse Controls | Population Dataa | |
| Highest level of education, %b | |||
| Junior secondary school | 12.8** | 7.0*** | 21.8 |
| Senior secondary school | 32.1 | 24.1** | 31.2 |
| Vocational | 25.8 | 27.8 | 25.3 |
| University | 29.3** | 41.2*** | 21.7 |
| Born in Australia, by study center, %b | |||
| Brisbane | 90.4*** | 96.1*** | 76.0 |
| Sydney | 80.9** | 88.3*** | 63.6 |
| Melbourne | 93.4** | 91.5*** | 70.6 |
| Index of relative socioeconomic disadvantage, by study center, meanc | |||
| Brisbane | 1,010.0 | 1,007.2 | 1,007.6 |
| Sydney | 1,040.0* | 1,050.4** | 1,016.8 |
| Melbourne | 1,039.3 | 1,030.5 | 1,020.6 |
Abbreviation: SEIFA, Socioeconomic Indexes for Areas.
* P < 0.05; **P < 0.01; ***P < 0.0001 (2-sided P values for comparison with population data, calculated by using a 1-sample test of proportion for categorical data and a 1-sample t test for continuous data).
Population data were obtained from the Australian Bureau of Statistics (www.abs.gov.au); country of birth was obtained from 2006 census data (ages 15–44 years), level of education from the 2002 Education and Work Survey (ages 20–44 years), and socioeconomic data from SEIFA (all ages).
Age-adjusted percentages using population data as the standard and excluding participants aged ≥45 years.
Based on 2001 SEIFA data published by the Australian Bureau of Statistics (www.abs.gov.au), unadjusted for age. The Index ranks areas on the level of social and economic well-being, and index scores have been standardized to have a mean of 1,000 and a standard deviation of 100 at the Collection District Level. A higher index value indicates less disadvantage. The mean SEIFA values for study participants were derived from the residential postcode at recruitment. For cases, the values were 1,005.1 (Brisbane), 1,047.1 (Sydney), and 1,034.6 (Melbourne).
Characteristics of relatives
Of the 355 eligible, ascertained relatives with reported melanoma, 177 (50%) were verified through official records as having had a melanoma. The proportion verified was 55% for first-degree relatives and 47% for second-degree relatives; in first-degree relatives, the proportion verified was 58% for case families, 57% for population-based control families, and 41% for spouse control families. The proportion verified for second-degree relatives was also similar for relatives of cases (47%) and population-based controls (45%).
Of the 629 cases, 97 (15%) reported melanoma and 58 (9%) had a verified melanoma in at least 1 first-degree relative and 187 (30%) and 105 (17%), respectively, in at least 1 first- or second-degree relative. For population-based controls and spouse/friend controls, a melanoma in at least 1 first-degree relative was reported for 21 (9%) and 27 (9%) families, respectively, and verified for 12 (5%) and 11 (4%) families, respectively. For population-based controls, 51 (21%) reported melanoma, and 28 (12%) had a verified melanoma in at least 1 first- or second-degree relative.
Associations of melanoma with known risk factors
Hair color, ability to tan, mole count, and family history were each significantly associated with odds of melanoma (Table 5). Results were similar when analyzed separately by using population-based controls, spouse/friend controls, and sibling controls.
Table 5.
Odds Ratios and 95% Confidence Intervals for Associations of Melanoma With Selected Known Risk Factors, for Different Control Groups, Australian Melanoma Family Study, 2001–2005
| Risk Factor | No. of Cases | Total Controls (Population and Spouse/Friend) |
Population Controls |
Spouse/Friend Controls |
Sibling Controls |
Meta-Analysisa |
||||||||||
| No. | Odds Ratiob | 95% Confidence Interval | No. | Odds Ratiob | 95% Confidence Interval | No. | Odds Ratiob | 95% Confidence Interval | No. of Casesc | No. of Controls | Odds Ratiod | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | ||
| Hair color | ||||||||||||||||
| Dark brown/black | 157 | 189 | 1.00 | 97 | 1.00 | 92 | 1.00 | 109 | 224 | 1.00 | 1.00 | |||||
| Light brown | 243 | 207 | 1.26 | 0.94, 1.71 | 98 | 1.46 | 1.00, 2.13 | 109 | 1.11 | 0.76, 1.62 | 157 | 253 | 1.37 | 0.95, 1.97 | 1.62 | 1.11, 2.34 |
| Fair or blonde | 140 | 63 | 2.34 | 1.59, 3.44 | 26 | 3.22 | 1.91, 5.43 | 37 | 1.71 | 1.06, 2.78 | 88 | 118 | 1.85 | 1.13, 3.01 | 1.96 | 1.41, 2.74 |
| Red | 72 | 25 | 3.02 | 1.79, 5.11 | 12 | 3.20 | 1.60, 6.37 | 13 | 2.88 | 1.44, 5.74 | 42 | 67 | 1.61 | 0.91, 2.83 | 3.64 | 2.56, 5.37 |
| Ptrend | <0.001 | <0.001 | <0.001 | 0.02 | ||||||||||||
| Ability to tan (repeated exposure) | ||||||||||||||||
| Very brown and deep tan | 84 | 119 | 1.00 | 63 | 1.00 | 56 | 1.00 | 50 | 123 | 1.00 | 1.00 | |||||
| Moderate tan | 271 | 203 | 1.91 | 1.35, 2.72 | 92 | 2.11 | 1.37, 3.27 | 111 | 1.60 | 1.02, 2.49 | 179 | 293 | 1.69 | 1.09, 2.61 | 1.77 | 1.23, 2.56 |
| Mild or occasional tan | 179 | 116 | 2.00 | 1.35, 2.94 | 55 | 2.20 | 1.36, 3.56 | 61 | 1.60 | 0.98, 2.63 | 123 | 189 | 2.01 | 1.25, 3.22 | 1.84 | 1.43, 2.36 |
| No tan or only freckles | 75 | 43 | 2.11 | 1.29, 3.45 | 22 | 2.20 | 1.18, 4.09 | 21 | 1.91 | 1.00, 3.65 | 45 | 60 | 2.53 | 1.35, 4.74 | 2.09 | 1.67, 2.58 |
| Ptrend | 0.002 | 0.007 | 0.06 | 0.002 | ||||||||||||
| Mole count on back | ||||||||||||||||
| None | 33 | 59 | 1.00 | 31 | 1.00 | 28 | 1.00 | 22 | 77 | 1.00 | 1.00a | |||||
| 1–4 | 88 | 103 | 1.37 | 0.80, 2.33 | 44 | 1.56 | 0.80, 3.03 | 59 | 1.25 | 0.65, 2.39 | 51 | 124 | 1.52 | 0.80, 2.87 | 1.44 | 1.29, 1.60 |
| 5–10 | 137 | 127 | 1.62 | 0.97, 2.71 | 68 | 1.62 | 0.87, 3.01 | 59 | 1.79 | 0.95, 3.36 | 90 | 167 | 2.22 | 1.17, 4.19 | 2.48 | 1.90, 3.23 |
| 11–15 | 67 | 43 | 2.39 | 1.31, 4.35 | 23 | 2.41 | 1.15, 5.01 | 20 | 2.25 | 1.05, 4.82 | 41 | 78 | 1.94 | 0.95, 3.96 | 4.82 | 3.05, 7.62 |
| ≥16 | 263 | 133 | 2.87 | 1.74, 4.71 | 60 | 3.33 | 1.81, 6.16 | 73 | 2.53 | 1.38, 4.65 | 172 | 171 | 4.72 | 2.47, 9.00 | ||
| Ptrend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| Reported family history of melanoma in first-degree relative | ||||||||||||||||
| None | 525 | 442 | 1.00 | 214 | 1.00 | 228 | 1.00 | 1.00 | ||||||||
| ≥1 | 97 | 46 | 1.98 | 1.34, 2.92 | 21 | 1.91 | 1.13, 3.22 | 25 | 1.96 | 1.19, 3.23 | 1.74 | 1.41, 2.14 | ||||
| P value | <0.001 | 0.02 | 0.008 | |||||||||||||
Results from meta-analyses by Gandini et al. (24, 30). For mole count, the odds ratios from the meta-analysis are not directly comparable because they are based on mole count on arms (not on the back as in our study); however, we have used the same cutpoints, and a different pooled analysis (60) has shown similar risk estimates for mole counts on the trunk and upper limbs.
Analyzed by using unconditional logistic regression. Adjusted for age, sex, state, ethnicity (Caucasian, other), reported family history (first degree) of melanoma (none, any), education (junior high school, senior high school, vocational, university), and lifetime cumulative time spent outdoors (quartiles). Excludes spouse controls aged ≥45 years (n = 33) and cases and controls missing values for any of the covariates (6 cases, 4 population controls, 3 spouse controls).
Excludes 222 cases with no sibling controls.
Analyzed by using conditional logistic regression (matched by family). Adjusted for age, sex, education (junior high school, senior high school, vocational, university), and lifetime cumulative time spent outdoors (quartiles). Excludes sibling controls aged ≥45 years and cases and controls missing values for any of the covariates (5 cases; 11 sibling controls).
DISCUSSION
This study design, which enables the conduct of family-based analyses in addition to traditional case-control analyses, provides several novel advantages and opportunities compared with previous genetic and epidemiologic studies of melanoma risk factors. Here, we expand briefly on the strengths and limitations of the design, some of which are outlined in Table 1, and discuss methodological issues, such as the potential for selection bias and measurement error.
We adopted a population-based, case-control-family design that, in contrast to the multiple-case, family-based study design (which generally represents high-risk mutation carriers), minimizes ascertainment bias and enables simple and direct inference to the population (1, 12). It can in some circumstances be used to derive more powerful and robust inferences than from a traditional case-control or multiple-case, family-based approach, for example, by selecting for characteristics that are associated with a putative underlying genetic cause, such as early age of onset (diagnosed before age 40 years in our study) (1). Given that familial risks are greater for earlier onset disease (20), this approach probably maximizes the prevalence of melanoma susceptibility alleles (1), should improve recall of early life sun exposure, and increases the chance of relatives in earlier generations being alive and willing to participate. We extended the sampling of relatives using the Cannings-Thompson sequential ascertainment scheme, which is a systematic, efficient, and unbiased method for ascertaining families that plausibly carry susceptibility genes (15). This study design allows study of both rare “high-risk” genetic mutations and common “low-moderate risk” genetic variants, as well as gene-environment interactions. The recent genes, environment, and melanoma multicenter population-based, case-control study (21) was motivated by the goal of evaluating the risk conferred by high-penetrance (high-risk) mutations. It used a different approach by including only participants with melanoma, “cases” had multiple primary melanoma and “controls” had single primary melanoma, and all were unselected for age.
The Australian population is advantageous for assessing the influence of genetic characteristics and gene-environment interactions on melanoma risk because the presence of strong environmental exposures (e.g., sunlight, range of ambient UV exposures, skin pigmentation characteristics) strengthens causal inference for genetic modifiers of risk for observed genetic characteristics (22). We maximized differences in ambient UV exposures by recruiting probands from 3 major cities at different latitudes, which differ substantially in ambient UV irradiance and melanoma incidence (3, 23).
Recruitment of first- and second-degree relatives of case and control probands ensures greater validity of family history and other data compared with most previous studies of melanoma that relied solely on proband reports of family history (24). In addition, we collected both self and parent reports of childhood sun exposure, enabling us to obtain estimates of sun exposure at very early ages (e.g., ages 3 and 5 years) and to compare proband and parent reports as a reliability measure. The first 10–15 years of life might be a period of particular susceptibility to melanoma initiation from sun exposure (25).
Poor participation is a potential disadvantage of population-based sampling (26) and in this study was a problem for both cases and controls, particularly population-based controls. An advantage of recruiting spouse/friend and sibling controls is the higher participation achieved. As in other case-control studies conducted with highly mobile, young adults (27), it was challenging to obtain up-to-date contact details for potentially eligible participants identified in the cancer registry or from the electoral rolls. Cases’ participation could depend on their perceptions of the role sun exposure might have played in causing their melanoma, and population-based controls might be more likely to participate if they have a family history of melanoma or other known risk factors, which could introduce differential selection bias (28). However, poor participation does not necessarily lead to selection bias (26). It is also probable that valid estimates of gene-environment interactions can be obtained from studies in which high nonparticipation raises concerns about selection bias (29). There is some evidence from examination of our data that selection bias might not be a major concern in our study. For example, the characteristics of the melanomas reported in cases were similar to those of cases from the same age group in the population. Our controls did differ from the population for some characteristics, such as country of birth; however, population-based controls and spouse/friend controls had similar proportions of first-degree relatives with reported melanomas. Thus, a family history of melanoma might not be overrepresented in our population-based controls. Our cases and controls were also of similar socioeconomic status. In addition, we demonstrate that the associations between known risk factors (hair color, ability to tan, mole count, family history) and melanoma are consistent with results of recent meta-analyses (24, 30) and were similar when analyzed separately by using population-based controls, spouse/friend controls, and sibling controls.
Measurement error is also a potential problem in our study, as in other studies of melanoma. Sun exposure is a complex, time-variable exposure that is difficult to measure accurately. Thus, although we collected detailed measures, it is possible that nondifferential measurement errors will attenuate modest associations with melanoma risk (31, 32). We tried to minimize measurement error by collecting objective clinical measures of skin phenotype and hair and eye color for selected subjects. Ambient UV irradiance estimates derived from erythemal UV measurements matched to places of residence should also be similarly accurate for cases and controls. The study questionnaire has been used elsewhere (16, 33–35) and has good reliability for capturing total lifetime sun exposure (36). Reliability is generally moderate for intermittent or single exposures, such as number of sunburns (36–40), and high for phenotype measures (38, 40–42). Moderate agreement has also been shown when comparing proxy and self-reports of risk factors (43). Differential recall bias is a threat to the validity of case-control studies of melanoma (44), especially when there is high public awareness of risk factors (28). However, most studies have found little evidence for differential recall of total and intermittent UV radiation-related exposures (36–39, 45–48). Some studies reported differential recall of specific exposures, such as ability to tan (41), but this was not consistent across studies (46). Recently, Parr et al. (46) quantified recall bias for melanoma risk estimates by comparing both prospective and retrospective measurements of phenotypic factors and UV exposures and found only minor indications of recall bias, which had a negligible impact on risk estimates. Recall bias was not observed in those exposures where it was most expected, such as for solarium use and other UV radiation-related exposures (46, 47).
The accuracy of self reports of melanoma is relatively poor compared with other cancers (49), particularly in Australia, mainly because of confusion with common skin neoplasms (49, 50). Thus, we attempted to verify all reports of melanoma for relatives where consent was provided. The proportion that could be verified (50%) is probably an underestimate as those not verified include 1) relatives who were found not to have melanoma, 2) relatives for whom medical records could not be located because the records were old or unavailable, and 3) relatives for whom consent was not given to check the records. A previous study in Queensland (49, 51) reported similar data for accuracy of melanoma reports in first-degree relatives and prevalence of a confirmed family history for cases. Although cases might be more likely to investigate their family history of disease following diagnosis of cancer and thus more accurately report it than controls (52, 53), this seems unlikely in our study as the proportion of confirmed melanoma reports for relatives was similar for case and population-based control families.
This population-based, case-control-family study provides a valuable resource for investigating the etiology of melanoma. Results of its analysis may lead to improved prediction of melanoma risk for young adults, indicate important interactions with early life sun exposure that might be relevant to melanoma risk in early and later adult life, and inform surveillance strategies and the rational use of genetic testing for the management of people with a personal or family history of melanoma.
Acknowledgments
Author affiliations: Centre for Molecular, Environmental, Genetic, and Analytic (MEGA) Epidemiology, School of Population Health, University of Melbourne, Melbourne, Australia (Anne E. Cust, Judith A. Maskiell, Mark A. Jenkins, John L. Hopper); Westmead Institute for Cancer Research and Melanoma Institute of Australia, University of Sydney at Westmead Millennium Institute, Sydney, Australia (Helen Schmid, Elizabeth A. Holland, Chantelle Agha-Hamilton, Richard F. Kefford, Graham J. Mann); Viertel Centre for Research in Cancer Control, the Cancer Council Queensland, Spring Hill, Brisbane, Australia (Jodie Jetann, Megan Ferguson, Joanne F. Aitken); Victorian Melanoma Service, Alfred Hospital, Melbourne, Australia (John Kelly); Cancer Epidemiology Centre, the Cancer Council Victoria, Melbourne, Australia (Graham G. Giles); and School of Public Health, University of Sydney, Sydney, Australia (Bruce K. Armstrong).
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) (project grants 107359 and 211172 and program grant 402761 to G. J. M. and R. F. K.); the Cancer Councils of New South Wales (project grants 77/00 and 06/10), Victoria, and Queensland (project grant 371); and the US National Institutes of Health (RO1 grant CA-83115-01A2 to the international Melanoma Genetics Consortium (GenoMEL)). A. E. C. is the recipient of a NHMRC public health postdoctoral fellowship (no. 520018) and a Victorian Cancer Agency Early Career Seed Grant (ECSG07_010). B. K. A.’s research was supported by a University of Sydney Medical Foundation Program Grant, and J. L. H. is an Australia Fellow of the NHMRC.
The authors gratefully acknowledge the work and dedication of the research interviewers, examiners, and data management staff, including Jackie Arbuckle, Steven Columbus, Michaela Lang, Helen Rodais, and Caroline Ellis (Victoria); Carol El Hayek, Lynne Morgan, Joanne Roland, Emma Tyler, Jodi Barton, Caroline Watts, and Lesley Porter (New South Wales); Michelle Hillcoat, Kellie Holland, Pamela Saunders, Joan Roberts, and Sheree Tait (Queensland); and Anil Kurien, Clare Patterson, Caroline Thoo, Sally de Zwaan, Angelo Sklavos, Shobhan Manoharan, Jenny Cahill, and Sarah Brennand (skin examiners). Vicky Thursfield, Peter Baade, Narelle Grayson, and Hui You helped to extract relevant summary data from the cancer registries. Dr. Michael Skilton provided assistance with graphics, and Chris Goumas provided assistance with analysis.
Conflict of interest: none declared.
Glossary
Abbreviation
- UV
ultraviolet
References
- 1.Hopper JL, Bishop DT, Easton DF. Population-based family studies in genetic epidemiology. Lancet. 2005;366(9494):1397–1406. doi: 10.1016/S0140-6736(05)67570-8. [DOI] [PubMed] [Google Scholar]
- 2.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150(2):179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 3.Australian Institute of Health and Welfare (AIHW) & Australasian Association of Cancer Registries (AACR) Cancer in Australia: An Overview, 2006. Canberra, Australia: AIHW; 2007. [Google Scholar]
- 4.Australian Institute of Health and Welfare (AIHW) & Australasian Association of Cancer Registries (AACR) Cancer in Australia, 2001. Canberra, Australia: AIHW; 2004. [Google Scholar]
- 5.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 6.Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313(5786):521–522. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 7.Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–812. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 8.Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22(20):3053–3062. doi: 10.1038/sj.onc.1206445. [DOI] [PubMed] [Google Scholar]
- 9.Box NF, Duffy DL, Chen W, et al. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet. 2001;69(4):765–773. doi: 10.1086/323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop DT, Demenais F, Goldstein AM, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma. J Natl Cancer Inst. 2002;94(12):894–903. doi: 10.1093/jnci/94.12.894. [DOI] [PubMed] [Google Scholar]
- 11.Brown KM, Macgregor S, Montgomery GW, et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat Genet. 2008;40(7):838–840. doi: 10.1038/ng.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopper JL, Chenevix-Trench G, Jolley DJ, et al. Design and analysis issues in a population-based, case-control-family study of the genetic epidemiology of breast cancer and the Co-operative Family Registry for Breast Cancer Studies (CFRBCS) J Natl Cancer Inst Monogr. 1999;1999(26):95–100. doi: 10.1093/oxfordjournals.jncimonographs.a024232. [DOI] [PubMed] [Google Scholar]
- 13.Australian Bureau of Statistics. Australian Demographic Statistics, March 2008. Canberra, Australia: Australian Bureau of Statistics; 2008. [Google Scholar]
- 14.Australian Bureau of Statistics. Census 2006. Canberra, Australia: Australian Bureau of Statistics; 2008. [Google Scholar]
- 15.Cannings C, Thompson EA. Ascertainment in the sequential sampling of pedigrees. Clin Genet. 1977;12(4):208–212. doi: 10.1111/j.1399-0004.1977.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 16.Kricker A, Armstrong BK, Goumas C, et al. Ambient UV, personal sun exposure and risk of multiple primary melanomas. Cancer Causes Control. 2007;18(3):295–304. doi: 10.1007/s10552-006-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee-Taylor J, Madronich S. Climatology of UV-A, UV-B, and Erythemal Radiation at the Earth's Surface, 1979–2000. Boulder, CO: National Center for Atmospheric Research; 2007. [Google Scholar]
- 18.English DR, MacLennan R, Rivers J, et al. Epidemiological Studies of Melanocytic Naevi: Protocol for Identifying and Recording Naevi. Lyon, France: International Agency for Research on Cancer; 1990. (IARC internal report no. 90/002) [Google Scholar]
- 19.Dwyer T, Blizzard L, Ashbolt R. Sunburn associated with increased number of nevi in darker as well as lighter skinned adolescents of northern European descent. Cancer Epidemiol Biomarkers Prev. 1995;4(8):825–830. [PubMed] [Google Scholar]
- 20.Leu M, Reilly M, Czene K. Evaluation of bias in familial risk estimates: a study of common cancers using Swedish population-based registers. J Natl Cancer Inst. 2008;100(18):1318–1325. doi: 10.1093/jnci/djn290. [DOI] [PubMed] [Google Scholar]
- 21.Berwick M, Orlow I, Hummer AJ, et al. The prevalence of CDKN2A germ-line mutations and relative risk for cutaneous malignant melanoma: an international population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1520–1525. doi: 10.1158/1055-9965.EPI-06-0270. [DOI] [PubMed] [Google Scholar]
- 22.Weiss NS. Assessing the influence of a genetic characteristic on disease in the presence of a strong environmental etiology. Epidemiology. 2007;18(4):429–430. doi: 10.1097/EDE.0b013e31806466cf. [DOI] [PubMed] [Google Scholar]
- 23.Gies P, Roy C, Javorniczky J, et al. Global Solar UV Index: Australian measurements, forecasts and comparison with the UK. Photochem Photobiol. 2004;79(1):32–39. [PubMed] [Google Scholar]
- 24.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma. III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41(14):2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12(1):69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- 26.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Cardis E, Richardson L, Deltour I, et al. The INTERPHONE study: design, epidemiological methods, and description of the study population. Eur J Epidemiol. 2007;22(9):647–664. doi: 10.1007/s10654-007-9152-z. [DOI] [PubMed] [Google Scholar]
- 28.de Vries E, Boniol M, Severi G, et al. Public awareness about risk factors could pose problems for case-control studies: the example of sunbed use and cutaneous melanoma. Eur J Cancer. 2005;41(14):2150–2154. doi: 10.1016/j.ejca.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto LM, White E, Newcomb PA. Selection bias in the assessment of gene-environment interaction in case-control studies. Am J Epidemiol. 2003;158(3):259–263. doi: 10.1093/aje/kwg147. [DOI] [PubMed] [Google Scholar]
- 30.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma. I. Common and atypical naevi. Eur J Cancer. 2005;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong BK, White E, Saracci R. Principles of Exposure Measurement in Epidemiology. Oxford, United Kingdom: Oxford University Press; 1992. [Google Scholar]
- 32.Parr CL, Hjartaker A, Laake P, et al. Parr et al. respond to “Recall bias in melanoma.”. Am J Epidemiol. 2009;169(3):271–272. doi: 10.1093/aje/kwn356. [DOI] [PubMed] [Google Scholar]
- 33.Kricker A, Armstrong BK, English DR, et al. A dose-response curve for sun exposure and basal cell carcinoma. Int J Cancer. 1995;60(4):482–488. doi: 10.1002/ijc.2910600410. [DOI] [PubMed] [Google Scholar]
- 34.Vajdic CM, Kricker A, Giblin M, et al. Sun exposure predicts risk of ocular melanoma in Australia. Int J Cancer. 2002;101(2):175–182. doi: 10.1002/ijc.10579. [DOI] [PubMed] [Google Scholar]
- 35.Hughes AM, Armstrong BK, Vajdic CM, et al. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;112(5):865–871. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 36.Kricker A, Vajdic CM, Armstrong BK. Reliability and validity of a telephone questionnaire for estimating lifetime personal sun exposure in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2427–2432. doi: 10.1158/1055-9965.EPI-05-0265. [DOI] [PubMed] [Google Scholar]
- 37.English DR, Armstrong BK, Kricker A. Reproducibility of reported measurements of sun exposure in a case-control study. Cancer Epidemiol Biomarkers Prev. 1998;7(10):857–863. [PubMed] [Google Scholar]
- 38.Rosso S, Miñarro R, Schraub S, et al. Reproducibility of skin characteristic measurements and reported sun exposure history. Int J Epidemiol. 2002;31(2):439–446. [PubMed] [Google Scholar]
- 39.Berwick M, Chen YT. Reliability of reported sunburn history in a case-control study of cutaneous malignant melanoma. Am J Epidemiol. 1995;141(11):1033–1037. doi: 10.1093/oxfordjournals.aje.a117367. [DOI] [PubMed] [Google Scholar]
- 40.Veierød MB, Parr CL, Lund E, et al. Reproducibility of self-reported melanoma risk factors in a large cohort study of Norwegian women. Melanoma Res. 2008;18(1):1–9. doi: 10.1097/CMR.0b013e3282f120d2. [DOI] [PubMed] [Google Scholar]
- 41.Weinstock MA, Colditz GA, Willett WC, et al. Recall (report) bias and reliability in the retrospective assessment of melanoma risk. Am J Epidemiol. 1991;133(3):240–245. doi: 10.1093/oxfordjournals.aje.a115868. [DOI] [PubMed] [Google Scholar]
- 42.Baxter AJ, Hughes MC, Kvaskoff M, et al. The Queensland Study of Melanoma: Environmental and Genetic Associations (Q-MEGA); study design, baseline characteristics, and repeatability of phenotype and sun exposure measures. Twin Res Hum Genet. 2008;11(2):183–196. doi: 10.1375/twin.11.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aitken JF, Green A, MacLennan R, et al. Comparability of surrogate and self-reported information on melanoma risk factors. Br J Cancer. 1993;67(5):1036–1041. doi: 10.1038/bjc.1993.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfahlberg AB, Gefeller O. Errors in assessing risk factors for melanoma: lack of reproducibility is the minor problem. Melanoma Res. 2008;18(4):300–301. doi: 10.1097/CMR.0b013e328308da8e. [DOI] [PubMed] [Google Scholar]
- 45.Walter SD, Marrett LD, From L, et al. The association of cutaneous malignant melanoma with the use of sunbeds and sunlamps. Am J Epidemiol. 1990;131(2):232–243. doi: 10.1093/oxfordjournals.aje.a115493. [DOI] [PubMed] [Google Scholar]
- 46.Parr CL, Hjartåker A, Laake P, et al. Recall bias in melanoma risk factors and measurement error effects: a nested case-control study within the Norwegian Women and Cancer Study. Am J Epidemiol. 2009;169(3):257–266. doi: 10.1093/aje/kwn363. [DOI] [PubMed] [Google Scholar]
- 47.Gefeller O. Invited commentary: recall bias in melanoma—much ado about almost nothing? Am J Epidemiol. 2009;169(3):267–270. doi: 10.1093/aje/kwn362. [DOI] [PubMed] [Google Scholar]
- 48.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses’ Health Study. Int J Epidemiol. 2006;35(6):1514–1521. doi: 10.1093/ije/dyl197. [DOI] [PubMed] [Google Scholar]
- 49.Aitken JF, Youl P, Green A, et al. Accuracy of case-reported family history of melanoma in Queensland, Australia. Melanoma Res. 1996;6(4):313–317. doi: 10.1097/00008390-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Green A, Battistutta D. Incidence and determinants of skin cancer in a high-risk Australian population. Int J Cancer. 1990;46(3):356–361. doi: 10.1002/ijc.2910460303. [DOI] [PubMed] [Google Scholar]
- 51.Aitken JF, Green AC, MacLennan R, et al. The Queensland Familial Melanoma Project: study design and characteristics of participants. Melanoma Res. 1996;6(2):155–165. doi: 10.1097/00008390-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Aitken J, Bain C, Ward M, et al. How accurate is self-reported family history of colorectal cancer? Am J Epidemiol. 1995;141(9):863–871. doi: 10.1093/oxfordjournals.aje.a117522. [DOI] [PubMed] [Google Scholar]
- 53.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292(12):1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 54.Witte JS, Gauderman WJ, Thomas DC. Asymptotic bias and efficiency in case-control studies of candidate genes and gene-environment interactions: basic family designs. Am J Epidemiol. 1999;149(8):693–705. doi: 10.1093/oxfordjournals.aje.a009877. [DOI] [PubMed] [Google Scholar]
- 55.Cui JS, Spurdle AB, Southey MC, et al. Regressive logistic and proportional hazards disease models for within-family analyses of measured genotypes, with application to a CYP17 polymorphism and breast cancer. Genet Epidemiol. 2003;24(3):161–172. doi: 10.1002/gepi.10222. [DOI] [PubMed] [Google Scholar]
- 56.Jenkins MA, Croitoru ME, Monga N, et al. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomarkers Prev. 2006;15(2):312–314. doi: 10.1158/1055-9965.EPI-05-0793. [DOI] [PubMed] [Google Scholar]
- 57.Antoniou AC, Pharoah PD, McMullan G, et al. A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. Br J Cancer. 2002;86(1):76–83. doi: 10.1038/sj.bjc.6600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dite GS, Jenkins MA, Southey MC, et al. Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst. 2003;95(6):448–457. doi: 10.1093/jnci/95.6.448. [DOI] [PubMed] [Google Scholar]
- 59.Gail MH, Pee D, Carroll R. Kin-cohort designs for gene characterization. J Natl Cancer Inst Monogr. 1999;1999(26):55–60. doi: 10.1093/oxfordjournals.jncimonographs.a024227. [DOI] [PubMed] [Google Scholar]
- 60.Olsen CM, Zens MS, Stukel TA, et al. Nevus density and melanoma risk in women: a pooled analysis to test the divergent pathway hypothesis. Int J Cancer. 2009;124(4):937–944. doi: 10.1002/ijc.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]