Abstract
OBJECTIVE: To investigate the age-specific relationship between body mass index (BMI) and risk of diabetes in a Japanese general population.
PARTICIPANTS AND METHODS: A cohort of Japanese men (N=19,926) and women (N=41,489) (aged 40-79 years) who under went community-based health checkups in 1993 and were free of diabetes was followed up by annual examinations with measurement of blood glucose concentrations until the end of 2006. Incident diabetes mellitus was defined as a blood glucose concentration of 126 mg/dL or greater under fasting conditions, 200 mg/dL or greater under nonfasting conditions, or diabetic medication use at baseline. Hazard ratios (HRs) for diabetes according to BMI were estimated using a Cox proportional hazard model. The model was adjusted for possible confounding variables.
RESULTS: A total of 4429 participants (7.2%) developed diabetes (2065 men and 2364 women) during a mean follow-up of 5.5 years. Compared with those with a BMI of less than 25.0, the multivariate HRs for diabetes among participants with a BMI of 30.0 or greater were 1.40 (95% confidence interval [CI], 0.89-2.20) for men aged 40 to 59 years and 1.26 (95% CI, 0.81-1.96) for men aged 60 to 79 years (P=.002 for interaction). The HRs were 2.50 (95% CI, 2.01-3.11) for women aged 40 to 59 years and 1.80 (95% CI, 1.41-2.30) for women aged 60 to 79 years (P=.04 for interaction).
CONCLUSION: The effect of obesity on the risk of diabetes is greater for middle-aged than for older adults.
In this investigation of the age-specific relationship between body mass index and risk of diabetes in 61,415 adults, 4429 individuals developed diabetes during a mean follow-up of 5.5 years. The effect of obesity on the risk of diabetes is greater for middle-aged adults than for older adults.
BMI = body mass index; CI = confidence interval; HR = hazard ratio
Diabetes mellitus is one of the major public health problems in Western countries and in Japan as a risk factor of cardiovascular diseases.1,2 Many previous prospective studies have shown that obesity or being overweight is related to the risk of diabetes mellitus.3-11 The Health Professionals' Follow-up Study8 of 51,529 US male dentists, veterinarians, osteopaths, podiatrists, optometrists, and pharmacists aged 40 to 75 years reported that the risk of diabetes mellitus increased continuously with increasing body mass index (BMI; calculated as the weight in kilograms divided by height in meters squared) among men with a BMI of 23 or greater. The Nurses' Health Study6 of 113,861 US female nurses aged 30 to 55 years also reported similar results among women with a BMI of 22 or greater. Because treating long-term diabetes mellitus is costly, the best approach to control diabetes is primary prevention. Examining the modifiable risk factors for diabetes mellitus, including obesity, is important because of its public health implications.
The relationship between obesity and diabetes mellitus has been reported to be age-dependent. A recent meta-analysis has shown an age-dependent relationship between BMI and the incidence of diabetes mellitus throughout the entire Asia-Pacific region.12 However, this age-dependent relationship has not been extensively studied in a large cohort of the general population in Japan only. Clarification of this issue may help by implementation of more effective public health and clinical efforts aimed at primary prevention of diabetes mellitus via weight control. The increasing prevalence of diabetes mellitus in all age groups in Japan highlights the need for such data. The purpose of the current study was to investigate whether aging affects the relationship between the degree of obesity and incident diabetes mellitus in a large Japanese cohort.
PARTICIPANTS AND METHODS
In this prospective cohort study, we enrolled 97,079 Japanese adults (33,139 men and 63,940 women) who underwent community-based health checkups conducted by the Ibaraki Health Service Association in 1993. These checkups were in accordance with regulations by local governments. Data were collected by the Ibaraki prefectural government from local governments after depersonalizing participant data to ensure anonymity. Data were collected regarding anthropometric measurements, blood pressure, blood samples, and interview questionnaires on smoking habits, daily alcohol intake, and medical history. We excluded 2624 adults (451 men and 2173 women) with incomplete data and 5101 adults (2557 men and 2544 women) with a fasting blood glucose concentration of 126 mg/dL or greater (to convert to mmol/L, multiply by 0.05551), a nonfasting blood glucose concentration of 200 mg/dL or greater, or diabetic medication use at baseline. Moreover, we excluded 27,939 adults (10,205 men and 17,734 women) who did not participate in the 1994 survey, thereby ensuring that the participants were followed up for at least 1 year. Of note, mean blood glucose concentrations were almost identical between participants who were and were not followed up.13
Thus, the study sample consisted of 61,415 adults (63.3% of initially recruited participants; 19,926 men and 41,489 women). These participants were followed up by annual examinations until diabetes mellitus had been diagnosed or until the end of 2006. Blood glucose concentrations were measured at annual follow-up examinations. Participants who did not undergo annual checkups during the follow-up periods were censored on the date of their latest checkup. The protocol for this cohort study was approved by the Ibaraki Epidemiology Study Union Ethics Review Committee.
Baseline Examinations
Height of participants in stocking feet and weight in light clothing were measured at baseline. With participants seated, blood samples were drawn into 2 polyethylene terephthalate tubes: 1 with an accelerator and 1 with sodium fluoride and ethylenediaminetetraacetic acid. Overnight fasting (≥8 hours) was not necessarily required. The blood glucose concentration was measured by means of a glucose oxidase electrode method with a GA1140 device (Kyoto Daiichi Kagaku, Kyoto, Japan) in a single laboratory of the Ibaraki Health Service Association. Serum total cholesterol and serum triglyceride values were measured by means of an enzyme method with an RX-30 device (Nihon Denshi, Tokyo, Japan). High-density lipoprotein cholesterol values were measured in the same laboratory by means of a phosphotungstic acid magnesium method with an MTP-32 device (Corona Electric, Ibaraki, Japan). The laboratory participated in external standardization and successfully met the criteria for precision accuracy for the measurement of blood samples by the Japan Medical Association, the Japanese Association of Medical Technologists, and the Japan Society of Health Evaluation and Promotion.
Baseline blood pressure was measured from the right arm of seated participants who had rested for more than 5 minutes, by trained observers using standard mercury sphygmomanometers. When systolic blood pressure was greater than 150 mm Hg or diastolic blood pressure was greater than 90 mm Hg, the second measurement was performed after several deep breaths, and the lower values, which were almost always observed after the second measurement, were used for analyses. An interview was conducted to ascertain medical history, smoking status (never, ex-smoker, and current smoker <20 cigarettes/day or ≥20 cigarettes/day), and alcohol intake (none, occasionally, or daily <66 g/d or ≥66 g/d).
End Point Determination
The blood glucose concentration was measured by the glucose oxidase electrode method with a GA1140 device in 1994-1996 and by the hexokinase/glucose-6-phosphate dehydrogenase method with a H7170 device (Hitachi, Tokyo, Japan) from l997-2006. Blood glucose concentrations determined by means of the glucose oxidase electrode method were compared with those determined by the hexokinase method, using 237 random samples of blood drawn in 1996. Comparability between blood glucose concentrations based on the 2 methods was excellent. In the linearity test, the regression line was Y = 1.017 × X + 0.802, in which Y is a hexokinase method value (mg/dL) and X is a glucose oxidase electrode method value (mg/dL) (r=0.999). The slope coefficient of 1.017 and the intercept of 0.802 were not statistically significantly different from 1.0 and 0, respectively. On the basis of the linearity test, calibration was performed every day.
Incident diabetes mellitus was defined as a fasting blood glucose concentration of 126 mg/dL or greater, a nonfasting blood glucose concentration of 200 mg/dL or greater, or initiation of treatment for diabetes mellitus. Fasting was defined as not having had a meal for at least 8 hours.
Statistical Analyses
Participants were classified with regard to their BMI as less than 25.0, 25.0 to 29.9, and 30.0 or greater according to the World Health Organization classification.14 Hazard ratios (HRs) with the corresponding 95% confidence interval (CI) for diabetes mellitus according to BMI were calculated with reference to BMI as less than 25.0 using a Cox proportional hazards regression model. Analyses were stratified by sex and age groups (40-59 and 60-79 years). We applied this age cut-point to maintain sufficient incidence data. Covariates included age, baseline blood glucose concentration, fasting status (yes/no), systolic blood pressure, antihypertensive medication use (yes/no), total cholesterol, high-density lipoprotein cholesterol, log-transformed triglyceride, lipid medication use (yes/no), smoking status (never, ex-smoker, current <20 cigarettes/day or ≥20 cigarettes/day), alcohol intake (none, occasionally, daily <60 g/d or ≥60 g/d), and BMI change from baseline to the end of the year of follow-up. The analysis was repeated with an interaction term of age groups and sex times BMI categories. P<.05 was regarded as statistically significant. All statistical analyses were conducted using SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Of the 61,415 adults (19,926 men and 41,489 women), 4429 (2065 men and 2364 women [7.2%]) developed diabetes mellitus during a mean 5.5 years of follow-up (5.1 years for men and 5.7 years for women).
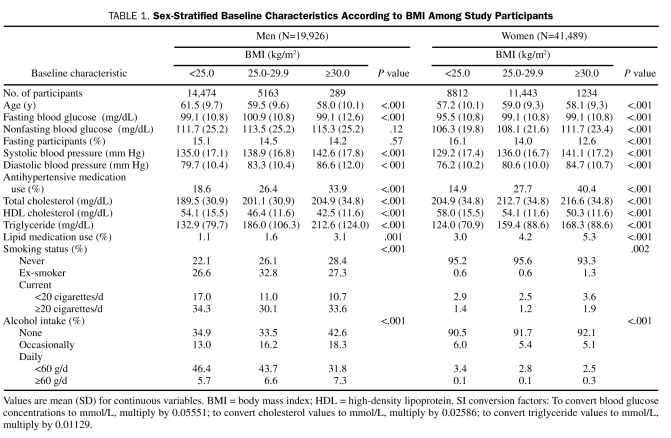
Sex-stratified baseline characteristics according to BMI categories are shown in Table 1. Approximately 15% of participants were in a fasting state at baseline. All covariates, except for nonfasting blood glucose in men and percentage of fasting male participants, were associated with BMI in both sexes.
TABLE 1.
Sex-Stratified Baseline Characteristics According to BMI Among Study Participants
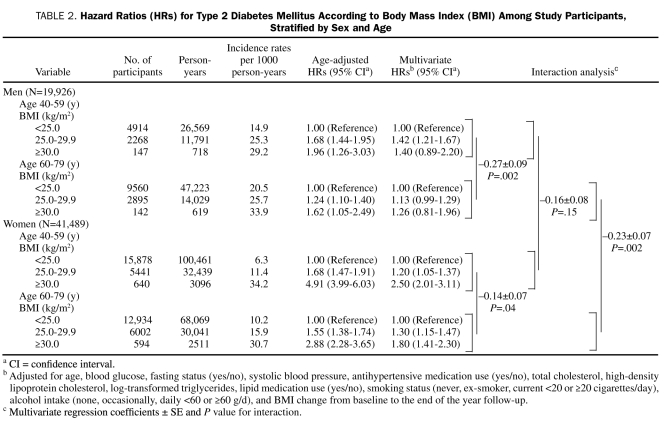
Table 2 presents sex- and age-stratified HRs for diabetes mellitus according to BMI categories. In men aged 40 to 59 years, compared with participants who had a BMI lower than 25.0, the multivariate HR for diabetes mellitus was significantly increased among participants who had a BMI of 25.0 to 29.9 but did not differ significantly among participants who had a BMI of 30.0 or greater. In men aged 60 to 79 years, compared with participants who had a BMI lower than 25.0, the multivariate HR for diabetes mellitus did not differ significantly among participants in either of the other BMI groups. The effect of BMI on risk of diabetes mellitus was significantly greater for men aged 40 to 59 years compared with men aged 60 to 79 years (P=.002 for interaction).
TABLE 2.
Hazard Ratios (HRs) for Type 2 Diabetes Mellitus According to Body Mass Index (BMI) Among Study Participants, Stratified by Sex and Age
In women aged 40 to 59 years and in women aged 60 to 79 years, compared with participants who had a BMI lower than 25.0, the multivariate HRs for diabetes mellitus were significantly greater among participants in each of the higher BMI groups. The effect of BMI on risk of diabetes was significantly greater for women aged 40 to 59 years compared with women aged 60 to 79 years (P=.04 for interaction).
Among participants aged 60 to 79 years, the effect of BMI on risk of diabetes was significantly greater for women compared with men (P=.002 for interaction). Among participants aged 40 to 59 years, the effect of BMI on risk of diabetes did not differ significantly between women and men (P=.15 for interaction).
DISCUSSION
The current large cohort study showed that the effect of BMI on diabetes mellitus, of which 99% was type 2 in Japan according to the published statistics,15 was significantly greater among middle-aged adults than among older adults. To our knowledge, this is the first large prospective cohort study to show that the relationship of the degree of obesity and the risk of diabetes mellitus is different between Japanese middle-aged and older adults. As judged by their HRs, this relationship might be more prominent in women than in men.
As we expected, a significant relationship between obesity and diabetes mellitus was observed in our cohort. This relationship was consistent with previous studies in white and Asian populations.2-10 Previous studies investigating the age-specific relationship between obesity and diabetes mellitus reported inconsistent results. Ishikawa-Takata et al3 found no age-specific relationship in Japanese male workers aged 18 to 59 years. Nagaya et al4 showed that for each 1-year increment of age, there was a 4% to 5% increased risk of developing diabetes mellitus in men and women aged 30 to 59 years. Our data did not corroborate the findings of these studies partly because of differences in age range. The Asia Pacific Cohort Studies Collaboration demonstrated age-specific relationships between BMI and risk of diabetes mellitus, showing stronger proportional relationships in younger age groups.12 The study reported that, for each reduction in BMI of 2.0, there was a 31% lower risk of diabetes mellitus in those younger than 60 years and a 19% lower risk in those aged 70 years or older. The Cardiovascular Health Study16 also revealed the effect of age and BMI on the development of diabetes mellitus in older adults (≥65 years). McNeely et al17 reported that overweight (BMI ≥25.0) Japanese Americans younger than 55 years, but not those aged 55 years or older, were at a significantly high risk of diabetes mellitus. These data corroborate with those in the current study. Because our study enrolled a larger sample population with a wide age range compared with previous studies, our results might provide stronger evidence of the effect of age on incident diabetes mellitus.
Several possible explanations exist for why an age-specific relationship between obesity and diabetes mellitus was observed. First, lower adiponectin levels in middle-aged adults may contribute to the age-specific effect on incident diabetes mellitus.18 Adiponectin is a novel cytokine specifically secreted from fat cells and has an antidiabetic effect by suppressing tumor necrosis factor α activity.19 Second, effects of genetic predisposition on differences in body weight might exist to a greater extent among middle-aged vs older adults.20 To the extent that diabetes mellitus and obesity share common genetic risk factors, being overweight in middle-age would seem to confer risk in itself. Third, recent work by our group showed that leanness in the elderly, but not in middle-aged adults, was also a risk factor for developing diabetes mellitus.21 Therefore, the relatively higher effect of obesity on diabetes mellitus in middle-aged adults may be evident because of an attenuated relative risk in obese elderly people. Fourth, the weaker relationship between obesity and the risk of diabetes mellitus in older age groups might be due in part to elevated risk among nonobese older individuals because of the effects of aging, such as increased fat mass and reduced physical activity.
We unexpectedly observed that the effects of obesity on incident diabetes mellitus were significantly greater in women than men. This might be accounted for by health risks induced by decreased production of sex hormones in postmenopausal women. However, the finding is inconsistent with a previous study,16 which reported that relative risks for diabetes were more prominent in older (≥65 years) men than in women. Possible explanations for this discrepancy might be differences in ethnicity or age range of participants (aged 40-79 years vs ≥65 years).
The strength of the current study is that it consists of a cohort large enough to allow subgroup analyses. Moreover, all blood samples were measured by the same laboratory, which was acknowledged by a known quality control program.22 We also ascertained the incidence of diabetes mellitus primarily by blood glucose concentrations, in contrast to many previous large cohort studies that used self-administered questionnaires.6,8
Our study has several limitations. First, external validity for the study may not be high because participants were community residents of a single prefecture in Japan. Second, the follow-up rates were moderate. However, mean blood glucose concentrations did not differ between individuals who were and were not followed up. Thus, the potential selection bias might have been small. Third, potential confounding variables were present that we could not assess, including fat distribution, physical activity, nutritional status, family history of diabetes mellitus, and duration of obesity. Physical activity is known to not substantially alter the relationship between BMI and the risk of diabetes mellitus.23 Fourth, even though the age-BMI interaction was statistically significant, the magnitude of differences showed that clinical importance was not necessarily high, especially in men. The HRs were 1.40 for obese men aged 40 to 59 years and 1.26 for obese men aged 60 to 79 years. However, the magnitude of differences in women may be clinically important because HRs for obese women aged 40 to 59 years and obese women aged 60 to 79 years were 2.50 and 1.80, respectively. Fifth, the diagnosis of diabetes was based on a single blood glucose measurement. Sixth, there were approximately 1-year differences in mean follow-up times between lean and obese participants. This was also observed between participants aged 40 to 59 years and those aged 60 to 79 years. Thus, screening bias might modify the differences in the effects of obesity on developing diabetes mellitus between participants aged 40 to 59 years and those aged 60 to 79 years.
Our results provide a better understanding of the age-specific relationship between obesity and the risk of diabetes mellitus and should guide public health and clinical efforts aimed at primary prevention by weight control. These results also highlight the importance of weight control for primary prevention of diabetes mellitus in middle-aged adults, even though the incidence rate was higher in older adults than in middle-aged adults.
CONCLUSION
This study shows that the effect of BMI on diabetes mellitus is greater among middle-aged than older adults. Moreover, we suggest that the relationship is more prominent in women than in men.
Acknowledgments
We thank all those who participated in this study.
Footnotes
This work was supported in part by a grant-in-aid from the Ministry of Health, Labour and Welfare, Health and Labor Sciences Research Grants, Japan (Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H18-Junkankitou [Seishuu]-Ippan-012; and H20-Junkankitou [Seishuu]-Ippan-013). These financial supports played an important role in the design and conduct of the study.
REFERENCES
- 1.Irie F, Sairenchi T, Iso H, Shimamoto T. Prediction of mortality from findings of annual health checkups utility for health care programs [in Japanese]. Nippon Koshu Eisei Zasshi 2001;48(2):95-108 [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229-234 [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa-Takata K, Ohta T, Moritaki K, Gotou T, Inoue S. Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in Japanese men. Eur J Clin Nutr. 2002;56(7):601-607 [DOI] [PubMed] [Google Scholar]
- 4.Nagaya T, Yoshida H, Takahashi H, Kawai M. Increases in body mass index, even within non-obese levels, raise the risk for type 2 diabetes mellitus: a follow-up study in a Japanese population. Diabet Med. 2005;22(8):1107-1111 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. Am J Epidemiol 1981;113(2):144-156 [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132(3):501-513 [DOI] [PubMed] [Google Scholar]
- 7.Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus: a prospective cohort study of men in the normative aging study. Am J Epidemiol 1992;136(12):1474-1486 [DOI] [PubMed] [Google Scholar]
- 8.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17(9):961-969 [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes 1990;39(3):283-288 [DOI] [PubMed] [Google Scholar]
- 10.Charles MA, Fontbonne A, Thibult N, Warnet JM, Rosselin GE, Eschwege E. Risk factors for NIDDM in white population: Paris prospective study. Diabetes 1991;40(7):796-799 [DOI] [PubMed] [Google Scholar]
- 11.Ohlson LO, Larsson B, Svärdsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985;34(10):1055-1058 [DOI] [PubMed] [Google Scholar]
- 12.Asia Pacific Cohort Studies Collaboration Body mass index and risk of diabetes mellitus in the Asia-Pacific region. Asia Pac J Clin Nutr 2006;15(2):127-133 [PubMed] [Google Scholar]
- 13.Sasai H, Sairenchi T, Irie F, Iso H, Tanaka K, Ota H. Development of a diabetes risk prediction sheet for specific health guidance [in Japanese]. Nippon Koshu Eisei Zasshi 2008;55(5):287-294 [PubMed] [Google Scholar]
- 14.World Health Organization Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253 [PubMed] [Google Scholar]
- 15.Tajima N, Matsushima M, Baba S, Goto Y. Japan. In: Ekoé J-M, Zimmet P, Williams R, eds. The Epidemiology of Diabetes Mellitus: An International Perspective New York, NY: John Wiley and Sons; 2001:253-260 [Google Scholar]
- 16.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity 2007;15(7):1827-1840 [DOI] [PubMed] [Google Scholar]
- 17.McNeely MJ, Boyko EJ, Shofer JB, Newell-Morris L, Leonetti DL, Fujimoto WY. Standard definitions of overweight and central adiposity for determining diabetes risk in Japanese Americans. Am J Clin Nutr 2001;74(1):101-107 [DOI] [PubMed] [Google Scholar]
- 18.Isobe T, Saitoh S, Takagi S, et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153(1):91-98 [DOI] [PubMed] [Google Scholar]
- 19.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab 2006;8(3):264-280 [DOI] [PubMed] [Google Scholar]
- 20.Stunkard AJ, Sørensen TI, Hanis C, et al. An adoption study of human obesity. N Engl J Med. 1986;314(4):193-198 [DOI] [PubMed] [Google Scholar]
- 21.Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes Care 2008;31(3):583-584 [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Sato S, Shimamoto T. Improvement in Japanese clinical laboratory measurements of total cholesterol and HDL-cholesterol by the US Cholesterol Reference Method Laboratory Network. J Atheroscler Thromb. 2003;10(3):145-153 [DOI] [PubMed] [Google Scholar]
- 23.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA 2004;292(10):1188-1194 [DOI] [PubMed] [Google Scholar]