Abstract
Vulvovaginal atrophy (VVA) is a common and underreported condition associated with decreased estrogenization of the vaginal tissue. Symptoms include dryness, irritation, soreness, and dyspareunia with urinary frequency, urgency, and urge incontinence. It can occur at any time in a woman's life cycle, although more commonly in the postmenopausal phase, during which the prevalence is close to 50%. Clinical findings include the presence of pale and dry vulvovaginal mucosa with petechiae. Vaginal rugae disappear, and the cervix may become flush with the vaginal wall. A vaginal pH of 4.6 or more supports the diagnosis of VVA. Even while taking systemic estrogen, 10% to 20% of women may still have residual VVA symptoms. Breast cancer treatment increases the prevalence of VVA because the surgical, endocrine, and chemotherapeutic agents used in its treatment can cause or exacerbate VVA. Local estrogen treatment for this group of women remains controversial.
AI = aromatase inhibitor; CI = confidence interval; ER = estrogen receptor; HT = hormone therapy; SERM = selective ER modulator; VMI = vaginal maturation index; VVA = vulvovaginal atrophy
Vulvovaginal atrophy (VVA) is a common condition, especially in postmenopausal women. Vaginal atrophy, atrophic vaginitis, and urogenital atrophy are other terms used to describe this constellation of symptoms associated with decreased estrogenization of the vulvovaginal tissue. Although treatment with topical estrogen is effective in alleviating symptoms, women frequently do not report symptoms and thus go untreated.1,2
Common symptoms include vaginal dryness, irritation, postcoital bleeding, and soreness. These symptoms may be associated with vaginal discharge and dyspareunia. Urinary symptoms associated with VVA include frequency, urgency, and urge incontinence.
PREVALENCE
Vulvovaginal atrophy can occur at any time in a woman's life cycle, although it is more common in the postmenopausal phase, a time of hypoestrogenism. Other causes of a hypoestrogenic state include lactation, various breast cancer treatments, and use of certain medications. In situations other than menopause, VVA may resolve spontaneously when estrogen levels are restored.
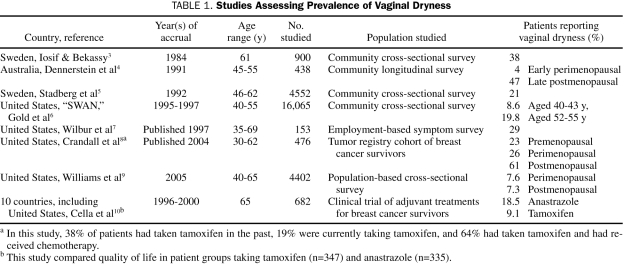
Numerous retrospective studies have evaluated the prevalence of symptoms of VVA (Table 1).3-10 Although these studies differ in type of symptoms elicited, study design, and study population, they provide a range of estimates of VVA prevalence. They all used self-reported symptoms of vaginal dryness to determine the prevalence of VVA. In general, the prevalence ranged from about 4% in the early premenopausal groups to 47% in the late postmenopausal group.
TABLE 1.
Studies Assessing Prevalence of Vaginal Dryness
The prevalence of VVA in some subgroups of women can be much higher. In a cohort of breast cancer survivors, vaginal dryness was present in 23.4% of the premenopausal patients and in 61.5% of the postmenopausal patients.8
PHYSIOLOGY
Vulvovaginal atrophy occurs under conditions of hypoestrogenism. In the premenopausal state, estradiol levels fluctuate from 10 to 800 pg/mL (to convert to pmol/L, multiply by 3.671),11 depending on when measured during the cycle. In the postmenopausal state, estradiol levels are typically less than 30 pg/mL. After menopause, circulating estradiol derives from estrone, which is peripherally converted in adipose tissue from adrenal androstenedione.
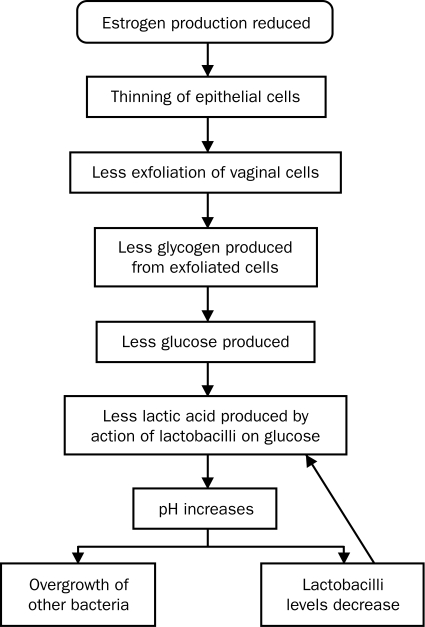
The vaginal epithelium is a stratified squamous epithelium, which until menopause is moist and thick with rugae. At menopause, with declining levels of estrogen, the vaginal epithelium thins. Fewer epithelial cells result in less exfoliation of cells into the vagina. As epithelial cells exfoliate and die, they release glycogen, which is hydrolyzed to glucose. Glucose, in turn, is broken down into lactic acid by the action of lactobacillus, a normal vaginal commensal organism. Without this cascade, the pH in the vagina rises, resulting in a loss of lactobacilli and an overgrowth of other bacteria, including group B streptococcus, staphylococci, coliforms, and diphtheroids12 (Figure 1). These bacteria can cause symptomatic vaginal infections and inflammation. After menopause, the elasticity of the vagina is reduced and connective tissue increases.13 A decline in estrogen level causes a decrease in vaginal blood flow and a decrease in vaginal lubrication. These changes can be reversed by the use of estrogens.14,15
FIGURE 1.
Proposed cascade-of-effects mechanism for vulvovaginal atrophy.
The effects of endogenous estrogens on vulvovaginal tissues are mediated through estrogen receptors (ERs) α and β, found at sites throughout the urogenital area, including the vagina, vulva, labia, urethra, and bladder trigone. These sites, in turn, regulate transcription at specific areas on the DNA.16
SYMPTOMS
The initial symptom is often lack of lubrication during intercourse. Eventually, persistent vaginal dryness may occur. Thinning of the epithelial lining may also cause pruritus, soreness, and a stinging pain in the vaginal and vulvar area, which, in turn, may further contribute to dyspareunia. Vaginal spotting, due to small tears in the vaginal epithelium, may also occur. Women with VVA may report a thin yellow or grey watery discharge secondary to the rise in pH that accompanies VVA.17
Women with VVA often report symptoms such as urgency, frequency, nocturia, and urge incontinence. Urinalysis may show microscopic hematuria. Recurrent urinary tract infections can also result. Stress incontinence is commonly found in this age group of women, but current evidence suggests it is not directly attributable to VVA.18
Women do not always report their symptoms of VVA. They are more likely to report vaginal discharge and urinary urgency but are less likely to report vaginal itching, soreness, or dyspareunia. Women may not report symptoms because they are self-treating, feel the symptoms are not important enough, or are embarassed.19
CLINICAL FINDINGS
Clinical findings include atrophy of the labia majora and vaginal introitus. The labia minora may recede. Vulvar and vaginal mucosae may appear pale, shiny, and dry; if there is inflammation, they may appear reddened or pale with petechiae. Vaginal rugae disappear, and the cervix may become flush with the vaginal wall. Vaginal shortening and narrowing tend to occur.20
A thin watery yellow vaginal discharge may be observed. A urethral caruncle, a small, soft, smooth friable red outgrowth along the edge of the urethra, may develop.
CLINICAL TESTS
The diagnosis of VVA is a clinical one. However, 2 tests may be used to support the diagnosis: a vaginal pH and a vaginal maturation index (VMI). To assess pH, a piece of litmus paper is placed on the lateral vaginal wall until moistened. A pH of 4.6 or greater indicates VVA, assuming the patient does not have bacterial vaginosis. Premenopausal women without VVA typically have a pH of 4.5 or less.12
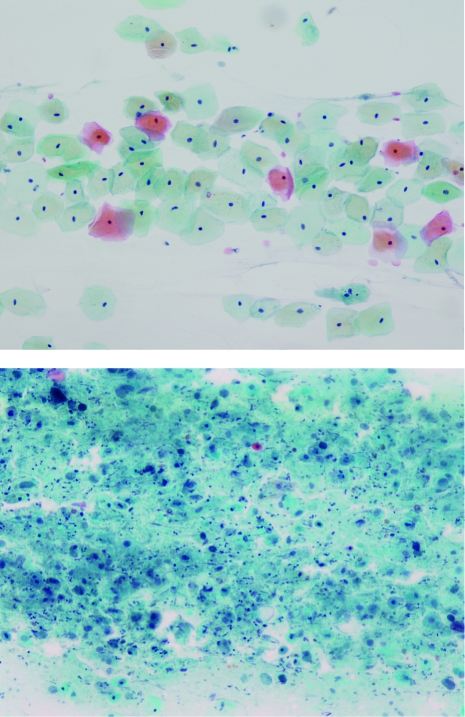
The VMI (Figure 2) is the criterion standard for VVA confirmation but is generally not used or needed in clinical practice. This test assesses the relative proportion of parabasal, intermediate, and superficial vaginal epithelial cell types. In premenopausal women, greater than 15% superficial cells would be considered normal; however, in postmenopausal women with VVA, the typical proportion would be less than 5%.
FIGURE 2.
Photomicrographs of superficial and intermediate cells (top) and atrophic cells (bottom). (Papanicolaou stain; original magnification x20). Courtesy of the Mayo Cytopathology Laboratory.
DIFFERENTIAL DIAGNOSIS
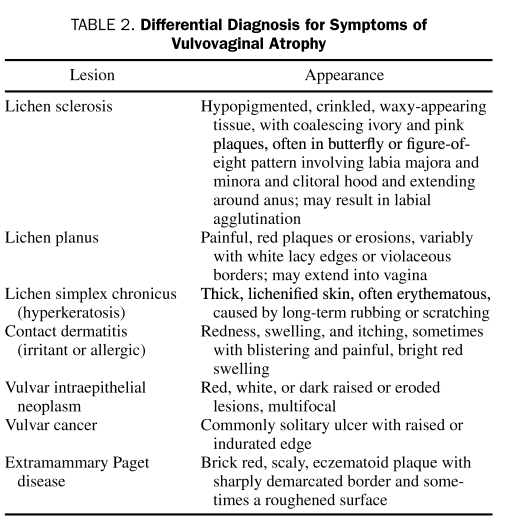
The differential diagnosis includes other conditions that cause chronic vaginal and vulvar itching, discharge, or pain (eg, vaginal infections, irritants, and vulvovaginal dermatoses). Vaginal infections can be caused by bacteria, viruses, protozoa, and fungi. The 3 most common vaginal infections are Candida vulvovaginitis, bacterial vaginosis, and trichomoniasis. Bacterial vaginosis may result from atrophic changes in the vagina. Irritants that can cause chronic vaginal itch include perfumes, any locally applied lubricant or moisturizer, and soaps. Vulvovaginal dermatoses, including lichen sclerosus, lichen planus, and lichen simplex chronicus, may cause similar symptoms.21 Cancer and precancerous lesions, including vulvar intraepithelial neoplasm, vulvar cancer, and extramammary Paget disease, are in the differential diagnosis of any localized areas of redness, thickening, or ulceration (Table 2). Biopsy should be performed if a malignancy is suspected or if the diagnosis is unclear.
TABLE 2.
Differential Diagnosis for Symptoms of Vulvovaginal Atrophy
TREATMENT
Nonhormonal Treatments
Current over-the-counter treatments include nonhormonal vaginal moisturizers for VVA symptoms and lubricants for dyspareunia. Vaginal moisturizers, which are water based, are available as liquids, gels, or ovules inserted every few days. Vaginal moisturizers can be safely used long term, but they need to be used regularly for optimal effect.
Vaginal lubricants are shorter acting than moisturizers and are applied at the time of sexual activity to reduce dyspareunia. They can be either water or silicone based, with the water-based products being the most widely available. They are applied to the vaginal opening and/or to the penis and often require repeated application during sexual activity. Silicone-based lubricants require application of only a very small amount and last longer; however, they can interfere with erectile function in male partners. The choice of lubricant may depend on individual preferences and product availability.
Hormonal Treatments
On the basis of an evidence-based review of clinical trials examining available low-dose vaginal estrogen preparations for the treatment of VVA in postmenopausal women, The North American Menopause Society (NAMS) 2007 position statement noted that “the choice of therapy should be guided by clinical experience and patient preference.”22 NAMS also stated it is generally unnecessary to prescribe a progestogen in combination with low-dose vaginal estrogen to prevent endometrial hyperplasia or cancer.
On the basis of a meta-analysis of 19 randomized clinical trials involving 4162 postmenopausal women, the 2006 Cochrane Database of Systematic Reviews concluded that vaginal estrogen is an effective treatment for VVA23 and that all forms, whether cream, ring, or tablet, appeared to relieve symptoms more effectively than nonhormonal gels and placebo. Differences observed between the treatments were in participant preferences.
Therefore, for symptomatic vaginal atrophy that does not respond to self-care measures, estrogen treatment is the standard of care, typically with vaginally administered local estrogens. Prescription vaginal estrogens are available as either estradiol or conjugated estrogens. In some countries outside the United States, vaginal estriol is also available.
SYSTEMIC ABSORPTION OF VAGINAL HORMONAL PREPARATIONS
An important concern about treatment safety relates to the extent of systemic absorption of vaginal estrogens. The conclusion from several studies comparing different doses of estradiol vaginal tablets24,25 or different vaginal estrogen preparations (conjugated estrogens and estradiol vaginal tablets)26 is that systemic absorption occurs, but to a limited extent. Labrie et al26 showed that levels of estradiol increased on average from a baseline (pretreatment) level of 3 pg/mL to 17 pg/mL on day 7 of treatment for both estradiol vaginal tablets (25 μg) and conjugated estrogen cream (0.625 mg). Nilsson and Heimer24 showed that, although plasma estradiol concentration diminished by the 14th day of daily treatment with 10 or 25 μg of vaginal estradiol, it was still statistically significantly higher than pretreatment levels. Some evidence shows that estradiol levels diminish over time when vaginal estrogens are used consistently.24,25
Although vaginal estrogens applied as a cream, vaginal tablets, or a low-dose vaginal ring are systemically absorbed, the rise in serum estrogen levels appears to remain well below premenopausal levels. Nonetheless, this may be of concern to women with a history of breast or other hormonally sensitive cancers
PRACTICAL ISSUES
Because all low-dose vaginal estrogens appear comparable in efficacy for the treatment of VVA, the choice of estrogen formulation is determined by the clinician and by each woman's preferences. Estrogen creams are currently the least costly and most widely used but require commitment to regular use for sustained effect. Dosing vaginal creams can be confusing because the dose of active estrogen cream is specified in milligrams, the dose of base cream in grams, and applicator volume in proportions. A simplified approach to dosing is provided in Table 3. The estradiol tablet is preferred by some to avoid the messiness of cream. The estradiol ring is long acting and requires less sustained effort to use; however, it requires dexterity to insert and remove and needs to be replaced every 3 months. The presence of a cystocele or rectocele may cause ring displacement.
TABLE 3.
Vaginal Estrogen Products
SYSTEMIC ESTROGEN AGENTS AND VVA
Systemic estrogen therapy, in the form of patches, oral agents, or a higher-dose vaginal ring, is sometimes used for VVA, especially when the patient also has hot flashes. However, 10% to 20% of women may have residual VVA symptoms even while taking systemic estrogen.27 These women will require administration of local vaginal estrogens alone or along with systemic therapy for relief of VVA symptoms.
Two studies have shown that oral hormone therapy (HT) may worsen symptoms of urinary incontinence. The Heart and Estrogen-Progestin Replacement Study found a higher risk of both urge (odds ratio, 1.5; 95% confidence interval [CI], 1.2-1.8; P<.001) and stress incontinence (odds ratio, 1.7; 95% CI, 1.5-2.1; P<.001) in the hormone-treated group vs the placebo group throughout the treatment period.28
It has been suggested that urge incontinence worsened with HT because progesterone was a component. However, in the Women's Health Initiative, 3 treatment groups were evaluated for urge incontinence (conjugated estrogens alone, conjugated estrogens with progesterone, and placebo). Women given conjugated estrogens alone were at increased risk of developing urge incontinence compared with those receiving placebo (relative risk, 1.32; 95% CI, 1.10-1.58), whereas the women given combination conjugated estrogens with progesterone were not (relative risk, 1.15; 95% CI, 0.99-1.34).29 Thus, whether oral HT adversely affects urge urinary incontinence remains unclear.
BREAST CANCER AND VVA
Currently, more than 2 million women in the United States have a history of breast cancer. In breast cancer survivors, the estimated prevalence of vaginal atrophy, by symptom report, ranges from 23% to 61%.8 Prescribing even very low-dose localized estrogen treatments for these patients can cause concern because of the potential for systemic absorption.
Concern about the provision of any form of estrogen, either systemic or local, to breast cancer survivors contributes to the high incidence of VVA in women with breast cancer. Discontinuation of HT may trigger the onset of VVA symptoms.
Many surgical, endocrine, and chemotherapeutic treatments for breast cancer can cause or exacerbate VVA.10,30-37 Tamoxifen acts as an estrogen antagonist or agonist depending on the target organ and menopausal status. In premenopausal women, tamoxifen may cause VVA by acting as an estrogen antagonist and blocking the naturally high levels of endogenous estrogen. In postmenopausal women, however, it acts as an estrogen agonist on the urogenital tract.38
Raloxifene does not appear to have an effect on the urogenital area in either premenopausal or postmenopausal women. Davies et al35 found no significant differences in incidence of VVA when comparing databases of postmenopausal women treated with raloxifene vs placebo.
Aromatase inhibitors (AIs) are prescribed as adjuvant systemic therapy to women with ER+ breast cancer. In the ATAC (Arimidex, Tamoxifen, Alone or in Combination) study, designed to compare outcomes in postmenopausal breast cancer survivors taking tamoxifen, anastrozole (an AI), or a combination of both, Cella et al10 demonstrated that vaginal dryness was more common in the group taking anastrozole than in the group taking tamoxifen (18.5% vs 9.1%). Dyspareunia was also more common in the anastrozole group (17.3% vs 8.1%).10
Chemotherapy itself can result in vaginal dryness and dyspareunia. In a randomized clinical trial comparing high-dose chemotherapy with standard-dose chemotherapy for breast cancer survivors, followed by radiotherapy and tamoxifen in all patients, more patients in the high-dose chemotherapy group experienced persistent vaginal dryness.37
Chemotherapeutic agents used in the treatment of breast cancer can also cause VVA because of chemotherapy-induced ovarian failure. Premenopausal women account for 25% of all diagnosed breast cancer cases and are more likely to need systemic chemotherapy. The risk of permanent chemotherapy-induced ovarian failure is more common in women older than 40 years (49%-100%) than in those younger than 40 years (21%-71%).39
Premenopausal women with hormone-sensitive (ER+) advanced breast cancer may be offered gonadotropin-releasing hormone agonists to induce a temporary menopause through suppression of ovarian function, with VVA as a potential adverse effect.40 High-risk premenopausal women who are BRCA1/2+ sometimes choose bilateral oophorectomy, which in turn increases their likelihood of developing VVA.
Whether breast cancer survivors with VVA can be safely treated with low-dose vaginal estrogens remains controversial. Kendall et al41 addressed this question when they followed up 7 breast cancer survivors taking AIs to suppress estrogen levels. These women took 25-μg vaginal tablets of estradiol daily for 2 weeks and then twice weekly for severe symptoms of VVA. After 2 weeks of treatment, mean serum estradiol levels increased from a pretreatment value of 1.4 pg/mL or less to 19.6 pg/mL. The estrogen levels had decreased to less than 9.5 pg/mL by 4 weeks of therapy in most women, but only 2 women had pretreatment estradiol levels at week 7. If third-generation AIs decrease the levels of circulating estrogens more than earlier-generation AIs and at the same time improve survival, Kendall et al41 hypothesized that a small increase in circulating estradiol could worsen survival outcomes.
COMPLEMENTARY AND ALTERNATIVE MEDICINE
Complementary and alternative medicine products have been extensively studied in the treatment of hot flashes, but less information is available on their use in VVA. One study found that Vitamin E and phytoestrogen applied locally as a gel improved the symptoms of VVA.42 An evaluation of VVA was undertaken in a cross-sectional study of 60 women, half of whom had taken 1,25-dihydroxyvitamin D (0.5 μg/d of calcitriol) orally for at least 1 year and half of whom had not. The prevalence of vaginal atrophy was significantly higher in the group who did not use vitamin D, as measured by VMI and symptoms.43
In a separate study, soy supplementation for the treatment of VVA was investigated. Phytoestrogens such as soy bind to ERs in the vagina and bladder. A randomized controlled trial evaluating dietary supplementation with 12 to 20 mg/d of soy showed no improvement in VMI.44
Currently, well-established effective complementary and alternative medicine treatments for VVA are lacking.
FUTURE STUDIES
Future studies will continue to explore the use of even lower doses of vaginal estrogens. The efficacy and safety of 10-μg vaginal tablets of low-dose estradiol for the treatment of VVA were evaluated in a 2009 study; after 12 weeks of therapy, significant improvements in symptoms, pH, and VMI were observed, with no adverse effects.45 In a recent study comparing treatment with 25 μg of estradiol, 10 μg of estradiol, and placebo, Bachmann et al46 found that both active groups experienced improvement in vaginal atrophy symptoms, vaginal pH, and VMI, with greater improvements seen for the higher dose.
Interest in vaginal estriol products is strong because of the inverse relationship with breast cancer risk at a population level. Studies have shown that estriol improves symptoms of VVA and reduces the incidence of urinary tract infection. Exogenous estriol administration does not alter serum estradiol or follicle-stimulating hormone levels.47-50
Studies are currently under way to evaluate newer selective ER modulators (SERMs), which specifically target urovaginal health without compromising breast health.51
Because moisturizers and lubricants also play a role in the treatment of VVA, better delivery systems for these nonhormonal vaginal treatments will likely be developed in the future.
CONCLUSION
Vulvovaginal atrophy, a common and often underreported condition, occurs in women who experience hypoestrogenic states. Systemic treatment, when prescribed for menopausal symptoms, may not be sufficient to control VVA. Local estrogens include creams, tablets, and rings, all of which are equally effective. Thus, patient preference will guide the choice. A growing number of women are at risk of developing VVA because of decreased use of systemic HT and increased use of SERMs, AIs, and chemotherapy in women with a history of breast cancer, for whom the safety of even low-dose vaginal estrogens has not been established.
Future research on VVA will likely explore the use of much lower doses of vaginal estrogens, seek to develop newer delivery systems for nonhormonal therapy, and develop SERMs that preferentially target urogenital tissues.
Supplementary Material
On completion of this article, you should be able to (1) recognize the common symptoms and signs of vulvovaginal atrophy, (2) evaluate the role of tests used in its identification, and (3) recommend effective treatment options.
CME Questions About Vulvovaginal Atrophy
-
A 58-year-old woman, who has been taking oral hormone therapy (HT) for successful control of hot flashes and night sweats, reports vaginal itching, burning, and dyspareunia. Clinical examination reveals a pale shiny vulva and petechiae in the vagina, consistent with vulvovaginal atrophy (VVA).
Which one of the following treatments would be most effective for managing this patient's symptoms?
Increase her current dose of oral HT
Decrease her current dose of oral HT, and recommend initiation of vaginal estrogen applied twice weekly and vaginal lubricant applied at time of intercourse
Continue current dose of oral HT, and recommend initiation of vaginal estrogen applied twice weekly and vaginal lubricant applied at time of intercourse
Substitute transdermal patch estrogen for oral estrogen and recommend initiation of vaginal lubricant applied at time of intercourse
Continue current dose of oral HT and advise use of vaginal lubricant at time of intercourse
-
A 65-year-old postmenopausal woman who is not taking HT therapy reports new-onset vaginal itching, burning, and dyspareunia. Clinical examination reveals a generalized pale shiny vulva and a white raised area on the vulva.
Which one of the following would be the best recommendation for this patient?
Apply low-dose estrogen cream to the vulvovaginal area
Apply low-dose estrogen cream to the vulvovaginal area and hydrocortisone cream to the whitened area
Apply hydrocortisone cream to the whitened area
Perform biopsy of the whitened area
Administer 1 dose of fluconazole (150 mg) orally, followed by application of low-dose estrogen cream to the vulvovaginal area
-
A 58-year-old woman, who has been taking oral HT for successful control of hot flashes and night sweats, reports vaginal itching, burning, and dyspareunia. Clinical examination reveals a pale shiny vulva and petechiae in the vagina, consistent with VVA.
Which one of the following tests is necessary to make the diagnosis?
pH of the vaginal mucosa
No test required
Urinalysis
Vaginal maturation index (VMI)
Vaginal culture
-
Which one of the following is not associated with VVA?
Lactation
Premenopausal use of tamoxifen
Postmenopausal use of tamoxifen
Premenopausal use of chemotherapeutic agents
Postmenopausal use of chemotherapeutic agents
-
Which one of the following medications would be most likely to cause VVA in a postmenopausal woman?
Raloxifene
Tamoxifen
Exemestane
Systemic estradiol
Medroxyprogesterone
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
Because the Concise Review for Clinicians contributions are now a CME activity, the answers to the questions will no longer be published in the print journal. For CME credit and the answers, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Moreira ED, Glasser DB, Nicolosi A, Nicolosi A, Duarte FG, Gingell C, GSSAB Investigators' Group Sexual problems and help-seeking behavior in adults in the United Kingdom and continental Europe. BJU Int 2008;101(8):1005-1011 [DOI] [PubMed] [Google Scholar]
- 2.Simon JA, Komi J. Postmenopausal women's attitudes: vulvovaginal atrophy and its symptoms [abstract LB-10]. Menopause 2007;14(6):1107 [Google Scholar]
- 3.Iosif CS, Bekassy Z. Prevalence of genito-urinary symptoms in the late menopause. Acta Obstet Gynecol Scand 1984;63(3):257-260 [DOI] [PubMed] [Google Scholar]
- 4.Dennerstein L, Dudley E, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obst Gynecol. 2000;96(3):351-358 [DOI] [PubMed] [Google Scholar]
- 5.Stadberg E, Mattsson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76(5):442-448 [DOI] [PubMed] [Google Scholar]
- 6.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152(5):463-473 [DOI] [PubMed] [Google Scholar]
- 7.Wilbur J, Miller AM, Montgomery A, Chandler P. Sociodemographic characteristics, biological factors, and symptom reporting in midlife women. Menopause. 1998;5(1):43-51 [PubMed] [Google Scholar]
- 8.Crandall C, Petersen L, Ganz P, Greendale GA. Association of breast cancer and its therapy with menopause-related symptoms. Menopause 2004;11(5):519-530 [DOI] [PubMed] [Google Scholar]
- 9.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58(4):348-358 [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Fallowfield L, Barker P, Cuzick J, Howell A, ATAC Trialists A9 Group Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Breast Cancer Res Treat 2006;100(3):273-284 [DOI] [PubMed] [Google Scholar]
- 11.North American Menopause Society (NAMS) Menopause Practice: A Clinician's Guide 3rd ed.Mayfield Heights, OH: North American Menopause Society; 2007. [Google Scholar]
- 12.Roy S, Caillouette JC, Roy T, Faden JS. Vaginal pH is similar to follicle-stimulating hormone for menopause diagnosis. Am J Obstet Gynecol 2004;190:1272-1277 [DOI] [PubMed] [Google Scholar]
- 13.Brown KH, Hammond CB. Urogenital atrophy. Obstet Gynecol Clin North Am 1987;14(1):13-32 [PubMed] [Google Scholar]
- 14.Semmens JP, Wagner G. Estrogen deprivation and vaginal function in postmenopausal women. JAMA 1982;248(4):445-448 [PubMed] [Google Scholar]
- 15.Gupta P, Özel B, Stanczyk FZ, Felix JC, Mishell DR., Jr The effect of transdermal and vaginal estrogen therapy on markers of postmenopausal estrogen status. Menopause 2008;15(1):94-97 [DOI] [PubMed] [Google Scholar]
- 16.Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol 2001;63:165-192 [DOI] [PubMed] [Google Scholar]
- 17.Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci 1997;314(4):228-231 [DOI] [PubMed] [Google Scholar]
- 18.Sultana CJ, Walters MD. Estrogen and urinary incontinence in women. Maturitas 1994;20(2-3):129-138 [DOI] [PubMed] [Google Scholar]
- 19.Barlow DH, Cardoza LD, Francis RM, et al. Urogenital ageing and its effect on sexual health in older British women. Br J Obstet Gynaecol 1997;104(1):87-91 [DOI] [PubMed] [Google Scholar]
- 20.Greendale GA, Zibecchi L, Peterson L, Ouslander JG, Kahn B, Ganz PA. Development and validation of a physical examination scale to assess vaginal atrophy and inflammation. Climateric 1999;2(3):197-204 [DOI] [PubMed] [Google Scholar]
- 21.O'Connell TX, Nathan LS, Satmary WA, Goldstein AT. Non-neoplastic epithelial disorders of the vulva. Am Fam Physician 2008;77(3):321-326 [PubMed] [Google Scholar]
- 22.North American Menopause Society The role of local vaginal estrogens for the treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3):357-369 [DOI] [PubMed] [Google Scholar]
- 23.Suckling J, Kennedy R, Lethaby A. Local oestrogens for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4):CD001500 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson K, Heimer G. Low-dose oestradiol in the treatment of urogenital oestrogen deficiency—a pharmacokinetic and pharmacodynamic study. Maturitas 1992;15(2):121-127 [DOI] [PubMed] [Google Scholar]
- 25.Notelovitz M, Funk S, Nanavati N, Mazzeo M. Estradiol absorption from vaginal tablets in postmenopausal women. Obstet Gynecol 2002;99(4):556-562 [DOI] [PubMed] [Google Scholar]
- 26.Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 2009;(16):30-36 [DOI] [PubMed] [Google Scholar]
- 27.Notelovitz M. Urogenital aging: solutions in clinical practice. Int J Gynaecol Obstet 1997;59(suppl 1):S35-S39 [DOI] [PubMed] [Google Scholar]
- 28.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy; does it cause incontinence? Obstet Gynecol 2005;1069(5, pt 1):940-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293(8):935-948 [DOI] [PubMed] [Google Scholar]
- 30.Mortimer JE, Boucher L, Baty J, Knapp DL, Ryan E, Rowland JH. Effect of tamoxifen on sexual functioning in patients with breast cancer. J Clin Oncol 1999;17(5):1488-1492 [DOI] [PubMed] [Google Scholar]
- 31.Nystedt M, Berglund G, Bolund C, Brandberg Y, Fornander T, Rutqvist LE. Randomized trial of adjuvant tamoxifen and/or goserelin in premenopausal breast cancer: self-rated physiological effects and symptoms. Acta Oncologica 2000;39(8):959-968 [DOI] [PubMed] [Google Scholar]
- 32.Lahti E, Vuopala S, Kauppila A, Blanco G, Ruokonen A, Laatikainen T. Maturation of vaginal and endometrial epithelium in postmenopausal breast cancer patients receiving long-term tamoxifen. Gynecol Oncol 1994;55(3, pt 1):410-414 [DOI] [PubMed] [Google Scholar]
- 33.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol 1998;16(2):501-514 [DOI] [PubMed] [Google Scholar]
- 34.Premkumar A, Venzon DJ, Avila N, et al. Gynecologic and hormonal effects of raloxifene in premenopausal women. Fertil Steril 2007;88(6):1637-1644 [DOI] [PubMed] [Google Scholar]
- 35.Davies GC, Huster WJ, Lu Y, Plouffe L, Jr, Lakshmanan M. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol 1999;93(4):558-565 [DOI] [PubMed] [Google Scholar]
- 36.Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 2005;23(28):6931-6940 [DOI] [PubMed] [Google Scholar]
- 37.Mourits MJ, Böckermann I, de Vries EG, et al. Tamoxifen effects on subjective and psychosexual well-being, in a randomised breast cancer study comparing high-dose and standard-dose chemotherapy. Br J Cancer 2002;86(10):1546-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornander T, Rutqvist L, Wilking N. Effects of tamoxifen on the female genital tract. Ann N Y Acad Sci 1991;622:469-476 [DOI] [PubMed] [Google Scholar]
- 39.Minton SE, Munster PN. Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control 2002;9(6)466-472 [DOI] [PubMed] [Google Scholar]
- 40.Higgins MJ, Davidson NE. What is the current status of ovarian suppression/ablation in women with premenopausal early-stage breast cancer? Curr Oncol Rep 2009;11(1):45-50 [DOI] [PubMed] [Google Scholar]
- 41.Kendall A, Dowsett M, Folkerd E, Smith I. Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 2006;17(4):584-587 [DOI] [PubMed] [Google Scholar]
- 42.Morali G, Polatti F, Metelitsa EN, Mascarucci P, Maqnani P, Marrè GB. Open, non-controlled clinical studies to assess the efficacy and safety of a medical device in form of gel topically and intravaginally used in postmenopausal women with genital atrophy. Arzneimittelforschung 2006;56(3):230-238 [DOI] [PubMed] [Google Scholar]
- 43.Yildirim B, Kaleli B, Düzcan E, Topuz O. The effects of postmenopausal vitamin D treatment on vaginal atrophy. Maturitas 2004;49(4):334-337 [DOI] [PubMed] [Google Scholar]
- 44.Reed SD, Newton KM, LaCroix AZ, Grothaus LC, Grieco VS, Ehrlich K. Vaginal, endometrial, and reproductive hormone findings: randomized, placebo-controlled trial of black cohosh, multibotanical herbs, and dietary soy for vasomotor symptoms: the Herbal Alternatives for Menopause (HALT) Study. Menopause 2008;15(1):51-58 [PubMed] [Google Scholar]
- 45.Simon JA. Vulvovaginal atrophy: new and upcoming approaches [editorial]. Menopause 2009;16(1):5-7 [DOI] [PubMed] [Google Scholar]
- 46.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008;111(1):67-76 [DOI] [PubMed] [Google Scholar]
- 47.Dessole S, Rubattu G, Ambrosini G, et al. Efficacy of low-dose intravaginal estriol on urogenital aging in postmenopausal women. Menopause 2004;11(1):49-56 [DOI] [PubMed] [Google Scholar]
- 48.Dugal R, Hesla K, Sørdal T, Aase K, Lilleeidet O, Wickstrøm E. Comparison of usefulness of estradiol vaginal tablets and estriol vagitories for treatment of vaginal atrophy. Acta Obstet Gynecol Scand 2000;79(4):293-297 [PubMed] [Google Scholar]
- 49.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med 1993;329:753-756 [DOI] [PubMed] [Google Scholar]
- 50.Weiderpass E, Baron JA, Adami HO, et al. Low-potency oestrogen and risk of endometrial cancer: a case-control study. Lancet 1999;353(9167):1824-1828 [DOI] [PubMed] [Google Scholar]
- 51.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet [published correction appears in Obstet Gyencol. 2008;112(6):1392] Obstet Gynecol 2008;112(5):1053-1060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.