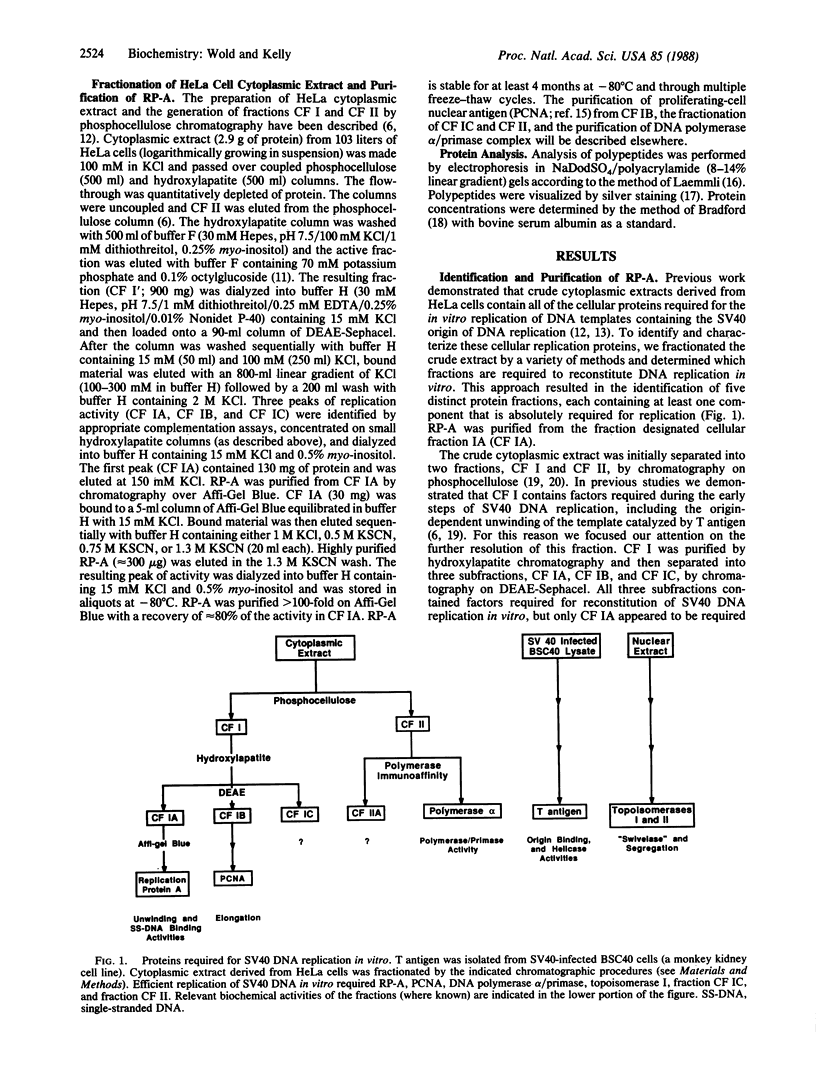
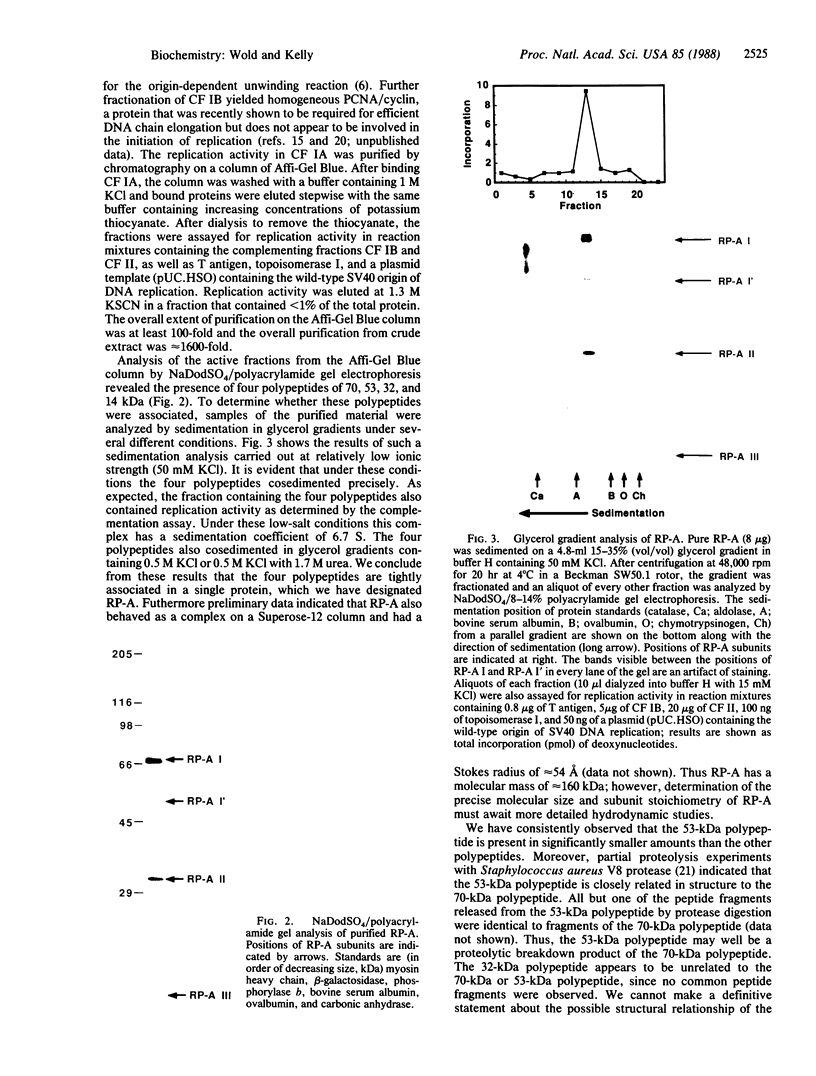
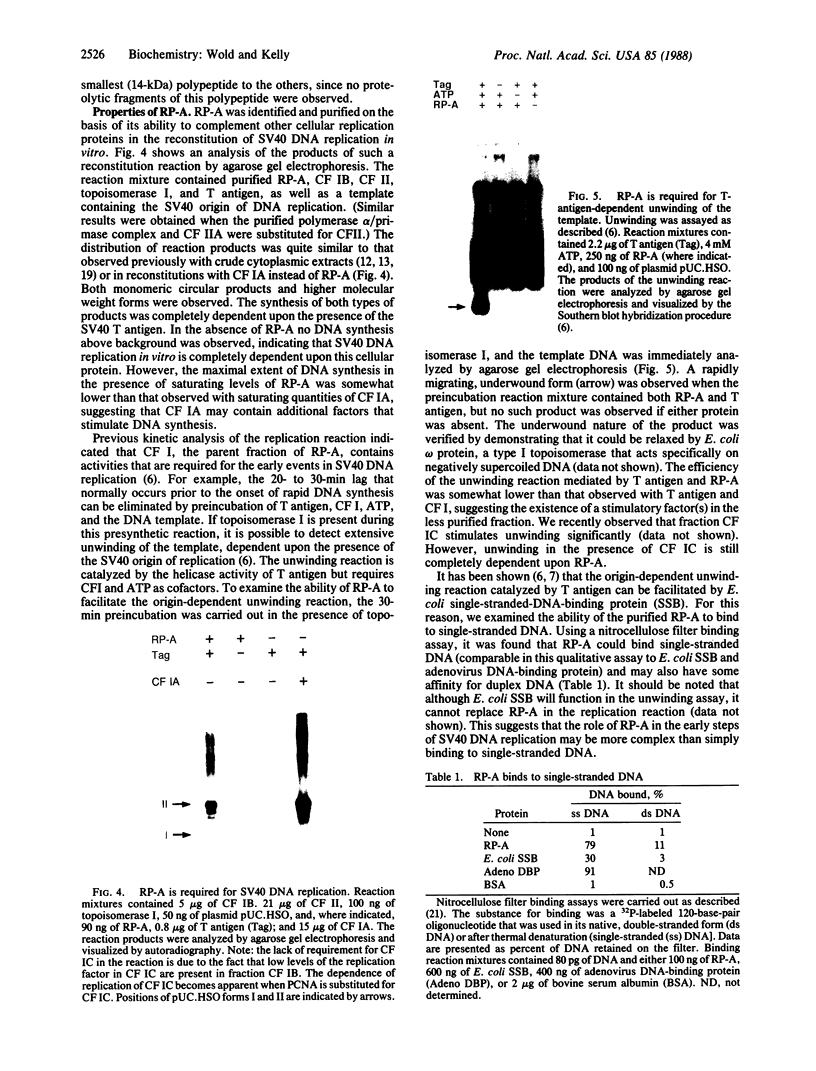
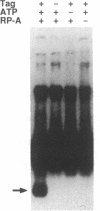
Abstract
The replication of simian virus 40 (SV40) DNA is largely dependent upon cellular replication proteins. To define these proteins we have made use of a cell-free system that is capable of replicating plasmid DNA molecules containing the SV40 origin of replication. Systematic fractionation-reconstitution experiments indicate that there are a minimum of six cellular proteins that are required for efficient viral DNA replication in vitro. We report here the purification of one of these proteins, replication protein A (RP-A), to homogeneity. RP-A is a multisubunit protein that contains four tightly associated polypeptides of 70, 53, 32, and 14 kDa. Partial proteolysis experiments indicate that the 53-kDa polypeptide is closely related to the 70-kDa polypeptide, suggesting that it may be a proteolytic fragment of the larger subunit. RP-A is absolutely required for reconstitution of SV40 DNA replication in vitro. The purified protein binds to single-stranded DNA and is required for the large tumor (T)-antigen-mediated unwinding of DNA molecules containing the SV40 origin of DNA replication. These properties are consistent with the possibility that RP-A plays a central role in the generation of a single-stranded region at the origin prior to initiation of DNA synthesis. The protein may also function to facilitate unwinding of the parental DNA strands during the elongation phase of SV40 DNA replication.
Full text
PDF