Abstract
Mechanical stress to alveolar walls may cause progressive damage after an early-life insult such as exposure to environmental tobacco smoke (ETS). This hypothesis was examined by using data from the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based cohort aged 45–84 years, free of clinical cardiovascular disease, recruited from 6 US sites in 2000–2002. The MESA-Lung Study assessed a fractal, structural measure of early emphysema (“alpha,” lower values indicate more emphysema) and a standard quantitative measure (“percent emphysema”) from cardiac computed tomography scans. Childhood ETS exposure was assessed retrospectively as a report of living with one or more regular indoor smokers. Analyses included 1,781 nonsmokers (<100 cigarettes, 20 cigars, or 20 pipefulls in their lifetime and urinary cotinine levels <100 ng/mL); mean age was 61 years (standard deviation, 10), and 65% were women. Childhood ETS exposure from 2 or more smokers (17%) compared with none (52%) was associated with 0.05 lower alpha and 2.8 higher percent emphysema (P for trend = 0.04 and 0.01, respectively) after adjustment for demographic, anthropometric, parental, and participant characteristics, as well as adult exposures (e.g., cumulative residential air pollution exposure, exposure to ETS as an adult). Childhood ETS exposure was associated with detectable differences on computed tomography scans of adult lungs of nonsmokers.
Keywords: cohort studies, diagnostic imaging, emphysema, lung, residence characteristics, spirometry, time, tobacco smoke pollution
Emphysema is defined anatomically as the destruction of alveolar walls and permanent enlargement of airspaces distal to the terminal bronchioles (1). Emphysema overlaps appreciably, but incompletely, with chronic obstructive pulmonary disease, which is defined by airflow obstruction that is not fully reversible (2). Combined, emphysema and chronic obstructive pulmonary disease are projected to become the third leading cause of death worldwide by 2020 (3, 4), and their global prevalence is estimated to be approximately 10% (2).
Emphysema can be diagnosed on histopathology or on computed tomography (CT) scans and can be subdivided into 2 major types (5). Centriacinar/centrilobular emphysema occurs predominantly in the lung apices. The major risk factor for centrilobular emphysema, as for chronic obstructive pulmonary disease, is cigarette smoking (6, 7). Panlobular emphysema generally occurs diffusely or in the lower lobes and occurs not infrequently in nonsmokers (7, 8). In a series of consecutive autopsies of older accident victims, for example, 10 of 72 lung specimens from nonsmokers exhibited moderate panlobular emphysema (7).
The major known cause of panlobular emphysema is alpha 1-antitrypsin deficiency (9), which affects fewer than 0.1% of individuals in population-based studies. Other risk factors for panlobular emphysema among nonsmokers have not been well described (6), in part because of the lack of population-based cohorts with quantitative measures of emphysema.
The mechanical hypothesis of emphysema posits that emphysema may develop and progress because of simple mechanical strain on alveolar walls (10, 11). The absence of a given alveolar wall increases mechanical strain on surrounding alveolar walls, predisposing them to rupture. Adjacent alveoli connected by loss of their interalveolar septum form an emphysematous sac, which places additional strain on neighboring alveolar walls. Emphysematous sacs therefore propagate and enlarge. Mishima et al. (10) found that the reduction in number of alveoli and increase in average sac size in a lattice model followed a fractal pattern. The same fractal relation between emphysematous hole number and size was observed on CT scans of patients with early chronic obstructive pulmonary disease.
Under this mechanical hypothesis, as well as other theories that frame early life as a sensitive period (12, 13), early insults should have a disproportionately large impact on the development of emphysema. One such early-life insult may be exposure to environmental tobacco smoke (ETS) in the childhood home. We therefore examined whether ETS exposure in childhood was associated with diffuse early emphysema and diffuse percent emphysema on CT scan in a large, population-based cohort of adults. We restricted the analysis to participants who denied a significant smoking history given our interest in emphysema in nonsmokers and to minimize potential confounding by smoking in adulthood.
MATERIALS AND METHODS
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter, prospective cohort study designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease. Details on the study design have been published previously (14). The study enrolled 6,814 men and women who were classified as African American, Chinese, Hispanic, or non-Hispanic white based on self-reported race and ethnicity. Participants were recruited from 6 communities in the United States: Forsyth County, North Carolina; northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles, California. All participants were aged 45–84 years when recruited in 2000–2002. Exclusion criteria included clinical cardiovascular disease, any cardiovascular procedure, weight >300 pounds (135 kg), pregnancy, or impediment to long-term participation. The protocols of MESA and all studies described herein were approved by the institutional review boards of all collaborating institutions and the National Heart, Lung, and Blood Institute (Bethesda, Maryland).
The MESA-Lung Study enrolled 3,965 participants of 4,483 randomly selected from all MESA participants who consented to genetic analyses, underwent baseline measures of endothelial function, and attended an examination during the MESA-Lung Study recruitment period in 2004–2006 (99%, 89%, and 91% of the MESA cohort, respectively). Chinese participants were oversampled, resulting in a sample that was 24% Chinese, 30% non-Hispanic white, 22% non-Hispanic black, and 23% Hispanic.
The present study included 1,781 MESA-Lung participants who met all of the following criteria. They reported that they had smoked fewer than 1) 100 cigarettes, 2) 20 cigars, and 3) 20 pipefulls in their lifetime. In addition, they confirmed this information at least a second time during a clinic visit at least 18 months later, and their urinary cotinine levels were <100 ng/mL at the time of the CT scan.
Early emphysema and percent emphysema
Quantitative measures of early emphysema were performed on the lung fields of cardiac CT scans, which imaged approximately 70% of the lung volume from the carina to the lung bases. Cardiac CT scans were performed at full inspiration on multidetector CT and electron-beam CT scanners in 2000–2002, following a standardized protocol (15). Two scans were performed on each participant; the scan with higher air volume was used for analyses, except that, when the 2 scans had discordant quality control scores, the higher quality scan was used (11).
Image attenuation was assessed by using modified Pulmonary Analysis Software Suite (16, 17) at a single reading center by trained readers without knowledge of other participant information. To correct for variations in scanner calibration and in the way different scanners handle scatter and beam hardening, we measured the attenuation of air outside and inside the body for each scan. Air outside the body, which should have a mean attenuation of −1,000 Hounsfield units (HU), was sampled in a region well away from the body, scanner table, and blankets. The attenuation of each pixel in the lung regions was then corrected to have the value equal measured pixel attenuation × (−1,000/mean air attenuation), from which emphysema measures were calculated. For our main analysis, emphysema measures were corrected for air attenuation outside the body and were calculated by using a threshold of −910 HU to define emphysema-like lung; sensitivity analyses were performed by using emphysema measures corrected for air attenuation within the body and by using an alternative threshold of −950 HU.
Early emphysema was defined by a measure termed “alpha” (10, 11). Emphysematous holes on CT scan were defined as connected voxels within a scanned slice falling below −910 HU. Alpha is defined as the slope of the log-log relation of hole size (x-axis) versus number of holes discernible in the lung field of a CT scan. This relation is linear (mean r2 on MESA scans = 0.97) and increases (approaching zero) with the degree of severity as multiple small holes merge to form fewer, larger holes. The slope of this log-log relation is termed “d” in studies by Mishima et al. (10), and alpha is equal to d × (−1). In our data, the coefficient of variation of alpha from paired cardiac scans was 4.3%, and the intraclass correlation coefficient was 0.88 (18).
Percent of emphysema-like lung (also known as percent low attenuation area and hereafter referred to as percent emphysema) was defined as the percentage of voxels in the lung that fell below −910 HU. This threshold was derived from comparisons to pathologic specimens (19). The intraclass correlation coefficient for percent emphysema was 0.94. Alpha and percent emphysema from cardiac scans correlated with those measures from full-lung scans from 42 MESA participants (e.g., 0.88 for alpha and 0.93 for percent emphysema on multidetector CT scanner data) (18).
For secondary analyses, apical and basal lung segments were defined as the cephalad or caudal eighths of the lung divided along the z-axis scan coverage of the cardiac CT scan, enabling estimation of apical-basilar difference in percent emphysema. The correlation of this measure with apical-basilar difference in percent emphysema from full lung scans is 0.76 (18).
Spirometry
Spirometry was conducted in 2004–2006 in accordance with American Thoracic Society/European Respiratory Society guidelines (20). Tests were conducted by using a dry-rolling-sealed spirometer and software that performed automated quality checks in real time (Occupational Marketing, Inc., Houston, Texas). All spirometry examinations were reviewed by one investigator, and each test was graded for quality (21). The intraclass correlation coefficient of both forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) on random 10% repeat testing was 0.99.
Childhood ETS exposure and parental smoking
Childhood ETS exposure was based on participants’ retrospective reports in response to the question, “In your childhood, did you live with a regular cigarette smoker who smoked in your home?” Participants also reported the number of smokers (“In your childhood, how many smokers lived in your home?”), and childhood ETS was categorized as none, one regular smoker in the home, or two or more regular smokers in the home. Parental smoking was classified as neither mother nor father, father only, mother only, or both mother and father based on a separate item that asked whether each parent smoked (“Did or does he/she smoke cigarettes?”).
Demographic characteristics and other covariates
Most covariates, including age, gender, race, ethnicity, and country of birth, were self-reported. Participants were asked to report their educational attainment (8 categories, from which years of education was estimated (22)). Asthma was defined as self-report of physician-diagnosed asthma, with symptom onset before age 45 years. Occupational exposure to dust was assessed by asking, “Have you ever been exposed at work to dust?” Environmental tobacco exposure in adulthood was measured with the following 2 items: “As an adult, have you ever lived with a regular cigarette smoker (not including yourself) who smoked in your home?” and “As an adult, have you ever regularly spent time, when you were not at home, indoors where people were smoking cigarettes (for example, at work)?”
Urinary cotinine was measured by immunoassay (Immulite 2000 Nicotine Metabolite Assay; Diagnostic Products Corp., Los Angeles, California). Air pollution was assessed by using a 20-year residential history to approximate the cumulative exposure to particles with a diameter smaller than 10 μm based on data from the US Environmental Protection Agency's Aerometric Information Retrieval System; exposure for the 20-year period was summarized by using an area-under-the-curve calculation (23). Height was measured to the nearest 0.1 cm, and weight was measured to the nearest pound (1 pound = 0.45 kg).
Statistical analysis
All outcomes were analyzed by using generalized linear regression with robust variance estimates. Models of percent emphysema, which was highly skewed, used a gamma error distribution.
Regression models were adjusted for personal, familial, and environmental characteristics that either 1) had the potential to confound the associations of interest because of their role as a common prior cause of both childhood ETS exposure and adult respiratory health or 2) were potential precision variables, outside of the causal pathway of interest but likely to explain some of the variance in the adult respiratory outcomes. The specific adjustment variables included age, sex, race/ethnicity, height, body mass index, whether the participant was born in the United States, mother's and father's educational attainment (whether each completed high school), participant's educational attainment (a grouped linear variable with 5 categories), history of asthma before the age of 45 years, cumulative residential air pollution exposure, living with a smoker as an adult, occupational exposures (dichotomous indicators for secondhand smoke and dust), and urinary cotinine (a dichotomous indicator of detectable levels to indicate recent ETS exposure). The continuous covariates age, height, and body mass index were modeled with linear and centered quadratic terms. Models also adjusted for CT scanner type and, if multidetector CT scanner, weight above 100 kg (220 pounds) since the dose of radiation, measured in milliampere seconds, was increased for these participants (15).
For model adjustment, missing data on covariates (father's education, n = 92; mother's education, n = 56; paternal smoking, n = 117; maternal smoking, n = 34; birth outside of the United States, n = 3; participant's education, n = 3; ETS in the adult household, n = 1; cumulative air pollution exposure, n = 406; occupational ETS exposure, n = 20; and occupational dust exposure, n = 5) were estimated by multiple imputation (24). Five imputed data sets were created, and their results were recombined such that regression confidence intervals reflected uncertainty from missing covariate data. Analyses were performed with Stata 10.0 software (Stata Corp., College Station, Texas).
RESULTS
The study sample included 1,781 participants who did not smoke (Table 1). The mean age was 61 years, and 65% were female. Participants were born between 1917 and 1957 (median: 1940). Approximately half of participants reported childhood ETS exposure from at least one regular smoker in their childhood home. Participants reporting childhood ETS exposure were somewhat younger, more likely to be non-Hispanic white, and less likely to have been born outside the United States. However, childhood ETS did not appear to be associated with adult educational attainment or an estimate of cumulative residential exposure to air pollution (specifically, particles with a diameter smaller than 10 μm). Participants who reported a smoker in the childhood home were more likely to report that their mother, father, or both smoked. For participants who reported childhood ETS exposure but no parental smoking, the regular smoker may have been another household member; however, we did not have additional information on number of members of the household.
Table 1.
Characteristics of Participants,a by Childhood ETSb Exposure, in the Multi-Ethnic Study of Atherosclerosis, United States, 2000–2006
| No Childhood ETS Exposure (n = 923) | One Smoker in the Childhood Home (n = 559) | Two or More Smokers in the Childhood Home (n = 299) | |
| Male sex | 35 | 34 | 37 |
| Age, years | 62 (10) | 61 (10) | 59 (10) |
| Adult height, cm | 163 (10) | 164 (9) | 165 (10) |
| Body mass index, kg/m2 | 27 (5) | 28 (6) | 28 (5) |
| White race/ethnicity | 24 | 33 | 39 |
| Chinese race/ethnicity | 28 | 25 | 16 |
| Black race/ethnicity | 22 | 22 | 23 |
| Hispanic race/ethnicity | 27 | 20 | 21 |
| Born outside of the United States | 55 | 42 | 37 |
| Father completed high school | 44 | 42 | 51 |
| Mother completed high school | 37 | 40 | 42 |
| Neither parent smoked | 74 | 6 | 7 |
| Only the father smoked | 20 | 75 | 19 |
| Only the mother smoked | 4 | 14 | 6 |
| Both the mother and father smoked | 2 | 5 | 68 |
| Asthma before age 45 years | 8 | 8 | 11 |
| Education, years | 13 (4) | 14 (4) | 14 (4) |
| Lived with a smoker as an adult | 30 | 41 | 38 |
| Occupational exposure to secondhand smoke | 32 | 38 | 44 |
| Occupational exposure to dust | 29 | 29 | 40 |
| AUC for cumulative residential PM10 exposure | 35 (8) | 35 (8) | 34 (7) |
| Cotinine levels below detection threshold (≤7.07 ng/mL) | 86 | 87 | 79 |
| Cotinine levels detectable (>7.07 and <100 ng/mL) | 14 | 13 | 21 |
Abbreviations: AUC, area under the curve; ETS, environmental tobacco smoke; PM10, particles with a diameter smaller than 10 μm.
Values are expressed as percentage or mean (standard deviation).
Childhood ETS was defined by retrospective reports in response to the question, “In your childhood, did you live with a regular cigarette smoker who smoked in your home?” and a follow-up question on the number of smokers in the childhood home.
Childhood ETS exposure
The number of smokers in the childhood home was classified as none, one, or two more and was monotonically associated with both alpha and percent emphysema after adjusting for all covariates (Table 2). The magnitude of the estimated differences in emphysema measures was modest, equal to approximately one-tenth of a standard deviation for alpha. In contrast, there was no association of childhood ETS exposure with apical-basilar difference in emphysema after adjustment.
Table 2.
Associations of Childhood ETSa Exposure With CT Measures of Early Emphysema in the Multi-Ethnic Study of Atherosclerosis, United States, 2000–2006
| No. | Mean (SD) | Unadjusted Model |
Adjusted Modelb |
P for Trend | |||
| Difference | 95% CI | Difference | 95% CI | ||||
| Alphac | |||||||
| No childhood ETS exposure | 923 | 1.24 (0.30) | Reference | Reference | 0.04 | ||
| One smoker in the childhood home | 559 | 1.22 (0.30) | −0.02 | −0.06, 0.01 | −0.01 | −0.04, 0.01 | |
| Two or more smokers in the childhood home | 299 | 1.19 (0.31) | −0.05 | −0.09, −0.01 | −0.03 | −0.07, 0.00 | |
| Percent emphysemac | |||||||
| No childhood ETS exposure | 923 | 17.1 (11.9) | Reference | Reference | 0.01 | ||
| One smoker in the childhood home | 559 | 18.3 (12.5) | 1.1 | −0.2, 2.4 | 0.7 | −0.4, 1.8 | |
| Two or more smokers in the childhood home | 299 | 20.0 (13.5) | 2.8 | 1.1, 4.5 | 2.1 | 0.4, 3.7 | |
| Apical-basilar difference in percent emphysema | |||||||
| No childhood ETS exposure | 922 | 2.77 (6.92) | Reference | Reference | 0.42 | ||
| One smoker in the childhood home | 558 | 2.36 (7.00) | −0.41 | −1.14, 0.33 | −0.21 | −0.91, 0.50 | |
| Two or more smokers in the childhood home | 299 | 1.67 (7.41) | −1.10 | −2.05, 0.14 | −0.34 | −1.24, 0.56 | |
Abbreviations: CI, confidence interval; CT, computed tomography; ETS, environmental tobacco smoke; SD, standard deviation.
Childhood ETS was defined by retrospective reports in response to the question, “In your childhood, did you live with a regular cigarette smoker who smoked in your home?” and a follow-up question on the number of smokers in the childhood home.
Adjusted for sex, race/ethnicity, age, height, body mass index, whether the participant was born in the United States, mother's and father's educational attainment, participant's educational attainment, history of asthma before age 45 years, cumulative residential air pollution exposure, living with a smoker as an adult, occupational exposures to secondhand smoke and dust, and urinary cotinine.
Refer to the Materials and Methods section of the text for a definition.
In contrast to findings for alpha and percent emphysema, there were no significant associations of childhood ETS exposure with lung function. The adjusted lung function differences comparing participants exposed to childhood ETS from 2 or more smokers with those without any childhood ETS exposure were 20 mL (95% confidence interval: −29, 69) for FEV1, 51 mL for FVC (95% confidence interval: 11, 114), and −0.41 (95% confidence interval: −1.28, 0.46) for FEV1/FVC ratio × 100% after adjusting for sex, race/ethnicity, age, height, body mass index, whether the participant was born in the United States, mother's and father's educational attainment, participant's educational attainment, history of asthma before age 45 years, cumulative residential air pollution exposure, living with a smoker as an adult, occupational exposures to secondhand smoke and dust, and urinary cotinine.
Parental smoking
Parental history of smoking was classified as paternal smoking only, maternal smoking only, or both paternal and maternal smoking. There was a trend for participants reporting that one or both of their parents smoked to have a lower mean alpha and a higher percent emphysema (Table 3). Although the statistical power to detect an interaction between childhood ETS exposure and parental smoking history was low, parental smoking appeared to be most associated with CT-based measures among those who reported childhood ETS exposure based on reports of living with a regular smoker who smoked in the home (Figure 1).
Table 3.
Associations of Parental Smoking With CT Measures of Early Emphysema in the Multi-Ethnic Study of Atherosclerosis, United States, 2000–2006
| No. | Mean (SD) | Unadjusted Model |
Adjusted Modela |
P for Trend | |||
| Difference | 95% CI | Difference | 95% CI | ||||
| Alphab | |||||||
| Neither parent smoked | 726 | 1.24 (0.31) | Reference | Reference | 0.04 | ||
| Only the father smoked | 655 | 1.22 (0.29) | −0.02 | −0.05, 0.01 | −0.01 | −0.04, 0.02 | |
| Only the mother smoked | 134 | 1.21 (0.30) | −0.04 | −0.09, 0.02 | −0.03 | −0.08, 0.01 | |
| Both parents smoked | 247 | 1.18 (0.32) | −0.06 | −0.11, −0.01 | −0.04 | −0.07, 0.00 | |
| Percent emphysemab | |||||||
| Neither parent smoked | 726 | 17.0 (12.0) | Reference | Reference | 0.03 | ||
| Only the father smoked | 655 | 18.1 (12.2) | 1.1 | −0.2, 2.4 | 0.7 | −0.4, 1.8 | |
| Only the mother smoked | 134 | 18.5 (11.9) | 1.5 | −0.7, 3.7 | 1.8 | −0.1, 3.7 | |
| Both parents smoked | 247 | 19.8 (14.1) | 2.8 | 0.9, 4.8 | 1.6 | −0.3, 3.5 | |
| Apical-basilar difference in percent emphysema | |||||||
| Neither parent smokedc | 726 | 3.19 (6.98) | Reference | Reference | 0.12 | ||
| Only the father smoked | 653 | 2.16 (6.90) | −1.04 | −1.77, −0.30 | −0.93 | −1.62, −0.24 | |
| Only the mother smoked | 134 | 1.67 (7.35) | −1.52 | −2.88, −0.18 | −0.91 | −2.24, 0.42 | |
| Both parents smoked | 247 | 1.62 (7.21) | −1.57 | −2.61, −0.54 | −0.60 | −1.59, 0.39 | |
Abbreviations: CI, confidence interval; CT, computed tomography; SD, standard deviation.
Adjusted for sex, race/ethnicity, age, height, body mass index, whether the participant was born in the United States, mother's and father's educational attainment, participant's educational attainment, history of asthma before age 45 years, cumulative residential air pollution exposure, living with a smoker as an adult, occupational exposures to secondhand smoke and dust, and urinary cotinine.
Refer to the Materials and Methods section of the text for a definition.
The reference group (neither parent smoked) is compared with 3 mutually exclusive categories of parental smoking (father only, mother only, both parents) modeled as indicator variables, and the trend is assessed for the order given.
Figure 1.
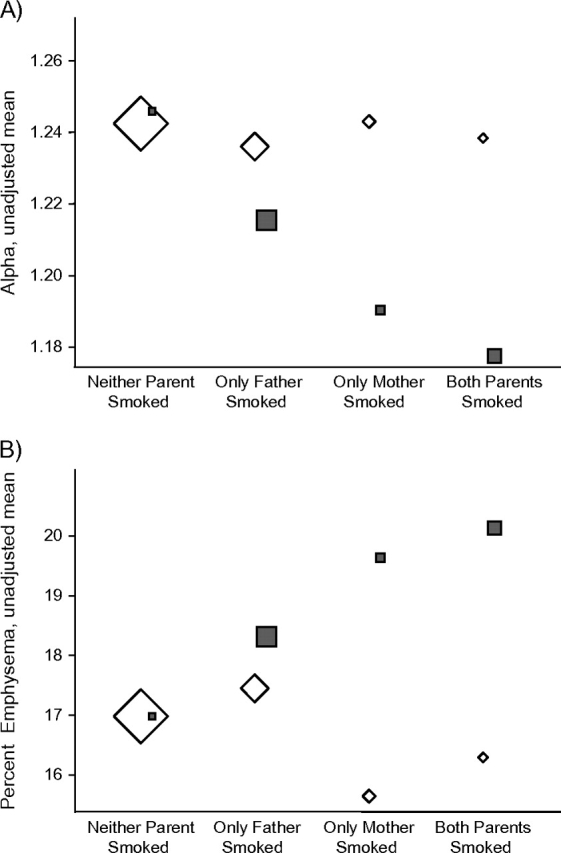
Association of childhood environmental tobacco smoke (ETS) exposure and parental smoking with early emphysema on computed tomography scan in the Multi-Ethnic Study of Atherosclerosis, United States, 2000–2006. Shown are unadjusted group means for A) alpha and B) percent emphysema (refer to the Materials and Methods section of the text for a definition of each) by parental smoking status and childhood ETS exposure. Squares: participants who reported childhood ETS exposure; diamonds: participants who did not report childhood ETS exposure. The sizes of the symbols correspond to the number of participants in each group.
Sensitivity analyses
We performed sensitivity analysis after excluding obese participants (body mass index >30 kg/m2), for whom CT scan of the lungs may be less accurate (25). Results for the 1,288 nonobese participants were similar to the associations in the entire sample. There was no significant effect modification by participants’ gender or race/ethnicity. A reanalysis of CT-based measures using inside-air-calibrated measures to better account for possible distortion due to body size did not alter the pattern of results. In an analysis using the threshold of −950 HU instead of −910 HU to define emphysema-like lung areas, the associations of childhood ETS exposure with alpha and percent emphysema were in the same direction but were attenuated; the association with percent emphysema remained monotonic and statistically significant (P = 0.001), but the association with alpha did not.
DISCUSSION
Compared with adult nonsmokers who reported no childhood ETS exposure, adult nonsmokers who reported greater childhood ETS exposure had greater degrees of diffuse emphysema measured quantitatively on CT scan. Reports that both parents smoked were likewise associated with early emphysema on CT scan. On the other hand, we found no difference in pulmonary function by degree of childhood ETS exposure.
To our knowledge, this population-based study is the first to examine the association of childhood ETS with early emphysema by CT scan in nonsmokers. One explanation for this observation can be found in Mishima et al.’s hypothesis (10) of mechanical tension with physical breakage of alveolar walls. In addition, local tension on alveolar walls may also increase the chance of their breakage because of decreased pulmonary blood flow. However, our cross-sectional CT scan data did not enable us to distinguish this pattern of vulnerability and breakage from the mere persistence of initial injury due to incomplete repair. Our findings are also compatible with other pathways that could link childhood ETS exposure with emphysema-like lung patterns in adulthood, such as altered gene expression or developmental plasticity (26).
Previous studies have found evidence that childhood ETS exposure affects perinatal and childhood health outcomes (27) along with adult respiratory health outcomes, including lung function (28–30) and respiratory symptoms (31); however, most of these studies have been limited to younger adults. Childhood ETS exposure has been linked to lower FEV1 (32, 33) or lower FEV1/FVC ratio (34–36) in childhood, and parental smoking has also been associated with worse lung function in young adulthood (28, 37). However, several studies have suggested that the effects of passive smoking on lung function may be heterogeneous across gender or racial groups (30, 35–37) or that childhood ETS is associated with higher FVC values in childhood (34, 36, 38). The lack of association between childhood ETS and lung function in our study does not contradict the results for early emphysema, since airflow obstruction and anatomic damage are theoretically and clinically distinguishable, but may suggest that emphysema is a more sensitive measure of damage compared with lung function in this relatively small and healthy cohort.
The unique strengths of this study—including the quantitative measures of emphysema in a racially and ethnically diverse, population-based cohort with a large number of nonsmokers—were offset by a number of limitations. First, our study relied on participants’ recall of cohabitation with one or more cigarette smokers as a child and of parental smoking for the assessment of childhood ETS exposure. Other sources of ETS, including pipe and cigar smoke, were not part of this assessment. This exposure information is subject to misclassification bias, which could be differential if participants perceiving respiratory symptoms were more likely to report childhood ETS exposure. However, the reliance on subclinical measures of early emphysema in a relatively healthy cohort of adults makes differential misclassification bias an unlikely explanation for our findings. The exposure also provides no information with regard to timing during childhood, making it difficult to distinguish cumulative exposure from exposure in utero or during other sensitive periods. The persistence of an association between childhood ETS and early emphysema after restricting our analyses to those whose mothers did not smoke and the monotonic relation between number of smokers in the home and early emphysema both suggest that the effect we are detecting is for smoking in the home during childhood, rather than in utero exposure alone.
Second, the CT-based measurements of early emphysema were taken at a single point in time and were based upon partial-lung scans. However, 2 scans were acquired at the examination, and we previously validated the measures from MESA cardiac scans against full-lung scans (18). In addition, associations with the measure alpha differed depending on the threshold used to define areas of emphysema-like lung, suggesting some sensitivity of our results to the specification of our CT-based measures. Since this is the first large study known to examine these CT-based measures in a large, healthy cohort of adults, correspondence with clinically relevant disease categories cannot be assumed. However, percent emphysema is a standard quantitative measure of emphysema that is close to US Food and Drug Administration approval as a surrogate marker for emphysema, and we previously found good agreement between measures of diffuse emphysema on partial- and full-lung scans (18).
This study is further limited by the cross-sectional and observational nature of the data, and we cannot exclude the possibility that the small differences in emphysema on CT scan were due to residual confounding or confounding by unmeasured characteristics. Survival bias could have affected these cross-sectional results if some individuals with childhood ETS exposure and early emphysema did not survive to be eligible participants; however, this possibility would likely have biased our results toward the null. We excluded smokers based on questionnaire and cotinine data so that individual smoking did not overwhelm the relatively modest effect of childhood ETS exposure. Some participants who were former smokers may not have accurately reported their prior smoking history. However, given that approximately 2% of the cohort misreported their current smoking status (comparing self-report with cotinine levels), it is likely that this group is small.
Childhood ETS exposure in childhood was associated with CT measures of early emphysema in adulthood in a large multiethnic cohort. This finding suggests that the lungs may not recover completely from the effects of early-life exposures and adds to the literature on detrimental effects of ETS exposure.
Acknowledgments
Author affiliations: Department of Epidemiology, Columbia University Mailman School of Public Health, New York, New York (Gina S. Lovasi); Center for Social Epidemiology and Population Health, University of Michigan, Ann Arbor, Michigan (Ana V. Diez Roux); Department of Radiology, University of Iowa, Iowa City, Iowa (Eric A. Hoffman); Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania (Steven M. Kawut); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); and Department of Medicine, Columbia University, New York, New York (R. Graham Barr).
This work was supported by the National Institutes of Health (grants NIH R01-HL077612, R01-HL075476, and N01-HC095159 to HC095169) and the Robert Wood Johnson Foundation Health & Society Scholars Program.
Presentation of this research at the 2009 American Thoracic Society International Conference, San Diego, California, May 15−20, 2009, was supported by a Travel Award from the American Thoracic Society Assembly on Behavioral Science.
Conflict of interest: none declared.
Glossary
Abbreviations
- CT
computed tomography
- ETS
environmental tobacco smoke
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HU
Hounsfield units
- MESA
Multi-Ethnic Study of Atherosclerosis
References
- 1.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364(9434):613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 2.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 4.The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. ( http://www.searo.who.int/LinkFiles/Reports_GBD_report_2004update_full.pdf) [Google Scholar]
- 5.Litmanovich D, Boiselle PM, Bankier AA. CT of pulmonary emphysema—current status, challenges, and future directions. Eur Radiol. 2009;19(3):537–551. doi: 10.1007/s00330-008-1186-4. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AE, Jr, Hernandez JA, Eckert P, et al. Emphysema in lung macrosections correlated with smoking habits. Science. 1964;144:1025–1026. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 8.Viegi G, Scognamiglio A, Baldacci S, et al. Epidemiology of chronic obstructive pulmonary disease (COPD) Respiration. 2001;68(1):4–19. doi: 10.1159/000050456. [DOI] [PubMed] [Google Scholar]
- 9.Perlmutter DH, Pierce JA. The alpha 1-antitrypsin gene and emphysema. Am J Physiol. 1989;257(4 pt 1):L147–L162. doi: 10.1152/ajplung.1989.257.4.L147. [DOI] [PubMed] [Google Scholar]
- 10.Mishima M, Hirai T, Itoh H, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999;96(16):8829–8834. doi: 10.1073/pnas.96.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adcock IM, Ford P, Ito K, et al. Epigenetics and airways disease. Respir Res. 2006;7:21. doi: 10.1186/1465-9921-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Reinhardt JM, Kitaoka H, et al. Biomedical Imaging, 2002: Proceedings of the IEEE International Symposium. Piscataway, NJ: IEEE; 2002. Integrated system for CT-based assessment of parenchymal lung disease; pp. 871–874. [Google Scholar]
- 17.Hoffman EA, Reinhardt JM, Sonka M, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of CT images of lung structure and function. Acad Radiol. 2003;10(10):1104–1118. doi: 10.1016/s1076-6332(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coxson HO, Mayo JR, Behzad H, et al. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol. 1995;79(5):1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Hankinson JL, Kawut SM, Shahar E, et al. Performance of spirometry reference values in a multiethnic population. The MESA-Lung Study [abstract] Presented at the International Conference of the American Thoracic Society, San Francisco, California, May 18–23, 2007. [Google Scholar]
- 22.Auchincloss AH, Diez Roux AV, Brown DG, et al. Association of insulin resistance with distance to wealthy areas: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2007;165(4):389–397. doi: 10.1093/aje/kwk028. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill MS, Diez-Roux AV, Auchincloss AH, et al. Airborne particulate matter exposure and urinary albumin excretion: the Multi-Ethnic Study of Atherosclerosis. Occup Environ Med. 2008;65(8):534–540. doi: 10.1136/oem.2007.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227–241. [Google Scholar]
- 25.Hoffman EA, Baumhauer H, Carr JJ, et al. CT lung density measures in a healthy multiethnic population: the MESA-Lung Study [abstract] Presented at the American Thoracic Society Annual Meeting, San Francisco, California, May 18–23, 2007. [Google Scholar]
- 26.Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88(12):1086–1090. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svanes C, Omenaas E, Jarvis D, et al. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59(4):295–302. doi: 10.1136/thx.2003.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tager IB, Weiss ST, Muñoz A, et al. Longitudinal study of the effects of maternal smoking on pulmonary function in children. N Engl J Med. 1983;309(12):699–703. doi: 10.1056/NEJM198309223091204. [DOI] [PubMed] [Google Scholar]
- 30.Landau LI. Tobacco smoke exposure and tracking of lung function into adult life. Paediatr Respir Rev. 2008;9(1):39–43. doi: 10.1016/j.prrv.2007.11.002. quiz 43–44. [DOI] [PubMed] [Google Scholar]
- 31.David GL, Koh WP, Lee HP, et al. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: the Singapore Chinese Health Study. Thorax. 2005;60(12):1052–1058. doi: 10.1136/thx.2005.042960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moshammer H, Hoek G, Luttmann-Gibson H, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173(11):1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 33.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122(2):409–415. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Wypij D, Gold DR, et al. A longitudinal study of the effects of parental smoking on pulmonary function in children 6–18 years. Am J Respir Crit Care Med. 1994;149(6):1420–1425. doi: 10.1164/ajrccm.149.6.8004293. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham J, Dockery DW, Gold DR, et al. Racial differences in the association between maternal smoking during pregnancy and lung function in children. Am J Respir Crit Care Med. 1995;152(2):565–569. doi: 10.1164/ajrccm.152.2.7633708. [DOI] [PubMed] [Google Scholar]
- 36.Vedal S, Schenker MB, Samet JM, et al. Risk factors for childhood respiratory disease. Analysis of pulmonary function. Am Rev Respir Dis. 1984;130(2):187–192. doi: 10.1164/arrd.1984.130.2.187. [DOI] [PubMed] [Google Scholar]
- 37.Jackson B, Kubzansky LD, Cohen S, et al. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. Int J Epidemiol. 2004;33(2):271–278. doi: 10.1093/ije/dyh003. [DOI] [PubMed] [Google Scholar]
- 38.Demissie K, Ernst P, Joseph L, et al. The role of domestic factors and day-care attendance on lung function of primary school children. Respir Med. 1998;92(7):928–935. doi: 10.1016/s0954-6111(98)90192-5. [DOI] [PubMed] [Google Scholar]


