Abstract
Stroke and head trauma are worldwide public health problems and leading causes of death and disability in humans, yet, no adequate neuroprotective treatment is available for therapy. Glutamate antagonists are considered major drug candidates for neuroprotection in stroke and trauma. However, N-methyl-d-aspartate antagonists failed clinical trials because of unacceptable side effects and short therapeutic time window. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) antagonists derived from the quinoxalinedione scaffold cannot be used in humans because of their insolubility and resulting renal toxicity. Therefore, achieving water solubility of quinoxalinediones without loss of selectivity and potency profiles becomes a major challenge for medicinal chemistry. One of the major tenets in the chemistry of glutamate antagonists is that the incorporation of phosphonate into the glutamate framework results in preferential N-methyl-d-aspartate antagonism. Therefore, synthesis of phosphonate derivatives of quinoxalinediones was not pursued because of a predicted loss of their selectivity toward AMPA. Here, we report that introduction of a methylphosphonate group into the quinoxalinedione skeleton leaves potency as AMPA antagonists and selectivity for the AMPA receptor unchanged and dramatically improves solubility. One such novel phosphonate quinoxalinedione derivative and competitive AMPA antagonist ZK200775 exhibited a surprisingly long therapeutic time window of >4 h after permanent occlusion of the middle cerebral artery in rats and was devoid of renal toxicity. Furthermore, delayed treatment with ZK200775 commencing 2 h after onset of reperfusion in transient middle cerebral artery occlusion resulted in a dramatic reduction of the infarct size. ZK200775 alleviated also both cortical and hippocampal damage induced by head trauma in the rat. These observations suggest that phosphonate quinoxalinedione-based AMPA antagonists may offer new prospects for treatment of stroke and trauma in humans.
Keywords: glutamate antagonists/ischemic brain damage/traumatic brain injury/therapeutic time window
Rescuing the brain from neuronal degeneration produced by acute stroke or head trauma remains an unmet goal of contemporary medicine despite the wealth of research efforts directed toward the discovery of neuroprotective drugs. A long track of failures in clinical trials of potential drug candidates such as Ca2+ channel or N-methyl-d-aspartate (NMDA) receptor antagonists raised serious questions about the feasibility of neuroprotective measures in stroke and trauma. Introduction of thrombolytic therapy with rt-PA (recombinant tissue plasminogen activator) offered the first successful step toward effective treatment for some stroke victims (1, 2).
Since the beginning of the 1980s, excitatory amino acids repeatedly have intrigued the scientific community through discoveries of their role in physiology and pathology (3). NMDA antagonists have been designed for the treatment of acute onset neurodegeneration such as stroke and trauma and recently reached the clinics. Unfortunately, the hopes for success of NMDA antagonists in patients so far has been limited by unacceptable side effects (4). The next generation of glutamate antagonists, the AMPA antagonists (named after the agonist AMPA), recently left the realm of drug research and moved toward the clinic. The first generation of selective AMPA antagonists such as 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX) (5) and 6-(1H-imidazol-1-yl)-7-nitro-2,3-(1H, 4H)-quinoxalinedione (YM90K) (6) failed clinical trials because of nephrotoxicity due to their limited solubility in water (4). Achieving water solubility of quinoxalinediones without loss of their selectivity and potency as AMPA antagonists has been a major challenge for medicinal chemistry. Incorporation of a polar phosphonate group into the l-glutamate framework was not pursued because of an expected loss of selectivity toward AMPA (7, 8). Here, we report the design of a class of quinoxalinediones that retain high selectivity at the AMPA receptor, are water-soluble, and are effective neuroprotective drugs in rodent models of ischemia and head trauma.
MATERIALS AND METHODS
In Vitro Binding.
The potency of quinoxalinediones in inhibiting the specific binding of 3H-AMPA (9), 3H-6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10), 3H-kainate (11), 3H-3-(2-carboxypiperazin-4-yl)-propyl-1phosphonate (CPP) (12), 3H-1-[1-(2-thienyl)cyclohexyl]piperidine (TCP) (13), 3H-dihydrochlorokynurenate (DCKA) (14), and 3H-glycine (15) was tested on rat cortical membranes.
Evoked Potentials in Cortical Slice Preparations and Spreading Depression.
To assess relative selectivity of quinoxalinediones in vitro, rat cingulate cortex slices trimmed to form wedges were subjected to quisqualate, kainate, or NMDA alone or with different concentrations of quinoxalinediones, and the evoked potentials were recorded by using Ag/AgCl electrodes (16). To confirm selectivity profiles in vitro, the latency to initiate spreading depression in chicken retina by quisqualate, kainate, NMDA, or glycine alone or with different concentrations of quinoxalinediones was measured (17).
Single-Cell Recordings in Cultured Hippocampal Neurons.
AMPA- and kainate-induced membrane currents were recorded at −60 mV in the whole cell patch–clamp configuration (18) from cultured neonatal rat hippocampal neurons. Patch electrodes were filled with a solution containing (in mM): CsCl 150, MgCl2 1, EGTA 10, and Hepes 10 (pH 7.4). The bathing medium contained (in mM): NaCl 150, KCl 5, CaCl2 1.8, MgCl2 2, and Hepes 10 (pH 7.4). For recording NMDA-induced currents, Mg2+ was omitted and 3 μM glycine was added. The concentration–response curves and inhibition curves by quinoxalinediones were fitted to the equation: I = 100/(1 + (K/[drug])n) where I = normalized current response in %, K = EC50 or IC50, and n = slope.
Pharmacological in Vivo Profile.
Anxiolytic activity of ZK200775 was assessed in NMRI mice (Schering AG) weighing 23–28 g and placed in the center of a chamber (23 × 18 × 30 cm) with a floor divided into four plates (19). For determination of the effect of ZK200775 on the threshold for clonic seizures, NMRI mice (20–24 g) received an intracerebroventricular infusion of AMPA, kainate, or NMDA until clonic seizures were triggered (20). The motor coordination of mice subjected to ZK200775 was estimated in the rota-rod test (21). To estimate muscle relaxant activity of ZK200775, spontaneous activity in the electromyogram was recorded from the gastrocnemius muscle of genetically spastic rats spa/spa (80–120 g) (Harlan–Winkelmann, Borchen, Germany) by using pairs of Teflon-insulated stainless steel wire electrodes (20). Effect of ZK200775 on spontaneous exploratory activity in nonhabituated NMRI mice (22–25 g) was estimated in a computerized Digiscan-16 monitoring system (AccuScan, Columbus, OH). The distance in centimeters travelled by the mouse and detected by interruptions of the horizontal sensors was taken as a measure of exploratory activity (22). The effect of ZK200775 on the body temperature was monitored by means of a rectal thermistor probe in male NMRI mice (25–30 g) or Wistar rats (100–120 g) (Charles River, Salzfeld, Germany) (23). For the monitoring of the effect of ZK200775 on arterial blood pressure and blood gases, Fischer 344 rats (230–250 g) (Charles River) had catheters placed in the femoral and tail arteries (24).
Global Ischemia in Mongolian Gerbils and Focal Ischemia in Rodents.
Transient global ischemia was induced in halothane-anesthetized Mongolian gerbils (60–70 g) (Møllegaard, Lille, Skendsved, Denmark) by bilateral occlusion of the common carotid arteries (CCAO) for 4 min (5). The permanent middle cerebral artery occlusion (MCAO) was established by means of microbipolar permanent coagulation in NMRI mice (35–45 g) anesthetized with tribromoethanol, 600 mg/kg i.p. (25), or in Fischer 344 rats (260–290 g) anesthetized with halothane (26). For determination of the therapeutic time window, ZK200775 was continuously infused i.v. at a dose of 3 mg/kg/h over 6 h beginning 1, 2, 4, 5, 6, 7, 12, or 24 h after MCAO. For the analysis of duration of neuroprotective action, Fischer 344 rats (250–390 g) were subjected to permanent MCAO by electrocoagulation and i.v. infusion of ZK200775, 1 and 3 mg/kg/h over 6 h, beginning immediately after MCAO. Seven days after MCAO, the size of infarct in the brain was estimated stereologically by means of advanced image analysis. For the assessment of neuroprotective action against focal cerebral reperfusion ischemia, Wistar rats (250–300 g) anesthetized with halothane, were subjected to temporary occlusion of the common carotid arteries and the right middle cerebral artery (CCA/MCAO) for 90 min. CCAs were occluded by means of silastic threads placed around the vessels, and MCA was occluded by means of a steel hook attached to a micromanipulator; blood flow stop was verified by microscopic examination of the MCA or laser doppler flowmetry. ZK200775 was infused continuously i.v. (external jugular vein) at doses of 0.01, 0.03, 0.1, 1, and 3 mg/kg/h over 6 h starting immediately after the beginning of reperfusion (dose–response relationship) or 2 h after the onset of reperfusion (time window). Seven days after CCA/MCAO, the size of infarct in the brain was estimated stereologically by means of image analysis.
Head Trauma.
Fischer 344 rats (300–340 g) anesthetized with tribromoethanol, 260 mg/kg i.p., were subjected to cortex contusion by means of a falling weight (20 g) with a force of 380 g × cm (24). ZK200775 was infused continuously i.v. in doses of 0.1, 0.3, 1, and 3 mg/kg/h over 6 h beginning 1 h after trauma. Three days after injury, the volume of the damage in cortex was determined by means of image analysis, and neuronal loss was assessed by using an unbiased stereological dissector technique to estimate the mean numerical density (Nv) in the hippocampal CA3 subfields (24). The differences in Nv of pyramidal neurons in the CA3 subfield between the damaged and the nondamaged side were analyzed statistically by means of ANOVA.
Vacuoles and Degeneration of Neurons in the Retrosplenial/Cingulate Cortex.
NMDA antagonists may induce vacuoles and eventually degeneration of sensitive neurons in rat cingulate and retrosplenial cortex (27). To determine whether ZK200775 produced vacuoles in the cingulate/retrosplenial cortex, Wistar rats (230–250 g) were subjected to i.v. infusion of ZK200775, 0.67, 2, or 6 mg/kg/h over 6 h and killed 12 h later. To determine whether ZK200775 induced neurodegeneration, neuronal density in the retrosplenial/cingulate cortex in Fischer 344 rats (220–340 g) subjected to i.v. infusion of ZK200775, 3 mg/kg/h over 6 h was assessed by using an unbiased stereological dissector technique (24).
Kidney Morphology in Rats and Dogs.
High doses of the AMPA antagonist NBQX induce reversible nephrotoxicity in the form of tubulointerstitial nephritis in rats and dogs because of unfavorable physicochemical properties and precipitation of crystals in the tubuli (28). Therefore, 24 rats were subjected to continuous i.v. infusion of ZK200775 in doses of 0.67, 2, and 6 mg/kg/h for 4 weeks whereas 9 Beagle dogs received continuous i.v. infusion of ZK200775 in doses of 0.05, 0.1, and 0.2 mg/kg/h for 4 weeks. Kidney morphology was evaluated by a blinded observer by light microscopy.
RESULTS
Chemistry of Phosphonate Quinoxalinediones.
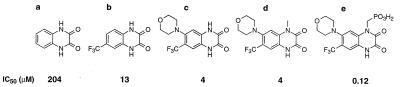
Unsubstituted quinoxaline-2,3-dione displaced AMPA from its binding sites in cortical membranes with an IC50 of 204 μM (Fig. 1a). The introduction of a CF3 group in position 6 (Fig. 1b) increased potency at the AMPA binding site 16-fold giving an IC50 value of 13 μM. Incorporation of an additional electron-donating moiety, such as a morpholino group (Fig. 1c), in position 7 of the quinoxalinedione framework increased AMPA receptor affinity 51-fold, in comparison with the unsubstituted quinoxaline-2,3-dione, resulting in an IC50 of 4 μM. The methyl substitution of 1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxaline at position 1 (Fig. 1d) did not result in a decrease of affinity at the AMPA binding site, yielding a similar IC50 value (4 μM) as the unsubstituted quinoxaline-2,3-dione and simultaneously demonstrating bulk tolerance at this position. It was surprising that additional prolongation of the chain length at position 1 by replacing the methyl group with a methylphosphonate group (Fig. 1e) led to a 34-fold increase of AMPA receptor affinity compared with the predecessor compound (Fig. 1d) and a 1,700-fold increase in comparison with the unsubstituted parent quinoxaline-2,3-dione (Fig. 1a). Furthermore, incorporation of a polar phosphonate group resulted in increased solubility in aqueous solutions reaching 25 mg/ml at pH 7.35 for [1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl]methylphosphonate (ZK200775) (Fig. 1e).
Figure 1.
Substitution patterns of quinoxalinediones leading to increase in water solubility and preservation of in vitro binding affinity to the AMPA receptor. (Lower) In vitro binding affinity to AMPA receptors in rat cortical membranes.
Receptor Binding Profile.
The receptor binding profile of ZK200775 showed high affinity to 3H-AMPA (120 nM) and 3H-CNQX (32 nM) binding sites (Table 1). It was 21-fold less potent at 3H-kainate (2.5 μM) and had weak affinity to the binding sites within the NMDA receptor complex such as 3H-CPP (2.8 μM), 3H-TCP (11 μM), 3H-dichlorokynurenate (2.8 μM), and 3H-glycine (5.15 μM) sites (Table 1). No affinity to nonglutamate receptor binding sites was detected up to concentrations of 20–100 μM in rat cortical membrane preparations.
Table 1.
In vitro binding affinity of ZK200775 to glutamate receptors in rat cortical membranes
| Glutamate receptor ligands | 3H-AMPA | 3H-CNQX | 3H-KA | 3H-CPP | 3H-TCP | 3H-DCKA | 3H-glycine |
|---|---|---|---|---|---|---|---|
| IC50, μM | 0.12 ± 0.09 | 0.032 ± 0.008 | 2.5 ± 0.2 | 2.8 ± 0.35 | 11 ± 2.1 | 2.8 ± 0.5 | 5.15 ± 0.21 |
Rat (Wistar; Møllegaard, Lille Skendsved, Denmark) frontoparietal cortex was homogenized in buffer containing 30 mM Tris⋅HCl, 2.5 mM CaCl2 (pH 7.1). The homogenate was centrifuged at 40,000 times g for 15 min, and the pellet was washed (3×) and suspended in buffer. Incubations were performed in triplicate at 0°C for 30 min and bound radioactivity separated by filtration through Whatman GF/C glass fiber filters. Nonspecific binding was defined by the addition of 680 μM l-glutamate, except for TCP binding, for which 3.5 μM phencyclidine was used. For glycine binding, the membranes were washed with buffer containing 5 mM Hepes/Tris and 2.5 mM MgCl2 (pH 7.1) supplemented with 0.08% Triton X-100. Nonspecific binding was defined by 100 μM d-serine, and membrane-bound tracer was separated by centrifugation. ZK200775 was dissolved in ethanol and added in at least four concentrations. The IC50 values were calculated by nonlinear regression analysis. Shown are means ± SEM of three determinations.
Evoked Potentials in Brain Slices and Spreading Depression.
Functional in vitro pharmacology assays using antagonism of quisqualate-, kainate-, and NMDA-induced evoked potentials in cingulate cortex slices or quisqualate-, kainate-, NMDA- and glycine-induced spreading depression in the chicken retina revealed that ZK200775 was a highly selective AMPA/kainate antagonist with little activity against NMDA. In the cortical slice preparation assay, ZK200775 gave Ki values of 3.2 nM, 100 nM, and 8.5 μM against quisqualate, kainate, and NMDA, respectively (Table 2). In the spreading depression assay, it gave IC50 values of 200 nM, 76 nM, 13 μM, and 18 μM against quisqualate, kainate, NMDA, and glycine (Table 2).
Table 2.
Effect of ZK200775 on evoked potentials in rat cortical slice preparations and spreading depression in chicken retina
| Quisqualate | Kainate | NMDA | Glycine | |
|---|---|---|---|---|
| Evoked potentials (EP) Ki, μM | 0.0032 | 0.1 | 8.5 | - |
| Spreading depression (SD) | 0.2 | 0.076 | 13 | 18 |
| IC50, μM | (0.05–0.90) | (0.015–0.379) | (7–23) | (6.68–48.25) |
EP: Agonists were applied to rat (Wistar; Møllegaard) cingulate cortex slices in concentrations of 3–100 μM for quisqualate, 0.5–5 μM for kainate, and 5–40 μM for NMDA for 2 min, and the evoked potentials were recorded. Ki values represent concentrations of ZK200775, which produced a two-fold shift to the right of the agonist concentration–response curves and were calculated using linear regression analysis. SD: The posterior chamber of each chicken (3 to 6-day-old, Copenhagen Serum Institute, Denmark) eye was placed in O2 saturated saline containing quisqualate (5 μM), kainate (5 μM), NMDA (100 μM), or glycine (100 μM) alone or with different concentrations of ZK200775 ranging from 0.25 to 75 μM. The occurrence of a white area (0.5 mm in diameter) was taken as the onset of spreading depression, and the latency to it was measured. Cut-off time was set at 60 s. An increase in the latency by 30 s was considered to represent maximal inhibition of spreading depression. The drug effects were expressed as the percentage of maximum inhibition at a given concentration. IC50 values were calculated by means of linear regression analysis.
Electrophysiology.
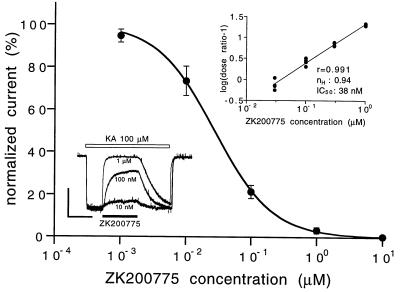
Currents evoked by kainate (nondesensitizing AMPA agonist at 100 μM) were inhibited by ZK200775 with an IC50 of 27 nM (Fig. 2). Currents evoked by AMPA (100 μM) were inhibited by ZK200775 with IC50s of 21 and 90 nM, for peak and plateau currents, respectively. Currents evoked by NMDA (100 μM) in Mg2+-free solutions were not affected by ZK200775 up to a concentration of 1 μM (IC50 40 μM).
Figure 2.
Concentration–response effect of ZK200775 on kainate-induced currents in neonatal rat hippocampal neurons. Current amplitudes were leak-subtracted, normalized to control, and plotted against antagonist concentration. Data represent means ± SEM from 5–13 determinations. (Lower Inset) Original current traces demonstrating effect of ZK200775, 0.01, 0.1, and 1 μM on kainate (100 μM) induced currents in a single cell. (Vertical scale bar = 100 pA; horizontal scale bar = 10 s.) (Upper Inset) Schild analysis of the antagonism by ZK200775 derived from partial concentration–response curves for kainate in the presence of increasing concentrations of ZK200775 (0.03, 0.1, 0.3, and 1 μM). Current amplitudes were leak-subtracted and related to the maximal current obtained at 10 mM kainate in the absence of the antagonist.
Pharmacology of ZK200775.
Pharmacological studies in rodents revealed anxiolytic, anticonvulsant, and muscle relaxant activity of ZK200775 (Fig. 3). In a four-plate test, which measures anxiolytic activity, ZK200775 in doses of 0.3–3 mg/kg i.p. significantly enhanced punished locomotor activity in mice, leaving spontaneous locomotor activity in unpunished mice unchanged (Fig. 3A). ZK200775 elevated the threshold for AMPA- and kainate-induced clonic seizures in mice with a THRD50 (threshold dose) of 2.9 (1.7–4.6) and 1.6 (1.3–2.0) mg/kg i.v., whereas the threshold for NMDA-induced seizures was elevated only in doses, THRD50 of 24.1 (21.9–26.5) mg/kg i.v. (Fig. 3B), which affected motor coordination in the rotating rod, ED50 14.6 (12.1–17.6) mg/kg. ZK200775 in doses of 10 and 30 mg/kg i.v. reduced muscle tone in genetically spastic rats (Fig. 3C). Exploratory activity in nonhabituated mice was reduced by ZK200775 with an ED50 of 14.2 (7.8–25.9) mg/kg i.v. (Fig. 3D). ZK200775 in the dose of 30 mg/kg i.v. induced initial short-lasting (up to 5 min) hyperthermia (up to +0.5°C) and a late time-dependent (30–180 min) hypothermia in rats (up to −2.8°C). ZK200775, 3 and 10 mg/kg i.v., had no effect on body temperature of rats. In mice, only hypothermia was recorded after i.v. administration of ZK200775 in a dose of 10 mg/kg (up to −2.7°C). ZK200775, 3 mg/kg i.v., did not induce any changes in body temperature in mice. In conscious rats, i.v. infusion of ZK200775 in a dose of 3 mg/kg/h for 6 h, a treatment regimen required for neuroprotection in rat permanent cerebral ischemia, had no effect on blood gases or hemodynamic status inducing only slight increases (<10%) in arterial blood pressure.
Figure 3.
Anxiolytic (A), anticonvulsant (B), muscle relaxant (C), and sedative (D) activity of ZK200775 in rodents. For assessment of anxiolytic activity (A) of ZK200775, 0.3, 1.0, and 3.0 mg/kg i.p. (30 min), mice were placed in the center of a chamber with a floor divided into four plates and, after 20 s of exploration, received a shock (1 mA, 60 ms) each time crossing from one plate to another. The number of crossings within 1 min were taken as a measure of exploratory activity. Experimental groups consisted of eight mice. ANOVA showed significant main effect [F(3, 30) = 4.8; P < 0.01] revealing that ZK200775 increased punished locomotor activity in mice. For assessment of the threshold for clonic seizures (B), AMPA, kainate or NMDA (1 nmol/5 μl) were infused continuously intracerebroventricularly to mice with a speed of 5 μl/min. ZK200775 was administered i.v. 5 min before the seizure test. The time in seconds to a clonic seizure was used as an endpoint determining susceptibility to convulsions. The THRD50 (threshold dose) was calculated in nanomols by means of regression analysis. Experimental groups consisted of five to eight mice. *P < 0.05; **P < 0.01; ***P < 0.001 vs. vehicle-treated mice. Muscle relaxant (C) effect of ZK200775 was measured in electromyogram (EMG) recorded from gastrocnemius muscle of genetically spastic rats after i.v. administration of ZK200775 (open circles), 1 (dotted), 3 (dash-dotted), 10 (dashed), and 30 (solid) mg/kg or vehicle (filled circles). The electrical signals were amplified, band-pass filtered (5–10 kHz), full wave rectified, and normalized. The electromyogram was recorded continuously, and the average integrated activity was determined in 5-min intervals over 2 h. Experimental groups consisted of 5–12 rats. ANOVA revealed that ZK200775 decreased muscle tone in genetically spastic rats in a dose- [Fdose(4, 36) = 15.88; P < 0.0001] and time-dependent [Ftime(4, 144) = 7.41, P < 0.0001] manner. Exploratory activity (D) in NMRI mice was monitored over 5 min beginning immediately after i.v. administration of ZK200775. Experimental groups consisted of eight mice. ED50 was calculated by means of regression analysis. *P < 0.05; ***P < 0.001 vs. vehicle-treated mice.
Global Ischemia in Gerbils.
Transient bilateral occlusion of the CCA in Mongolian gerbils for 4 min induced damage of the CA1 hippocampal subfield and produced an increase in locomotor activity after 3–4 days of reperfusion. Treatment of gerbils with 10 mg/kg ZK200775 i.p. 30, 90, 150, and 210 min after occlusion significantly reduced the damage in the hippocampus and prevented the increase in locomotor activity (Table 3).
Table 3.
Effect of ZK200775 on neuronal loss in the hippocampal CA1 subfield and locomotor activity in gerbils subjected to bilateral occlusion of common carotid arteries (CCAO) for 4 min
| Surgery | Vehicle | ZK200775 |
|---|---|---|
| Neuronal loss score in hippocampal CA1 subfield | ||
| CCAO | 5.97 (17) | 4.89* (13) |
| Exploratory activity (counts) | ||
| CCAO | 12804 ± 1307 | 7296 ± 1200** |
| Sham | 7382 ± 1274 (10) | 5679 ± 603 (10) |
Mongolian gerbils (Møllegaard) were subjected to i.p. administration of ZK200775 in the dose of 10 mg/kg 30, 90, 150, and 210 min after CCAO. Body temperature was maintained at 37.5 ± 0.5°C. Locomotor activity was measured 3 days after CCAO for 30 min. The extent of degeneration in the hippocampus was assessed 24 h later in cresyl violet and hematoxilin/eosin-stained coronal sections of the entire brain and blindly scored on each site from 0 (no cell loss) to 3 (total cell loss). The maximal damage score for both hippocampal CA1 subfields was 6.0
P < 0.05 (Mann–Whitney U test); **P < 0.01 (Student’s t test) vs vehicle-treated gerbils.
Middle Cerebral Artery Occlusion in Rodents.
In mice subjected to focal ischemia, i.v. infusion of ZK200775 at a dose of 10 mg/kg/h over 6 h, starting immediately after permanent MCAO, reduced the infarct volume by 34% (Table 4A). In rats subjected to the permanent MCAO, ZK200775 reduced the infarct volume by 24% at 3 and by 29% at 10 mg/kg/h when infused i.v. over 6 h (Table 4A). Neuroprotection in focal ischemia offered by treatment with ZK200775 was permanent; the reduction in infarct volume, after treatment of rats with 3 mg/kg/h over 6 h starting immediately after MCAO, was still seen after 7 days and amounted to 45% (Table 4A). It was surprising that delayed treatment of rats with ZK200775 at a dose of 3 mg/kg/h for 6 h, beginning 1, 2, 4, or 5 h after permanent MCAO, reduced the infarct volume by 20–30% (Table 4B), suggesting a long therapeutic time window of >4 h. When treatment was initiated 6 h after permanent occlusion, infarct volume was reduced by only 6%. Little protection (up to 8%) was seen when therapy with ZK200775 was initiated between 7 and 24 h after occlusion (Table 4B). Neuroprotective action of ZK200775 was confirmed in the rat focal cerebral ischemia with reperfusion based on temporary occlusion of the CCA/MCA for 90 min. The infarct size was reduced at a dose of 0.1 mg/kg/h by 45% (Table 4C) when i.v. infusion was initiated at the onset of reperfusion. Treatment with ZK200775, 1 and 3 mg/kg/h for 6 h, beginning at the onset of reperfusion, reduced the infarct volume by 19% (93.30 ± 7.58 mm3; n = 11) and 35% (74.83 ± 7.49 mm3; n = 13, P < 0.05), respectively. When infusion of ZK200775 in the dose of 0.1 mg/kg/h was initiated 2 h after onset of reperfusion, morphometric assessment performed 7 days later revealed that the infarct volume was reduced by 44% (117.08 ± 12.36 mm3; n = 12 vs. 62.29 ± 9.63 mm3; n = 16, P < 0.05).
Table 4.
Dose–response relationship and time window of ZK200775 in rodent models of ischemia and head trauma
| A MCAO |
Infarct volume (mm3; means ± SEM), n
|
|||
|---|---|---|---|---|
| Treatment | Vehicle | 1 mg/kg/h | 3 mg/kg/h | 10 mg/kg/h |
| Mouse, 1 d | 26.05 ± 1.55 (10) | – | 28.80 ± 3.01 (8) | 17.18 ± 1.44** (9) |
| Rat, 1 d | 160.79 ± 10.56 (25) | 142.07 ± 6.88 (9) | 122.92 ± 9.97* (16) | 113.64 ± 13.7* (12) |
| Rat, 7 d | 84.39 ± 9.95 (27) | 82.51 ± 9.70 (22) | 46.23 ± 7.45** (17) | – |
| B Time window, rat MCAO | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment delay | 1 h | 2 h | 4 h | 5 h | 6 h | 7 h | 12 h | 24 h |
| Vehicle | 206.20 ± 16.36 | 194.57 ± 19.28 | 213.42 ± 15.66 | 236.59 ± 11.90 | 218.22 ± 16.91 | 210.06 ± 20.06 | 194.01 ± 18.57 | 216.42 ± 12.28 |
| (12) | (9) | (8) | (19) | (14) | (14) | (15) | (15) | |
| 3 mg/kg/h | 146.82 ± 16.43** | 144.32 ± 14.15* | 168.57 ± 8.80* | 190.65 ± 14.13* | 204.64 ± 13.92 | 208.66 ± 14.38 | 179.58 ± 15.08 | 199.27 ± 19.37 |
| (12) | (13) | (8) | (18) | (15) | (18) | (16) | (18) | |
| % protection | 28.80 | 25.83 | 21.01 | 19.42 | 6.22 | 0.67 | 7.44 | 7.92 |
| C Common carotid/middle cerebral artery occlusion (CCA/MCAO) | ||||
|---|---|---|---|---|
| Treatment | Vehicle | 0.01 mg/kg/h | 0.03 mg/kg/h | 0.1 mg/kg/h |
| Rat CCA/MCAO transient, 7 d | 115.10 ± 17.41 (12) | 114.92 ± 16.61 (13) | 112.14 ± 9.99 (11) | 63.33 ± 9.78** (13) |
| % protection | – | 0.16 | 2.57 | 44.98 |
| D Rat traumatic brain injury, 3 d |
Numerical density of pyramidal cells in the hippocampal CA3 subfield (NV; means × 106/mm3 ± SEM), n
|
||||
|---|---|---|---|---|---|
| Treatment | Vehicle | 0.03 mg/kg/h | 0.3 mg/kg/h | 1 mg/kg/h | 3 mg/kg/h |
| Ipsilateral side | 0.067 ± 0.004 (18) | 0.067 ± 0.010 (5) | 0.049 ± 0.013 (7) | 0.063 ± 0.010 (7) | 0.108 ± 0.009*** (8) |
Body temperature of NMRI mice (Schering AG), Fischer 344, or Wistar rats (Charles River, Sulzfeld, Germany/Iffa Credo, Arbresle, France) was kept at 37.5 ± 0.5°C; 24 h or 7 days after ischemia, the brains were stained with triphenyltetrazolium chloride or with vanadium acidic fuchsin, and the infarct size was determined stereologically by using image analysis. ZK200775 was administered i.v. over 6 h beginning immediately after MCAO or CCA/MCAO. For determination of therapeutic time window, ZK200775 was infused continuously i.v. in the dose of 3 mg/kg/h over 6 h beginning 1-24 h after MCAO. In trauma experiments, ZK200775 was infused i.v. over 6 h beginning 1 h after the head injury. Three days after traumatic brain injury, volume of damage in the cortex was determined by means of volumetry, and neuronal loss in the hippocampal subfield CA3 was assessed by using an unbiased stereological dissector technique (24). On the side contralateral to traumatic injury, numerical density (NV) of pyramidal cells in the CA3 subfield varied from 0.171 to 0.184 × 106/mm3. The differences in NV of pyramidal cells in the CA3 subfield between the damaged and nondamaged side were analyzed statistically by means of ANOVA.
*P < 0.05; **P < 0.01; ***P < 0.001 vs. vehicle-treated animals.
Traumatic Brain Injury.
Rats subjected to percussion trauma of the cortex showed primary damage in the cortex and secondary damage in the hippocampus. Morphometric analysis demonstrated that both cortical and hippocampal damage was mitigated by treatment with ZK200775. Delayed treatment of rats with ZK200775 at a dose of 0.3 mg/kg/h over 6 h, beginning 1 h after cortex trauma, reduced the volume of cortical damage by 41% (16.03 ± 0.96 mm3; n = 10 vs. 9.52 ± 1.67 mm3; n = 6, F(3, 25) = 5.70, P < 0.005), whereas the dose of 3 mg/kg/h reduced loss of pyramidal neurons in the hippocampal CA3 subfield by 37% [0.067 ± 0.004; n = 18 vs. 0.108 ± 0.009; n = 8, F(4, 40) = 8.06, P < 0.0001, ANOVA] (Table 4D).
Effect of ZK200775 on Morphology of Retrosplenial/Cingulate Cortex in Rats.
Neuronal vacuolization was not observed in rats killed 12 h after i.v. infusion of ZK200775 in doses of 0.67, 2, and 6 mg/kg/h for 6 h. Treatment of rats with ZK200775 at a dose of 3 mg/kg/h for 6 h also did not affect density of neurons in both the cingulate and retrosplenial cortex after 72 h (Table 5).
Table 5.
Numerical density of neurons in cingulate and retrosplenial cortex in rats subjected to i.v. infusion of ZK200775, 3 mg/kg/h, or vehicle for 6 h
| Numerical density (NV; means × 106/mm3 ± SEM), n
|
|||
|---|---|---|---|
| Vehicle | ZK200775 | % | |
| Cingulate cortex | 0.158 ± 0.004 (17) | 0.168 ± 0.004 (8) | 6 |
| Retrosplenial cortex | 0.310 ± 0.004 (19) | 0.320 ± 0.005 (11) | 3 |
To quantitatively assess possible neuronal loss in the retrosplenial and posterior cingulate cortex 3 days after beginning of the i.v. infusion of ZK200775 or vehicle in Fischer 344 rats (Charles River), an unbiased stereological dissector technique (24) was used to estimate the mean numerical density (NV). An unbiased counting frame (0.05 × 0.05 mm; dissector height 0.01 mm) and a high aperture (100×) oil-immersion objective were used for the sampling. The NV for each field was determined with 5-9 dissectors. ANOVA revealed no significant difference between vehicle- and ZK200775-treated rats: Fcing(1,24) = 1.99, NS; Fretro(1,29) = 1.96, NS.
Effect of ZK200775 on Renal Morphology.
Continuous infusion of ZK200775 in doses of up to 6 mg/kg/h over 4 weeks in rats and up to 0.2 mg/kg/h over 4 weeks in dogs did not produce signs of kidney toxicity.
DISCUSSION
The successful incorporation of the methylphosphonate group into the quinoxalinedione framework represents a breakthrough in the design of competitive glutamate antagonists because the resulting derivative ZK200775 is a preferential AMPA/kainate antagonist with little or no affinity to NMDA channel-associated binding sites such as those labeled by 3H-CPP, 3H-TCP, 3H-DCKA, or 3H-glycine. The effects of ZK200775 on amino acids induced evoked potentials, spreading depression, or currents in rat hippocampal neurons confirm its preferential AMPA/kainate selectivity profile.
Another new feature is the excellent solubility of ZK200775 in water at physiological pH, promising a lack of renal toxicity. In fact, no kidney toxicity was observed during long term infusions of high doses of ZK200775 in rats and dogs.
An additional advantage of ZK200775 over other quinoxalinediones such as NBQX or YM90K and NMDA antagonists is the favorable safety profile allowing for effective protection against ischemic and traumatic brain injury with only minimal systemic side effects. No major deleterious effects on motor behavior, cardiovascular status, or respiratory system were detected in doses up to 10 mg/kg/h i.v. over 6 h. Body temperature was affected by ZK200775 only in doses higher than those required for neuroprotection in ischemia and trauma models. Sedation and muscle relaxation were observed in doses higher than those required for neuroprotection. Psychotomimetic effects such as hyperactivity, jumping, head weaving, or stereotypy, which may be induced in rodents by NMDA antagonists (29), were not observed during treatment with ZK200775 (30). Induction of vacuoles in retrosplenial/cingulate cortex or degeneration of neurons, typical neurotoxic side effect of NMDA antagonists in the rodent brain (27), were not observed after treatment with ZK200775.
The therapeutic time window is a key to the clinical use of neuroprotective drugs (31). ZK200775 protected rat brain from the damage induced by permanent focal ischemia when delayed up to 5 h after the occlusion, suggesting a superb opportunity for therapeutic intervention in stroke patients. It was surprising that delayed treatment with ZK200775 commencing 2 h after the onset of reperfusion protected rat brain from the damage induced by transient focal ischemia. The maximum efficacy of ZK200775 in transient focal ischemia required 0.1 mg/kg/h over 6 h only, the dose being 30–100 times lower than that necessary for protection in the rat or mouse permanent focal ischemia. Such an efficacy profile has not been reported previously for any other glutamate antagonist. Furthermore, no other glutamate antagonist has been shown to be active in transient focal ischemia when treatment was delayed by 2 h after the onset of reperfusion.
The elevated glutamate concentration in the brain of rodents subjected to focal ischemia or head trauma is transient, lasting for up to 2 h only (32). However, a sustained elevation of glutamate concentration can be observed in stroke and trauma patients for up to 1–4 days after the insult (33, 34), suggesting that the therapeutic time window for glutamate antagonists in humans may be longer than that in rodent models. The extent of protection offered by ZK200775 in permanent focal ischemia in rats was similar over the initial 5 h after the MCAO predicting an unusual efficacy profile in stroke patients. Furthermore, in permanent and transient focal ischemia, ZK200775 appears to provide permanent protection because it is still detectable after 7 days; the protective effect of NMDA antagonists often decreases or disappears with time (35). It was surprising that the extent of protection produced by ZK200775 increased with time and was more pronounced after 7 days than after 24 h.
Broad spectrum of neuroprotective activity of ZK200775 was confirmed in global ischemia in gerbils and in the rat head trauma model. In the gerbil model of global ischemia, ZK200775 offered morphological and functional improvement decreasing hyperactivity triggered by hippocampal damage. In the contusion brain trauma model, ZK200775 efficiently mitigated primary damage in the cortex and secondary damage in the hippocampus on treatment delayed by 1 h.
In summary, the favorable efficacy profile of ZK200775 in ischemia and trauma models and its physicochemical properties justify its clinical development and offer new prospects for the future treatment of stroke and trauma victims.
Acknowledgments
The unfailing technical assistance of K. Behn, P. Böttcher, I. Bredmose, K. Bressler, F. Fiske, M. Gieseler, L. Igel, H. Lam, A. Meincke, T. Møller, R. Neumeister, W. Niemann, V. Petersen, P. Pietzuch, E. Richter, J. Seidler, A. Seltz, O. Schmücker, C. Schneider, and K. Zessin is acknowledged gratefully. We also thank Drs. K. Höhlmann, B. Voet, and R. Vonk for expert statistical advice.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- NMDA
N-methyl-d-aspartate
- Nv
numerical density
- ZK200775
[1,2,3,4-tetrahydro-7-morpholinyl-2,3-dioxo-6-(trifluoromethyl)quinoxalin-1-yl]methylphosphonate
- MCAO
middle cerebral artery occlusion
- CCAO
common carotid artery occlusion
References
- 1.Barinaga M. Science. 1996;272:664–666. [PubMed] [Google Scholar]
- 2.Schehr R S. Nature Biotech. 1996;14:1549–1554. doi: 10.1038/nbt1196-1549. (1996). [DOI] [PubMed] [Google Scholar]
- 3.Seeburg P H, Bresink I, Turski L. Excitatory Amino Acids: From Genes to Therapy. Heidelberg: Springer; 1998. [Google Scholar]
- 4.Herrling P L. Excitatory Amino Acids: Clinical Results with Antagonists. New York: Academic; 1997. [Google Scholar]
- 5.Sheardown M J, Nielsen E O, Hansen A J, Jacobsen P, Honor T. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu-Sasamata M, Kawasaki-Iatsugi S, Okada M, Sakamoto S, Yatsugi S-I, Togami J, Hatanaka K-I, Ohmori J, Koshija K, Usuda S, Murase K. J Pharmacol Exp Ther. 1996;276:84–92. [PubMed] [Google Scholar]
- 7.Olverman H J, Jones A W, Watkins J C. Nature (London) 1984;307:460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- 8.Watkins J C, Krogsgaard-Larsen P, Honoré T. Trends Pharmacol Sci. 1990;11:25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- 9.Honoré T, Drejer J, Nielsen M. J Neurochem. 1988;51:457–461. doi: 10.1111/j.1471-4159.1988.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 10.Honoré T, Drejer J, Nielsen E O, Nielsen M. Biochem Pharmacol. 1989;38:3207–3212. doi: 10.1016/0006-2952(89)90615-1. [DOI] [PubMed] [Google Scholar]
- 11.Honoré T, Drejer J, Nielsen M. Neurosci Lett. 1989;65:47–52. doi: 10.1016/0304-3940(86)90118-7. [DOI] [PubMed] [Google Scholar]
- 12.Olverman H J, Monaghan D T, Cotman C W, Watkins J C. Eur J Pharmacol. 1986;131:161–162. doi: 10.1016/0014-2999(86)90533-9. [DOI] [PubMed] [Google Scholar]
- 13.Stirling J M, Cross A J, Green A R. Neuropharmacology. 1989;28:1–7. doi: 10.1016/0028-3908(89)90059-2. [DOI] [PubMed] [Google Scholar]
- 14.Canton T, Doble A, Miquet J M, Jimonet P, Blanchard J C. J Pharm Pharmacol. 1992;44:812–816. doi: 10.1111/j.2042-7158.1992.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 15.Haring R, Stanton P K, Scheideler M A, Moskal J R. J Neurochem. 1991;57:323–332. doi: 10.1111/j.1471-4159.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison N L, Simmonds M A. Br J Pharmacol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheardown M J. Brain Res. 1993;607:189–194. doi: 10.1016/0006-8993(93)91506-n. (1993). [DOI] [PubMed] [Google Scholar]
- 18.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 19.Stephens D N, Kehr W. Psychopharmacology. 1985;85:143–147. doi: 10.1007/BF00428403. [DOI] [PubMed] [Google Scholar]
- 20.Steppuhn K G, Turski L. Proc Natl Acad Sci USA. 1993;90:6889–6893. doi: 10.1073/pnas.90.14.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunham M S, Miya T A. J Am Pharm Ass Sci Ed. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 22.Pinna G, Galici R, Schneider H H, Stephens D N, Turski L. Proc Natl Acad Sci USA. 1997;94:2719–2723. doi: 10.1073/pnas.94.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turski L, Kleinrok Z. Psychopharmacology. 1980;71:35–39. doi: 10.1007/BF00433249. [DOI] [PubMed] [Google Scholar]
- 24.Bernert H, Turski L. Proc Natl Acad Sci USA. 1996;93:5235–5240. doi: 10.1073/pnas.93.11.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikonomidou C, Turski L. Ann NY Acad Sci. 1997;827:394–402. doi: 10.1111/j.1749-6632.1997.tb48450.x. [DOI] [PubMed] [Google Scholar]
- 26.Lippert K, Welsch M, Krieglstein J. Eur J Pharmacol. 1994;253:207–213. doi: 10.1016/0014-2999(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 27.Olney J W, Labruyere J, Price M T. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 28.Xue D, Huang Z-G, Barnes K, Lesiuk H, Smith K E, Buchan A, M. J Cereb Blood Flow Metab. 1994;14:251–259. doi: 10.1038/jcbfm.1994.32. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch S I, Rosse R B, Mastropaolo J. Clin Neuropharmacol. 1997;20:375–384. doi: 10.1097/00002826-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Turski L, Huth A, Sheardown M J, Jacobsen P, Ottow E. Soc Neurosci Abstr. 1996;22:1529. [Google Scholar]
- 31.Pulsinelli W. Lancet. 1992;339:533–536. doi: 10.1016/0140-6736(92)90347-6. [DOI] [PubMed] [Google Scholar]
- 32.Takagi K, Ginsberg M D, Globus M Y T, Dietrich D, Martinez E, Kraydieh S, Busto R. J Cereb Blood Flow Metab. 1993;13:575–585. doi: 10.1038/jcbfm.1993.75. [DOI] [PubMed] [Google Scholar]
- 33.Bullock R, Zauner A, Woodward J, Young H F. Stroke. 1995;26:2187–2189. doi: 10.1161/01.str.26.11.2187. [DOI] [PubMed] [Google Scholar]
- 34.Dávalos A, Castillo J, Serena J, Noya M. Stroke. 1997;28:708–710. doi: 10.1161/01.str.28.4.708. [DOI] [PubMed] [Google Scholar]
- 35.Buchan A. Cerebrovasc Brain Metab Rev. 1990;2:1–56. [PubMed] [Google Scholar]