Abstract
The authors explored the association of cigarette smoking with tuberculosis incidence, recurrence, and mortality. A 14-year prospective cohort study (1992–2006) was carried out in 1,294,504 South Koreans. Participants were grouped by smoking history, and the authors assessed tuberculosis incidence, mortality, and recurrence risk for each group. Unadjusted and adjusted Cox proportional hazards models were used to investigate the association between smoking history and the 3 outcomes of interest, adjusting for age and alcohol use. Compared with never smokers, current smokers had increased mortality from tuberculosis among both men (adjusted hazard ratio (HR) = 1.6, 95% confidence interval (CI): 1.3, 2.0) and women (HR = 1.6, 95% CI: 1.0, 2.4). Current male smokers had greater risk of incident tuberculosis than former smokers (HR = 1.4, 95% CI: 1.3, 1.5), and risk among current smokers increased with number of cigarettes smoked daily. In females, cigarette smoking was not associated with incident tuberculosis. There was interaction between smoking and sex for incidence (P = 0.00047). The effect of smoking was generally reduced with adjustment for body mass index. Among men, the highest alcohol consumption category (≥100 g/day) was associated with risk of incident tuberculosis (HR = 1.5, 95% CI: 1.3, 1.7). This study provides longitudinal evidence that smoking increases risk of incident tuberculosis, mortality from tuberculosis, and tuberculosis recurrence.
Keywords: alcohol drinking, incidence, mortality, recurrence, smoking, tuberculosis
The possibility that smoking increases risk of tuberculosis has significant disease control implications, as there are presently 1.3 billion smokers in the world, and 9 million incident tuberculosis cases and 1.7 million tuberculosis deaths occur annually (1, 2). A number of studies, largely in developing countries, have indicated that smoking may contribute substantially to mortality from tuberculosis. Based on a case-control study conducted in India from 2001 to 2003, Jha et al. (3) estimated that 50% of tuberculosis deaths in men were due to smoking. Smoking has been associated with mortality from tuberculosis in other studies carried out in India (4) and in studies conducted in China (5), Hong Kong (6, 7), Taiwan (8), South Africa (9), and the United Kingdom (10). Several recent literature reviews and meta-analyses have synthesized evidence on risks of tuberculosis infection, disease, and mortality among smokers (11–16). These reviews found that cigarette smoking is associated with an approximate doubling of risk for a positive skin test, for having clinical evidence of disease, and for tuberculosis mortality (15). Few studies have addressed incidence, and most recent evidence on tuberculosis and smoking comes from countries where tuberculosis is still epidemic and health care is limited.
We explored the association between smoking and tuberculosis in a cohort study of 1.3 million people in South Korea, the Korean Cancer Prevention Study. Study participants are largely employed and middle-class but still subject to the persistently high risk of tuberculosis found in South Korea (annual incidence rate = 178/100,000 population in 2006) (2). South Korea provides screening and state-of-the-art treatment for tuberculosis to all of its citizens. Because of the richness of clinical data available, we were able to assess relations between smoking and the risks of incident tuberculosis, recurrent tuberculosis, and tuberculosis mortality.
MATERIALS AND METHODS
Study population
A description of the Korean Cancer Prevention Study has been previously published (17). The study includes 1,329,525 South Koreans aged 30–95 years who participated in 1 biennial National Health Insurance Corporation medical evaluation between 1992 and 1995. Enrollment took place in 1992 (n = 784,870), 1993 (n = 367,903), 1994 (n = 98,417), and 1995 (n = 78,335).
To avoid bias due to preexisting disease in assessing the association of smoking with tuberculosis mortality risk, we excluded 904 participants who died before 1993, as well as 34,117 participants with missing information on alcohol use or chest radiography. The final sample included 1,294,504 participants.
Because the study involved routinely collected data, consent was not specifically obtained. The institutional review boards of Yonsei University (Seoul, South Korea) and Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland) approved the study.
Data collection
Enrollees in the National Health Insurance Corporation undergo standardized biennial examinations at local hospitals.
Questionnaire survey.
During biennial visits made from 1992 through 1995 and from 1997 through 2000, participants reported their smoking habits, including number of cigarettes smoked per day and duration of cigarette smoking (in years) for current smokers, along with other health information, including alcohol consumption. History of past or prevalent pulmonary tuberculosis was also included in the questionnaire. Participants were asked whether they had ever had any of a series of diseases, including pulmonary tuberculosis, and whether they had ever received at least 3 months of treatment for tuberculosis.
Medical examination.
Participants had a chest radiograph (x-ray) taken at each visit, and findings were classified as normal, nonactive, light pulmonary tuberculosis, moderate pulmonary tuberculosis, severe pulmonary tuberculosis, suspected pulmonary tuberculosis, or nontuberculosis disease. Prevalent tuberculosis based on chest radiography was defined as a chest radiograph showing light, moderate, or severe pulmonary tuberculosis. Weight and height measurements were recorded while participants were wearing light clothing (17).
Medication data on pulmonary tuberculosis.
Between 2001 and 2005, the following 8 primary antituberculosis medications were listed in the medication database of the Health Insurance Review and Assessment Service: isoniazid, rifampin, ethambutol, pyrazinamide, prothionamide, cycloserine, p-aminosalicylic acid, and streptomycin. Patients receiving at least 3 medications were considered to have active tuberculosis. Of those receiving 3 medications, 73.8% received rifampin, isoniazid, and either ethambutol or pyrazinamide.
Outpatient and hospitalization records.
All hospitalization and outpatient records from 1993–2006 were captured by the National Health Insurance Corporation. However, not all outpatient records for 1993–1997 were collected.
Death data.
The vital status of all participants was tracked from 1993 to 2006. For deceased participants, the underlying cause of death as reported to the national statistical office was obtained.
Follow-up and outcome classification
The principal outcome variables were mortality from tuberculosis, incident tuberculosis, and recurrence of tuberculosis (Table 1). Mortality, outpatient, and hospitalization codes for tuberculosis were International Classification of Diseases, Tenth Revision, codes A15–A19.
Table 1.
Definitions of Tuberculosis Prevalence, Incidence, Recurrence, and Mortality Used in the Korean Cancer Prevention Study, 1992–2006
| Variable | Definition | Time Period |
| Prevalence upon entry | History of past or prevalent pulmonary TB as assessed by standard questionnaire. Participants were asked whether they had ever had any of a series of diseases, including pulmonary TB, and whether they had ever received at least 3 months of treatment. | 1992–2000 |
| Incidence | Limited to participants who provided information on their smoking history during 1997–2000. Four measures of incident TB were used: 1) at least 1 hospitalization for TB; 2) 2 or more outpatient visits for TB; 3) receipt of at least 3 anti-TB medications; and 4) at least 1 of the above indicators. | 2001–2005 |
| Recurrence | Limited to participants who provided information on their smoking history during 1997–2000. Participants with prior or prevalent TB reported at the initial visit (1992–1995) or a follow-up visit (1997–2000) were considered to have recurrent TB if they had any of the above indicators for incident TB between 2001 and 2005. | 2001–2005 |
| Mortality | Underlying cause of death as reported to the national statistical office. | 1993–2006 |
Abbreviation: TB, tuberculosis.
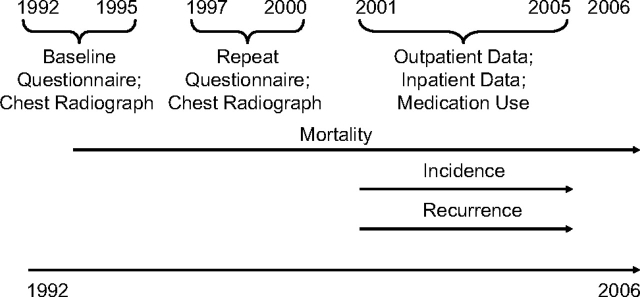
The time frames over which these outcomes could be assessed varied with data availability (Figure 1 and Table 1). Mortality data were available for the full follow-up period, while incidence and recurrence of tuberculosis could be tracked only during the years 2001–2005, when medication and outpatient visit data were available.
Figure 1.
Timeline for data collection in the Korean Cancer Prevention Study, 1992–2006.
Analysis of incidence and recurrence was limited to participants providing information on their smoking history during 1997–2000; this resulted in the exclusion of 129,121 participants from the full cohort used for analysis of mortality. Three measures of incident tuberculosis were constructed: at least 1 hospitalization for tuberculosis, 2 or more outpatient visits for tuberculosis, and receipt of at least 3 antituberculosis medications. We also created a summary measure for having at least 1 of these indicators. For recurrent tuberculosis, participants with prior or prevalent tuberculosis reported at the initial visit (1992–1995) or a follow-up visit (1997–2000) were considered to have recurrent tuberculosis if they had had any indicators of incident tuberculosis between 2001 and 2005.
Statistical analysis
Smoking status was classified as never, current, or former smoking, and current smokers were further classified as smokers of 1–9, 10–19, or ≥20 cigarettes per day. Body mass index (BMI; weight (kg)/height (m)2) was categorized as <18.5, 18.5–19.9, 20.0–21.4, 21.5–22.9, 23.0–24.9, 25.0–26.4, 26.5–27.9, 28.0–29.9, 30–31.9, or ≥32.0. Indicator variables corresponding to these categories were used for the analysis. Cox proportional hazards models were used to evaluate the association of baseline smoking status with risk of tuberculosis incidence or death. For death from tuberculosis, data collected between 1993 and 2006 were used. In analyzing incident tuberculosis from 2001 to 2005, our tuberculosis case definition included a report of tuberculosis at hospitalization, a record of more than 2 outpatient visits involving tuberculosis, and a record of use of at least 3 types of antituberculosis medication (Table 1).
Analyses were stratified by sex, and results were adjusted for age at enrollment (using age and the square of age) and alcohol consumption (in 5 categories based on grams consumed per day: 0, 1–24, 25–49, 50–99, and ≥100). Because BMI is associated with both smoking and respiratory disease mortality, we also adjusted for BMI in the main models, using the categorical variables. All Cox models were tested for and met the proportional hazards assumption. In a sensitivity analysis for mortality, we excluded the first 2 years of follow-up to assess the potential role of reverse causality (i.e., that smoking status changed because of diagnosis of tuberculosis). We assessed modification of the effect of smoking by including terms for the interaction of smoking category indicators with indicator variables for sex and alcohol drinking (in 3 categories: 0, 1–<50, and ≥50 g/day). All analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
The study population of 1,294,504 persons (827,333 men and 467,171 women) was largely middle-aged (median age, 45 years; interquartile range, 37–55). Cigarette smoking and alcohol consumption were common among men but not among women (Table 2); consistent with national data at the time, the majority of men were smokers (18). At enrollment, based on questionnaire reports and chest radiographs, 29,360 persons (23,106 men and 6,254 women) had current or previous tuberculosis. Among males, those with prevalent or past tuberculosis upon enrollment comprised 2.4% of current smokers and 3.8% of former smokers, while corresponding figures for women were 1.0% and 1.9%, respectively. During 14 years of follow-up, 827 deaths (659 in men and 168 in women) from tuberculosis occurred (crude mortality rate = 5.0 per 100,000 person-years).
Table 2.
General Characteristics of Participants by Tuberculosis Status Upon Enrollment, Korean Cancer Prevention Study, 1992–1995
| Men |
Women |
|||||||||||||||
| Total Population |
Ever Having Had TB at Enrollmenta |
Death From TBb |
Incident TBc |
Total Population |
Ever Having Had TB at Enrollmenta |
Death From TBb |
Incident TBc |
|||||||||
| No. | %d | No. | % | No. | % | No. | % | No. | %d | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||||||||
| 30–49 | 538,469 | 65.1 | 12,448 | 2.3 | 107 | 0.0 | 4,107 | 0.8 | 238,148 | 51.0 | 3,548 | 1.5 | 7 | 0.0 | 1,599 | 0.7 |
| 50–64 | 241,578 | 29.2 | 8,373 | 3.5 | 248 | 0.1 | 3,552 | 1.8 | 171,313 | 36.7 | 1,879 | 1.1 | 43 | 0.0 | 2,086 | 1.3 |
| ≥65 | 47,286 | 5.7 | 2,285 | 4.8 | 304 | 0.6 | 998 | 3.1 | 57,710 | 12.3 | 827 | 1.4 | 118 | 0.2 | 924 | 1.9 |
| Smoking status | ||||||||||||||||
| Nonsmoker | 172,040 | 20.8 | 4,736 | 2.8 | 107 | 0.1 | 1,561 | 1.0 | 437,885 | 93.7 | 5,873 | 1.3 | 119 | 0.0 | 4,200 | 1.0 |
| Ex-smoker | 171,732 | 20.8 | 6,565 | 3.8 | 188 | 0.1 | 1,846 | 1.2 | 9,997 | 2.1 | 191 | 1.9 | 22 | 0.2 | 144 | 1.7 |
| Current smoker | 483,561 | 58.4 | 11,805 | 2.4 | 364 | 0.1 | 5,250 | 1.2 | 19,289 | 4.1 | 190 | 1.0 | 27 | 0.1 | 265 | 1.6 |
| Alcohol drinking, g/day | ||||||||||||||||
| Nondrinker | 197,578 | 23.9 | 6,454 | 3.3 | 262 | 0.1 | 2,283 | 1.4 | 401,170 | 85.9 | 5,491 | 1.4 | 147 | 0.0 | 3,955 | 1.1 |
| <50 | 565,977 | 68.4 | 15,244 | 2.7 | 370 | 0.1 | 5,679 | 1.1 | 65,936 | 14.1 | 762 | 1.2 | 21 | 0.0 | 653 | 1.1 |
| ≥50 | 63,778 | 7.7 | 1,408 | 2.2 | 27 | 0.0 | 695 | 1.2 | 65 | 0.0 | 1 | 1.5 | 0 | 0.0 | 1 | 1.6 |
Abbreviation: TB, tuberculosis.
Information on prevalent TB by self-report and prevalent TB upon radiography was obtained from baseline data (1992–1995).
Information on TB death was obtained from follow-up data (1993–2006).
Information on incident TB was obtained from follow-up data (2001–2005).
The percentages in the “Total Population” columns were calculated vertically; all other percentages in the table were calculated horizontally.
Initially, we assessed the relations of smoking and alcohol consumption to mortality from tuberculosis (Table 3). Among both men and women, smoking was associated with increased mortality from tuberculosis. Risks were similar in current and former smokers, and there was no indication of increasing risk with increasing amount smoked daily for current smokers. The estimates dropped substantially with adjustment for BMI, particularly in current smokers. Risks were similar in males and females, without evidence of effect modification by sex.
Table 3.
Effect of Smoking and Alcohol Drinking on Mortality From Tuberculosis, Korean Cancer Prevention Study, 1993–2006
| Men |
Women |
|||||||||
| No. of Cases | Model 1a |
Model 2b |
No. of Cases | Model 1a |
Model 2b |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Smoking status | ||||||||||
| Nonsmoker | 107 | 1.0 | 1.0 | 119 | 1.0 | 1.0 | ||||
| Ex-smoker | 188 | 1.45 | 1.14, 1.85 | 1.37 | 1.07, 1.75 | 22 | 2.16 | 1.35, 3.46 | 1.98 | 1.21, 3.24 |
| Current smoker | 364 | 1.58 | 1.27, 1.97 | 1.21 | 0.96, 1.51 | 27 | 1.55 | 1.00, 2.41 | 1.08 | 0.67, 1.74 |
| Amount of smoking (among current smokers), cigarettes/day | ||||||||||
| 1–9 | 119 | 1.55 | 1.19, 2.00 | 1.17 | 0.89, 1.53 | 13 | 1.34 | 0.75, 2.41 | 1.02 | 0.55, 1.88 |
| 10–19 | 149 | 1.62 | 1.26, 2.08 | 1.24 | 0.96, 1.60 | 11 | 1.93 | 1.00, 3.70 | 1.10 | 0.50, 2.38 |
| ≥20 | 96 | 1.59 | 1.20, 2.10 | 1.22 | 0.91, 1.62 | 3 | 1.59 | 0.50, 5.02 | 1.33 | 0.42, 4.21 |
| Alcohol drinking, g/day | ||||||||||
| Nondrinker | 262 | 1.0 | 1.0 | 147 | 1.0 | 1.0 | ||||
| <50 | 370 | 0.83 | 0.70, 0.97 | 0.91 | 0.77, 1.08 | 21 | 0.72 | 0.45, 1.15 | 0.78 | 0.48, 1.26 |
| ≥50 | 27 | 1.00 | 0.66, 1.51 | 1.31 | 0.86, 1.97 | No data | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Results were adjusted for age and age squared.
Results were adjusted for age, age squared, and body mass index.
Among men, we found that smoking was associated with incident tuberculosis during 2001–2005 (Table 4). The findings were similar for each of the 3 separate indicators of incident tuberculosis (see Web Tables 1 and 2, which are posted on the Journal’s Web site (http://aje.oxfordjournals.org/)). Current smokers had greater risk of incident tuberculosis than former smokers, and risk among current smokers increased with amount smoked daily. In men, the hazard ratios for current smokers were somewhat lower with adjustment for BMI. Drinking at least 50 g of alcohol daily increased the risk of incident tuberculosis, and persons consuming at least 100 g daily had a 50% increased risk. There was no indication of interaction between cigarette smoking and alcohol consumption (P for interaction = 0.368). By contrast, in women, cigarette smoking was not associated with incident tuberculosis (Table 4), and there was significant interaction between smoking and sex for risk of incident tuberculosis (P for interaction = 0.00047).
Table 4.
Effect of Smoking and Alcohol Drinking on Incident Tuberculosis in Men and Women, Korean Cancer Prevention Study, 2001–2005
| Men |
Women |
|||||||||
| No. of Cases | Model 1a |
Model 2b |
No. of Cases | Model 1a |
Model 2b |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Smoking status | ||||||||||
| Nonsmoker | 1,451 | 1.0 | 1.0 | 4,179 | 1.0 | 1.0 | ||||
| Ex-smoker | 2,112 | 1.2 | 1.1, 1.3 | 1.2 | 1.1, 1.3 | 155 | 1.1 | 0.9, 1.3 | 1.1 | 0.9, 1.3 |
| Current smoker | 5,094 | 1.4 | 1.3, 1.5 | 1.2 | 1.2, 1.3 | 275 | 1.1 | 1.0, 1.2 | 1.0 | 0.9, 1.1 |
| Amount of smokingc (among current smokers), cigarettes/day | ||||||||||
| 1–9 | 1,064 | 1.2 | 1.1, 1.3 | 1.1 | 1.0, 1.2 | No data | ||||
| 10–19 | 2,497 | 1.4 | 1.3, 1.5 | 1.2 | 1.2, 1.3 | No data | ||||
| ≥20 | 1,527 | 1.5 | 1.4, 1.6 | 1.4 | 1.3, 1.5 | No data | ||||
| Alcohol drinking, g/day | ||||||||||
| Nondrinker | 2,045 | 1.0 | 1.0 | 3,829 | 1.0 | 1.0 | ||||
| <25 | 5,087 | 0.9 | 0.9, 1.0 | 1.0 | 0.9, 1.1 | 778 | 1.0 | 0.9, 1.1 | 1.0 | 1.0, 1.1 |
| 25–49.9 | 722 | 1.0 | 0.9, 1.1 | 1.1 | 1.0, 1.2 | No data | ||||
| 50–99.9 | 607 | 1.2 | 1.1, 1.3 | 1.3 | 1.2, 1.4 | 2 | 0.6 | 0.2, 2.5 | 0.6 | 0.2, 2.5 |
| ≥100.0 | 196 | 1.5 | 1.3, 1.7 | 1.6 | 1.4, 1.9 | No data | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Results were adjusted for age and age squared.
Results were adjusted for age, age squared, and body mass index.
Six cases among men had missing information on amount of smoking.
We also examined risk of tuberculosis recurrence among 67,003 participants (52,503 men and 14,500 women) with prior tuberculosis on the basis of questionnaires and chest radiographs completed in 1992–1995 or 1997–2000. During follow-up between 2001 and 2005, 5,546 recurrent tuberculosis diagnoses were reported (4,529 in men and 1,017 in women) (Table 5). Estimates were slightly attenuated by BMI adjustment. Among men and women, smoking was associated with increased risk of recurrence of tuberculosis, though the increase was not significant for women. Among men, consumption of at least 50 g of alcohol daily increased risk of recurrence.
Table 5.
Effect of Smoking and Alcohol Drinking on Risk of Recurrent Tuberculosis in Men and Women, Korean Cancer Prevention Study, 2001–2005
| Men |
Women |
|||||||||
| No. of Cases | Model 1a |
Model 2b |
No. of Cases | Model 1a |
Model 2b |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Smoking status | ||||||||||
| Nonsmoker | 720 | 1.0 | 1.0 | 942 | 1.0 | 1.0 | ||||
| Ex-smoker | 1,400 | 1.3 | 1.1, 1.4 | 1.3 | 1.2, 1.4 | 34 | 1.2 | 0.9, 1.8 | 1.2 | 0.9, 1.8 |
| Current smoker | 2,409 | 1.3 | 1.2, 1.4 | 1.2 | 1.1, 1.3 | 41 | 1.2 | 0.8, 1.6 | 1.0 | 0.7, 1.4 |
| Amount of smokingc (among current smokers), cigarettes/day | ||||||||||
| 1–9 | 571 | 1.3 | 1.2, 1.5 | 1.2 | 1.1, 1.4 | 22 | 1.1 | 0.7, 1.7 | 0.9 | 0.6, 1.4 |
| 10–19 | 1,188 | 1.3 | 1.2, 1.4 | 1.2 | 1.1, 1.3 | 12 | 1.1 | 0.6, 2.0 | 0.8 | 0.5, 1.6 |
| ≥20 | 645 | 1.4 | 1.2, 1.5 | 1.3 | 1.2, 1.5 | 6 | 1.6 | 0.7, 3.6 | 1.4 | 0.6, 3.2 |
| Alcohol drinking, g/day | ||||||||||
| Nondrinker | 1,139 | 1.0 | 1.0 | 843 | 1.0 | 1.0 | ||||
| <50 | 3,008 | 0.9 | 0.9, 1.0 | 1.0 | 0.9, 1.1 | 174 | 1.0 | 0.8, 1.2 | 1.0 | 0.9, 1.2 |
| ≥50 | 382 | 1.2 | 1.0, 1.3 | 1.3 | 1.1, 1.4 | No data | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Results were adjusted for age and age squared.
Results were adjusted for age, age squared, and body mass index.
Five cases among men and 1 case among women had missing information on amount of smoking.
DISCUSSION
In this prospective cohort study of over 1.3 million South Koreans, male current cigarette smokers had a 40% increased risk of incident tuberculosis compared with nonsmokers and were 55% more likely to die of tuberculosis. In women, findings for mortality risk were similar, but current smoking was not associated with incidence. Former smokers, both males and females, were at increased risk of tuberculosis mortality and incidence. Smokers also had greater risk of recurrence.
After receiving little emphasis for decades, the effect of smoking on risk of tuberculosis has recently been addressed in several large studies and in systematic reviews. Overall, the various recent syntheses of evidence concur in finding that smoking is associated with increased risk of tuberculosis, regardless of the disease outcome measure used: infection, clinical disease, or mortality (12, 13, 15). Using a prospective cohort study design, we have added to this evidence by demonstrating an association between smoking and tuberculosis incidence among men and confirming prior findings on mortality and recurrence.
Only 2 other cohort studies have addressed the incidence of tuberculosis. Leung et al. (7) tracked incidence of tuberculosis in a cohort of 42,655 elderly persons in Hong Kong. The rate of case notification was 6 times higher in current smokers than in never smokers; the adjusted hazard ratio for pulmonary tuberculosis was 2.9 (95% confidence interval: 2.0, 4.1). The greater risk in the Hong Kong cohort as compared with the South Korean cohort may reflect its greater age or other differences between the 2 populations. In a cohort study of 17,699 participants in a national survey in Taiwan, current smoking was associated with a near-doubling of risk of incident tuberculosis (8).
Patients with a history of a tuberculosis diagnosis are at risk of a subsequent tuberculosis event (19), and smoking has been reported to be an independent risk factor for relapse in studies carried out in South India (20) and Brazil (21). In men, we found significantly increased risks of recurrent tuberculosis among current smokers and former smokers (Table 5). The risks were similar in women, though not statistically significant. While we cannot determine whether smoking increases risk of treatment failure or reactivation of a second infection with Mycobacterium tuberculosis, the heightened risk among smokers needs to be recognized in providing treatment and follow-up for tuberculosis.
A smoking-associated increase in risk of mortality could reflect an effect of smoking on incidence, risk of recurrence, or disease severity. We found that smoking was associated with increased risk of both incidence and recurrence, but we could not address disease severity. Smoking has widespread effects on lung structure and function and affects host defenses both in the lung and systemically; therefore, it could plausibly act to increase incidence or worsen the prognosis of tuberculosis (22). Smoking is the dominant cause of chronic obstructive pulmonary disease, and tuberculosis is associated with airflow obstruction (23). Smoking-caused obstruction in conjunction with obstruction related to tuberculosis may worsen prognosis. With regard to incidence, smoking increases the risk of becoming infected with M. tuberculosis (24), and recently infected patients are at greatest risk of progression to active disease (25). Smoking has been causally associated with the risk of other respiratory infections, including pneumonia and influenza (26). Postulated mechanisms include effects on clearance mechanisms and immunologic responses to inhaled pathogens, as well as the structural changes in the lung caused by smoking.
Regardless of the underlying mechanisms by which smoking increases incidence of tuberculosis and affects its natural history, our study and others document that smoking is 1 modifiable contributor to mortality. In men and women, smoking was associated with an approximately 50% increase in risk of mortality from tuberculosis. Estimates in other studies have been as high as a 4-fold increase (5), although there is significant heterogeneity in the available estimates for mortality (15). Thus, even in a country as affluent as South Korea, which has a national health care system, we estimate from this study's data (assuming a smoking prevalence of 60% and a hazard ratio of 1.5) that approximately 25% of tuberculosis deaths in South Korean men can be attributed to smoking (27).
We found that sex modified the risk of incident tuberculosis associated with smoking, such that women had no increase in risk while male current smokers had an approximately 50% increase in risk. However, male and female current smokers had equally heightened risks of death from tuberculosis (Table 2). Epidemiologic characteristics of tuberculosis differ by sex, with men having a higher prevalence at all ages in surveys conducted around the world (28, 29). Thus, the effect modification of incidence by sex could reflect the higher background rate of dormant infection in males. The comparability of mortality risks associated with smoking in males and females is consistent with this hypothesis. Other studies published to date have not addressed modification of the effect of smoking by sex.
Alcohol abuse, which is more frequent among smokers, is a well-known risk factor for tuberculosis (30). In the present study, heavy alcohol consumption was found to be significantly associated with tuberculosis incidence in men, though not with mortality. However, sample size posed a limitation in the analyses of mortality and alcohol use. We did not find any indication of interaction between smoking and alcohol consumption.
Potential limitations of this study primarily reflect the need to rely on self-reports for data on tobacco and alcohol use and on medical database information for establishing the diagnosis of tuberculosis, leading to concern about potential misclassification of exposures and outcomes. Information on smoking status was updated, however, and self-reporting of smoking in South Korea has been shown to be valid when compared with cotinine measurements (31). For establishing the occurrence of incident tuberculosis, we used 3 different indicators based on outcomes unlikely to be subject to misclassification and found similar results (Table 1). Our definitions were intended to exclude persons being evaluated for tuberculosis who did not actually have the disease. Consequently, for incident disease, we required at least 2 outpatient visits or the prescription of at least 3 antituberculosis agents. For establishing a diagnosis of past tuberculosis, we relied partly on self-reports, which may be subject to misclassification.
Smokers are more likely to stop smoking when they develop respiratory symptoms above and beyond those normally associated with cigarette smoking (32). Thus, assessments of smoking among patients recently diagnosed with tuberculosis may underestimate the association between current smoking and active tuberculosis disease. Of those participants with prevalent or past tuberculosis upon enrollment, a greater percentage were former smokers (28.9%) and a lower percentage were current smokers (50.1%) compared with those without such a history (20.5% and 58.7%, respectively). This pattern suggests that reverse causality is present, in which smokers stop smoking when tuberculosis symptoms arise or a tuberculosis diagnosis is confirmed.
We conducted the analyses with and without adjustment for BMI, a covariate not considered in other studies of smoking and tuberculosis. We had previously shown a strong inverse association between respiratory mortality and BMI in this cohort (17), an association probably reflecting reverse causation in part, and other investigators have found a similar and quite strong inverse association for tuberculosis mortality specifically (33, 34). In a study carried out in Norway, however, Tverdal (33) did not find an indication of reverse causality; the inverse association of BMI with tuberculosis incidence persisted with little change through the longest duration of follow-up, 10–19 years after enrollment. While the basis for the association with BMI is uncertain, smokers weigh somewhat less than nonsmokers, and consequently low BMI is a potential confounder. Additionally, the effect of smoking on tuberculosis risk could be mediated, at least in part, by the lower BMI of smokers.
In our study, BMI adjustment had a greater impact for mortality than for incidence, which is consistent with a possible role of reverse causation in the association between BMI and respiratory mortality (Tables 3 and 4). There is also a potential for confounding. We observed that relations of BMI with risk of both tuberculosis incidence and tuberculosis mortality were approximately linear, and among men and women, current smokers had average BMIs 0.36 and 0.52 units lower than those of never smokers, respectively. Decreases in effect estimates with BMI adjustment might reflect either confounding by BMI or mediation of smoking's effect by lowering BMI. The adjusted models provide an assessment of the sensitivity of our estimates of the effect of smoking, assuming that there is either confounding or an indirect effect of smoking. Regardless, there is a persistent effect of smoking on incidence with adjustment. The association of BMI with risk of tuberculosis needs further research.
Supplementary Material
Acknowledgments
Author affiliations: Institute for Health Promotion, Graduate School of Public Health, Yonsei University, Seoul, South Korea (Sun Ha Jee, Jaeseong Jo); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Jonathan E. Golub); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Jonathan E. Golub); National Health Insurance Corporation, Seoul, South Korea (Il Su Park); Department of Preventive Medicine and Public Health, College of Medicine, Yonsei University, Seoul, South Korea (Heechoul Ohrr); and Department of Preventive Medicine and Institute for Global Health, Keck School of Medicine, University of Southern California, Los Angeles, California (Jonathan M. Samet).
This study was partially supported by the Seoul Research and Business Development Program (grant 10526), Seoul, South Korea. Dr. Jonathan E. Golub was supported by National Institutes of Health grant AI066994.
The authors thank the staff of the Korean National Health Insurance Corporation. They also thank Charlotte Gerczak and Athena Foong for editorial assistance.
The Johns Hopkins Bloomberg School of Public Health is one of 5 partner organizations in the Bloomberg Initiative; its mission is to promote freedom from smoking worldwide.
Conflict of interest: none declared.
Glossary
Abbreviation
- BMI
body mass index
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030 [electronic article] PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control 2008: Surveillance, Planning, Financing. Geneva, Switzerland: World Health Organization; 2008. (Publication no. WHO/HTM/TB/2008.393) [Google Scholar]
- 3.Jha P, Jacob B, Gajalakshmi V, et al. A nationally representative case-control study of smoking and death in India. N Engl J Med. 2008;358(11):1137–1147. doi: 10.1056/NEJMsa0707719. [DOI] [PubMed] [Google Scholar]
- 4.Gajalakshmi V, Peto R, Kanaka TS, et al. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet. 2003;362(9383):507–515. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317(7170):1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam TH, Ho SY, Hedley AJ, et al. Mortality and smoking in Hong Kong: case-control study of all adult deaths in 1998 [electronic article] BMJ. 2001;323(7309):361. doi: 10.1136/bmj.323.7309.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung CC, Li T, Lam TH, et al. Smoking and tuberculosis among the elderly in Hong Kong. Am J Respir Crit Care Med. 2004;170(9):1027–1033. doi: 10.1164/rccm.200404-512OC. [DOI] [PubMed] [Google Scholar]
- 8.Lin HH, Ezzati M, Chang HY, et al. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med. 2009;180(5):475–480. doi: 10.1164/rccm.200904-0549OC. [DOI] [PubMed] [Google Scholar]
- 9.Sitas F, Urban M, Bradshaw D, et al. Tobacco attributable deaths in South Africa. Tob Control. 2004;13(4):396–399. doi: 10.1136/tc.2004.007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll R, Peto R, Wheatley K, et al. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309(6959):901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai M, Mohan A, Dheda K, et al. Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther. 2007;5(3):385–391. doi: 10.1586/14787210.5.3.385. [DOI] [PubMed] [Google Scholar]
- 12.Slama K, Chiang CY, Enarson DA, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11(10):1049–1061. [PubMed] [Google Scholar]
- 13.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis [electronic article] PLoS Med. 2007;4(1):e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies PD, Yew WW, Ganguly D, et al. Smoking and tuberculosis: the epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg. 2006;100(4):291–298. doi: 10.1016/j.trstmh.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Bates MN, Khalakdina A, Pai M, et al. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167(4):335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 16.Chiang CY, Slama K, Enarson DA. Associations between tobacco and tuberculosis. Int J Tuberc Lung Dis. 2007;11(3):258–262. [PubMed] [Google Scholar]
- 17.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355(8):779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 18.Chung MH, Chung KK, Chung CS, et al. Health-related behaviors in Korea: smoking, drinking, and perinatal care. Asia Pac J Public Health. 1992–1993;6(1):10–15. doi: 10.1177/101053959200600105. [DOI] [PubMed] [Google Scholar]
- 19.Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis. 2007;11(8):828–837. [PubMed] [Google Scholar]
- 20.Thomas A, Gopi PG, Santha T, et al. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis. 2005;9(5):556–561. [PubMed] [Google Scholar]
- 21.d'Arc Lyra Batista J, de Fátima Pessoa Militão de Albuquerque M, de Alencar Ximenes RA, et al. Smoking increases the risk of relapse after successful tuberculosis treatment. Int J Epidemiol. 2008;37(4):841–851. doi: 10.1093/ije/dyn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Office of the Surgeon General, US Public Health Service. The Health Effects of Active Smoking: A Report of the Surgeon General. Washington, DC: US GPO; 2004. [Google Scholar]
- 23.Menezes AM, Hallal PC, Perez-Padilla R, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30(6):1180–1185. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 24.den Boon S, van Lill SW, Borgdorff MW, et al. Association between smoking and tuberculosis infection: a population survey in a high tuberculosis incidence area. Thorax. 2005;60(7):555–557. doi: 10.1136/thx.2004.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comstock GW. Frost revisited: the modern epidemiology of tuberculosis. Am J Epidemiol. 1975;101(5):363–382. doi: 10.1093/oxfordjournals.aje.a112105. [DOI] [PubMed] [Google Scholar]
- 26.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Rothman KJ, Lash TL. Measures of effects and measures of association. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 51–70. [Google Scholar]
- 28.Borgdorff MW, Nagelkerke NJ, Dye C, et al. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;4(2):123–132. [PubMed] [Google Scholar]
- 29.Ottmani SE, Uplekar MW. Gender and TB: pointers from routine records and reports. Int J Tuberc Lung Dis. 2008;12(7):827–828. [PubMed] [Google Scholar]
- 30.Friedman LN, Williams MT, Singh TP, et al. Tuberculosis, AIDS, and death among substance abusers on welfare in New York City. N Engl J Med. 1996;334(13):828–833. doi: 10.1056/NEJM199603283341304. [DOI] [PubMed] [Google Scholar]
- 31.Park SS, Lee JY, Cho SI. Validity of expired carbon monoxide and urine cotinine using dipstick method to assess smoking status [in Korean] J Prev Med Public Health. 2007;40(4):297–304. doi: 10.3961/jpmph.2007.40.4.297. [DOI] [PubMed] [Google Scholar]
- 32.Langhammer A, Johnsen R, Holmen J, et al. Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study (HUNT) J Epidemiol Community Health. 2000;54(12):917–922. doi: 10.1136/jech.54.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis. 1986;69(5):355–362. [PubMed] [Google Scholar]
- 34.Singh M, Mynak ML, Kumar L, et al. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child. 2005;90(6):624–628. doi: 10.1136/adc.2003.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.