Abstract
Objective
To determine the pharmacokinetics and safety of RAD001 (everolimus) in Japanese patients with advanced solid tumors.
Methods
An open-label, non-randomized, dose-escalation Phase I study of RAD001 administered continuously once daily in a 28-day cycle was performed. The study had a ‘3 + 3’ design, with three patients recruited to each of three successive cohorts treated with RAD001 at 2.5, 5.0 or 10.0 mg/day.
Results
The pharmacokinetics of RAD001 in Japanese patients were similar to those previously determined in Caucasians. The drug safety profile was consistent with that of a mammalian target of rapamycin inhibitor. No dose-limiting toxicities were observed. One patient with esophageal cancer and one with gastric cancer treated with RAD001 at 10 mg/day showed marked tumor responses.
Conclusions
Treatment of Japanese cancer patients with RAD001 may be undertaken with the expectation that previously determined pharmacokinetic and safety profiles apply. The drug may hold promise for treatment of esophageal and gastric cancer.
Keywords: Phase I study, pharmacokinetics, mTOR, RAD001, everolimus
INTRODUCTION
Mammalian target of rapamycin (mTOR) is an intracellular protein kinase that mediates cellular responses to growth factors, nutrients and changes in energy status and thereby plays an important role in the regulation of cell growth, cell division and angiogenesis. It controls ribosome biosynthesis and the transcription of genes for many proteins that participate in the cell cycle, metabolism, nutrient transport or utilization, or the response to hypoxia. Various signaling defects upstream of mTOR, some of which are relatively common, have been identified in cancer cells and result in loss of cell growth control, unrestrained proliferation, tumor angiogenesis, and other malignant characteristics. Defects in mTOR itself have not been identified in cancer, rendering this kinase both a well-situated and stable target for therapeutic intervention in cancers driven by defects in the mTOR signaling pathway (1–3).
RAD001 (everolimus) blocks the mTOR pathway by forming a complex with the immunophilin FK506-binding protein-12, which also binds mTOR with high affinity. This drug has exhibited antitumor activity with a variety of cancer cells both in vitro (4–9) and in vivo (10–12). In addition, the anticancer effects of RAD001 complement those of chemotherapy, radiation, hormonal agents and targeted therapeutics (13–15). RAD001 inhibits tumor growth dependent on angiogenesis by inhibiting the production of angiogenic growth factors and thereby reducing the proliferation of neovascular endothelial cells (3). Phase I studies of RAD001 have shown sustained inhibition of mTOR activity in tumor tissue at oral doses of ≥20 mg weekly or 5–10 mg daily (16). Continuous daily dosing with RAD001 has been found to result in a more profound and sustained inhibition of mTOR than that achieved with an intermittent weekly schedule (17,18).
We have now performed a Phase I trial of RAD001 administered daily to Japanese patients with advanced solid tumors. The purpose of our study was to assess the pharmacokinetics, safety and tolerability of escalating oral doses of RAD001 in this patient population. An additional objective included evaluation of antitumor activity.
We herein report that RAD001 can be safely administered at daily doses up to 10 mg to Japanese patients with advanced solid malignancies. A dosage of 10 mg/day is recommended for further development.
PATIENTS AND METHODS
Patient Population
Japanese individuals ≥20 years of age with a histologically confirmed diagnosis of an advanced tumor refractory to or unsuitable for existing standard therapy were included in the study if they had >1 measurable lesion, a life expectancy of ≥3 months, and adequate or acceptable renal [serum creatinine concentration of ≤1.5× the upper limit of normal (ULN)], liver (serum bilirubin concentration of ≤1.25× ULN, serum transaminase activity of ≤3× ULN and serum albumin concentration of ≥3.5 g/dl) and bone marrow (absolute neutrophil count of ≥1500/mm3, platelet count of ≥1 × 105/mm3 and hemoglobin concentration of ≥9 g/dl) function. Patients with tumors or metastases in the central nervous system, uncontrolled infection, gastrointestinal impairment disease, active bleeding diathesis, other concurrent or uncontrolled medical disease, or a history of coagulation disorders as well as those under treatment with strong inhibitors or inducers of isoenzyme CYP3A4 were excluded from the study. All subjects provided written informed consent to participation in the study, which was approved by the Institutional Review Board of each participating center and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Study Design
The study was an open-label, non-randomized, dose-escalation Phase I trial of RAD001 administered on a continuous once-daily schedule in a 28-day cycle to adult Japanese patients, with continuation of therapy after 28 days in the absence of progressive disease. The primary objective was to evaluate the tolerability/safety and dose-limiting toxicity (DLT) of RAD001, up to the dose level of 10 mg/day which is being used in the global study. The study had a ‘3 + 3’ design, with three patients recruited to each of three successive cohorts treated with RAD001 at 2.5, 5.0 or 10.0 mg/day. Patients were allowed to receive a higher RAD001 dose, at the investigator's discretion, if the higher dose had been confirmed as tolerable. Treatment was discontinued in the event of progressive disease, DLT, a dose delay of >14 days (or >42 days for hematologic DLTs), or withdrawal of consent. A DLT was defined as a hematologic (anemia, leukopenia, thrombocytopenia or neutropenia) or non-hematologic adverse event with a grade of ≥3 or a laboratory abnormality with a grade of ≥3 that occurred within the first 4 weeks of treatment and was suspected to be related to RAD001. Standard antiemetic prophylaxis and anti-hyperlipidemia therapy were allowed. Recruitment was permitted for Cohort 2 if DLTs were observed in 0/3 or ≤1/6 patients in Cohort 1, and for Cohort 3 if DLTs were observed in 0/3 or ≤1/6 patients in Cohort 2. DLTs in ≥2/6 patients in Cohort 1 would result in study discontinuation; DLTs in ≥2/6 patients in Cohort 2 or 3 would result in additional patient enrollment in Cohorts 1 and 2, respectively. The maximum-tolerated dose was defined as the dose at which two or more patients experienced a DLT in the first cycle.
Assessments
Blood samples for pharmacokinetic analysis were collected on days 1 and 15 of cycle 1 at 0, 1, 2, 4, 6, 8 and 24 h after RAD001 administration. Blood samples for assessment of the trough concentration (Cmin) of RAD001 were obtained immediately before administration of the next dose on days 2, 8, 11, 15 and 16 of cycle 1 and on day 1 of cycle 2 as well as at the end of the study. Pharmacokinetic parameters of RAD001 determined for each cohort included the maximum blood concentration (Cmax), time of maximum concentration (tmax), area under the concentration-versus-time curve from time 0 to 24 h after drug administration (AUCτ, dosing interval) and apparent systemic clearance (CL/F). Drug safety and tolerability were assessed according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) scale, version 3.0. Patients were monitored for adverse events throughout the study. Tumor volume was evaluated every 2 months and at the end of the study according to RECIST. Data were recorded for up to 28 days after discontinuation of treatment.
Statistics
The number of patients in each proposed cohort was based on the standard ‘3 + 3’ design for dose-escalation studies. A total of 9–18 patients were planned to assess the safety and tolerability of RAD001, depending on observed toxicities. Descriptive statistics were used for evaluation of safety, efficacy and pharmacokinetic outcomes.
RESULTS
Patient Characteristics
Between November 2005 and December 2006, nine patients with advanced, refractory solid tumors were enrolled in the study at the two participating centers (Kinki University School of Medicine and National Cancer Center Hospital East) (Table 1). The median age was 64 years (range, 49–74). All patients had received prior chemotherapy for their disease, and most of them had previously undergone cancer-related surgery. The median durations of RAD001 therapy were 57 days in the 2.5 mg/day cohort, 42 days in the 5 mg/day cohort and 98 days in the 10 mg/day cohort. Treatment was discontinued in all nine patients as a result of either progressive disease (n = 4), toxicities (n = 2), consent withdrawal (n = 2) or death (n = 1, hemorrhage). All patients were evaluable for drug safety and pharmacokinetics.
Table 1.
Patient characteristics
| Characteristic | No. of patients |
|---|---|
| Sex | |
| Male | 4 |
| Female | 5 |
| Performance status (ECOG) | |
| 0 | 5 |
| 1 | 4 |
| Previous therapy | |
| Surgery | 8 |
| Chemotherapy | 9 |
| Radiotherapy | 3 |
| Tumor type | |
| Colorectal cancer | 3 |
| Lung cancer | 3 |
| Esophageal cancer | 1 |
| Gastric cancer | 1 |
| Thyroid cancer | 1 |
The median (range) age was 64 (49–74) years. ECOG, Eastern Cooperative Oncology Group.
Safety
DLTs were not observed for any patient in the first cycle of treatment (28 days). Overall, the most common adverse events of all grades were thrombocytopenia (56% of patients), leukopenia (33%), anorexia (44%) and rash (44%) (Table 2). One patient with colon cancer and both lung and liver metastases was treated at the RAD001 dose of 5 mg/day experienced grade 2 pneumonitis after 142 days of therapy. The patient developed cough, and computed tomographic scan of the chest revealed new ground-glass opacities. The patient was hospitalized with PaO2 of 72.7 mmHg. Steroid treatment and discontinuation of RAD001 resulted in marked improvement of the patient within days. All toxicities of Grade 3 or 4 occurred at the dose of 10 mg/day, but none occurred in the first cycle and therefore did not qualify as DLTs. One patient with advanced esophageal carcinoma at a dose of 10 mg/day developed Grade 3 fatigue and stomatitis on day 58 and RAD001 was interrupted. The study drug was restarted on day 66 at a reduced dose of 5 mg/day. On day 71, the patient visited the hospital because of hemorrhage from the right supraclavicular tumor which was a metastatic focus. Although the patient was treated as an inpatient, the Grade 4 hemorrhage could not be controlled and the patient died on day 78. Since RAD001 markedly diminished the size of the patient's metastatic focus, the cause of death was hemorrhage from either the right supraclavicular metastatic focus or the enriched vessels. The study drug did not seem to be the direct cause of hemorrhage. The other two patients in the 10 mg/day cohort took RAD001 for >3 months. One patient with colorectal cancer was treated with 10 mg/day and experienced Grade 3 hyperglycemia on day 98. The patient was determined to have progressive disease on the same day. Another patient in the 10 mg/day cohort did not have any Grade 3 or 4 toxicities and discontinued RAD001 due to disease progression on day 154.
Table 2.
Number of patients with adverse events in all courses thought to be attributable to RAD001
| Adverse event | RAD001 dose (mg/day) |
Total | |||||
|---|---|---|---|---|---|---|---|
| 2.5 (n = 3) |
5 (n = 3) |
10 (n = 3) |
|||||
| G1/2 | G3/4 | G1/2 | G3/4 | G1/2 | G3/4 | ||
| Thrombocytopenia | 0 | 0 | 2 | 0 | 3 | 0 | 5 |
| Leukopenia | 1 | 0 | 2 | 0 | 0 | 0 | 3 |
| Neutropenia | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Anemia | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Anorexia | 1 | 0 | 2 | 0 | 1 | 0 | 4 |
| Rash | 0 | 0 | 1 | 0 | 3 | 0 | 4 |
| Stomatitis | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Nausea | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Mucosal inflammation | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Diarrhea | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Fatigue | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Weight decreased | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Elevated ALT or AST | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Pneumonitis | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Hypertension | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Glucose tolerance impaired | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
Includes all adverse events occurring in two or more patients or were ≥Grade 2. G, grade; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Pharmacokinetics
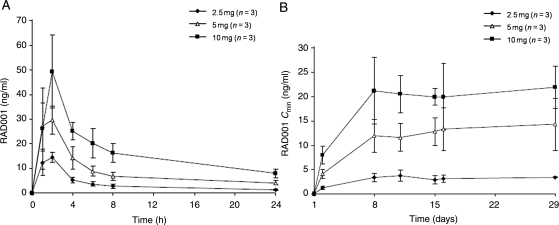
Pharmacokinetic parameters of RAD001 are summarized in Table 3. The Cmax of RAD001 was apparent 2 h after administration of a single dose of the oral drug (Table 3 and Fig. 1A). The Cmin of RAD001 indicated that a steady state was attained after ∼8 days of repeated once-daily oral dosing (Fig. 1B). Determination of the AUCτ on days 1 and 15 revealed that the exposure to RAD001 achieved after multiple dosing was about twice that achieved after a single dose (Table 3). On day 1, Cmax and AUCτ increased almost dose-proportionally. At steady state (day 15), Cmax and AUCτ increased with increment of dose but dose-proportionality was not clear due to large inter-individual variability in the 5 mg/day cohort.
Table 3.
Pharmacokinetic parameters of RAD001
| RAD001 dose (mg/day) |
|||
|---|---|---|---|
| 2.5 (n = 3) | 5 (n = 3) | 10 (n = 3) | |
| Day 1 | |||
| tmax (h) | |||
| Median | 1.98 | 1.00 | 2.00 |
| Range | 0.98–2.00 | 1.00–1.95 | 1.92–2.00 |
| Cmax (ng/ml) | 15.1 ± 2.48 | 31.5 ± 3.40 | 49.4 ± 14.8 |
| AUCτ (ng h/ml) | 85.2 ± 18.7 | 211 ± 50.0 | 401 ± 51.6 |
| Day 15 | |||
| tmax (h) | |||
| Median | 1.92 | 1.98 | 2.02 |
| Range | 1.00–1.98 | 1.93–1.98 | 2.00–2.20 |
| Cmax (ng/ml) | 16.8 ± 1.33 | 57.6 ± 17.6 | 65.9 ± 1.40 |
| AUCτ (ng h/ml) | 134 ± 24.1 | 543 ± 189 | 711 ± 113 |
| CL/F (l/h) | 19.1 ± 3.26 | 9.94 ± 3.21 | 14.3 ± 2.23 |
Data are means ± SD unless indicated otherwise. tmax, time of maximum concentration; Cmax, maximum blood concentration; AUCτ, area under the concentration-versus-time curve from time 0 to 24 h after drug administration; CL/F, apparent systemic clearance.
Figure 1.
Pharmacokinetics of RAD001. (A) Blood concentration of RAD001 after administration of a single oral dose (2.5, 5 or 10 mg) on day 1 of cycle 1. Data are means ± SD. (B) Blood trough concentration (Cmin) of RAD001 during continuous oral dosing for 29 days (cycle 1). Data are means ± SD.
Tumor Response
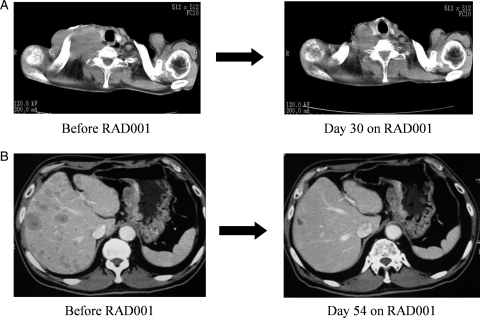
Among seven patients evaluable for tumor response, obvious tumor shrinkage was observed in two patients treated at the dose level of 10 mg/day. A 60-year-old male with advanced esophageal carcinoma who had been treated with seven prior chemotherapy regimens started treatment with RAD001 at 10 mg/day. After one cycle of RAD001 treatment, computed tomography revealed that lymph nodes with metastases in the right supraclavicular region had shrunk markedly (Fig. 2A). A 64-year-old male with gastric adenocarcinoma and liver metastases who had undergone four prior chemotherapy regimens showed a partial response to RAD001 that persisted for >4 months at the dose of 10 mg/day (Fig. 2B).
Figure 2.
Computed tomography images of tumor response to RAD001 treatment. (A) Shrinkage of metastases in supraclavicular lymph nodes in a patient with esophageal cancer. (B) Shrinkage of liver metastases in a patient with gastric cancer.
DISCUSSION
Evidence implicating the phosphatidylinositol 3-kinase–Akt–mTOR signaling pathway in the pathogenesis of a variety of malignancies has prompted the development of therapeutic strategies to modulate this pathway. RAD001 is an oral inhibitor of the mTOR pathway, and we have now performed a dose-escalation Phase I study of this drug in Japanese patients with advanced solid tumors in order to evaluate its safety and pharmacokinetics. Therapy with RAD001 at oral doses of up to 10 mg once daily was relatively well tolerated in the study subjects. Indeed, the safety and tolerability of RAD001 in the Japanese patients were similar to those observed in previous studies with larger populations of Caucasian patients, for whom the most common drug-related toxicities included rash, stomatitis and fatigue. Previous studies have reported that patients receiving RAD001 manifested hyperglycemia and hyperlipidemia, probably as a result of inhibition of mTOR-regulated glucose and lipid metabolism (16,18,19). Grade 3 hyperglycemia was observed in one patient treated with 10 mg/day, whereas hyperlipidemia was not observed in our study. One patient in our study developed pneumonitis of Grade 2, with this condition having previously been identified as a potential class-related toxicity for mTOR inhibitors that should be monitored in clinical trials with these agents (16–20). However, the condition of pneumonitis in our study was reversible after discontinuation of RAD001 treatment. The pharmacokinetic profile of RAD001 in Japanese patients was also similar to that in Caucasian patients. RAD001 was absorbed rapidly, with the Cmax being achieved as early as 1–2 h after oral administration. A recent Phase I study of RAD001 performed in Europe and the USA showed that the mean (±SD) Cmax in patients with advanced cancer was 32 ± 9 and 61 ± 17 ng/ml at daily doses of 5 and 10 mg, respectively, with a mean AUCτ of 238 ± 77 and 514 ± 231 ng h/ml, respectively (16). These results for Caucasian patients are similar to those obtained here with Japanese patients, especially for the dose level of 10 mg/day (Cmax of 65.9 ± 1.40 ng/ml and AUCτ of 711 ± 113 ng h/ml. Given the limited number of patients in both studies, these results suggest that there are no substantial differences in the pharmacokinetics of RAD001 between the two populations. RAD001 has already undergone extensive clinical testing in the setting of renal and cardiac transplantation (21,22). Our present data are also supported by observations with 673 renal transplant patients who received RAD001 (23). This large cohort included 80% Caucasian patients and 2.5% patients of Asian origin with no significant differences in clearance of RAD001 being apparent between the Asian and Caucasian patients. The data from this study, combined with those from previous studies, suggest that the pharmacokinetic and safety data for RAD001 obtained in larger clinical trials with Caucasian patients are likely applicable to the Japanese population.
Although response was not a primary outcome of our study, two of three patients treated with RAD001 at a daily dose of 10 mg manifested marked tumor shrinkage. This antitumor activity occurred in patients with esophageal and gastric cancer. One esophagogastric cancer patient also exhibited a partial response to RAD001 treatment at a daily dose of 5 mg in a previous Phase I study (16). The likelihood that these findings will extend to other patients is supported by recent studies suggesting that defects in the mTOR signaling pathway are important in the pathogenesis of these cancers. mTOR is an upstream regulator of hypoxia-inducible factor-1α, which is a key mediator of gastric cancer growth (24). Pre-clinical studies have shown that the mTOR inhibitor rapamycin inhibits the growth of human gastric adenocarcinoma cell lines, gastric cancer, gastrointestinal tumors, and the development of peritoneal carcinomatosis from gastric cancer in vitro or in vivo (24–27).
In conclusion, the results of our Phase I study suggest that RAD001 can be safely administered at a daily dose of 10 mg to Japanese patients with advanced solid malignancies. The pharmacokinetic characteristics of RAD001 in Japanese patients did not appear to differ from those previously observed in Caucasian patients. The safety profile and potential broad-spectrum efficacy of RAD001 thus warrant additional clinical evaluation of this new agent.
Funding
This study was sponsored by Novartis Pharma K.K.
Conflict of interest statement
The authors Katsutoshi Kurei and Ken Kobayashi are employed by Novartis Pharma.
Acknowledgements
We thank Richard McCabe and Nelson Erlick for comments on the manuscript.
References
- 1.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–37. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 2.Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol Ther. 2006;5:1065–73. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 3.Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ. Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26:611–21. doi: 10.1007/s10555-007-9077-8. [DOI] [PubMed] [Google Scholar]
- 4.Stracke S, Ramudo L, Keller F, Henne-Bruns D, Mayer JM. Antiproliferative and overadditive effects of everolimus and mycophenolate mofetil in pancreas and lung cancer cells in vitro. Transplant Proc. 2006;38:766–70. doi: 10.1016/j.transproceed.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 6.Haritunians T, Mori A, O'Kelly J, Luong QT, Giles FJ, Koeffler HP. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–9. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 7.Tuncyurek P, Mayer JM, Klug F, Dillmann S, Henne-Bruns D, Keller F, et al. Everolimus and mycophenolate mofetil sensitize human pancreatic cancer cells to gemcitabine in vitro: a novel adjunct to standard chemotherapy? Eur Surg Res. 2007;39:380–7. doi: 10.1159/000107356. [DOI] [PubMed] [Google Scholar]
- 8.Zitzmann K, De Toni EN, Brand S, Goke B, Meinecke J, Spottl G, et al. The novel mTOR inhibitor RAD001 (everolimus) induces antiproliferative effects in human pancreatic neuroendocrine tumor cells. Neuroendocrinology. 2007;85:54–60. doi: 10.1159/000100057. [DOI] [PubMed] [Google Scholar]
- 9.Calabro A, Tai J, Allen SL, Budman DR. In-vitro synergism of m-TOR inhibitors, statins, and classical chemotherapy: potential implications in acute leukemia. Anticancer Drugs. 2008;19:705–12. doi: 10.1097/CAD.0b013e328304ae19. [DOI] [PubMed] [Google Scholar]
- 10.Jundt F, Raetzel N, Muller C, Calkhoven CF, Kley K, Mathas S, et al. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein {beta} and NF-{kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood. 2005;106:1801–7. doi: 10.1182/blood-2004-11-4513. [DOI] [PubMed] [Google Scholar]
- 11.Khariwala SS, Kjaergaard J, Lorenz R, Van Lente F, Shu S, Strome M. Everolimus (RAD) inhibits in vivo growth of murine squamous cell carcinoma (SCC VII) Laryngoscope. 2006;116:814–20. doi: 10.1097/01.mlg.0000210544.64659.35. [DOI] [PubMed] [Google Scholar]
- 12.Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–13. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 13.Goudar RK, Shi Q, Hjelmeland MD, Keir ST, McLendon RE, Wikstrand CJ, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–12. [PubMed] [Google Scholar]
- 14.Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K, et al. The antitumor effects of sunitinib (formerly SU11248) against a variety of human hematologic malignancies: enhancement of growth inhibition via inhibition of mammalian target of rapamycin signaling. Mol Cancer Ther. 2006;5:2522–30. doi: 10.1158/1535-7163.MCT-06-0071. [DOI] [PubMed] [Google Scholar]
- 15.Manegold PC, Paringer C, Kulka U, Krimmel K, Eichhorn ME, Wilkowski R, et al. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (Everolimus) increases radiosensitivity in solid cancer. Clin Cancer Res. 2008;14:892–900. doi: 10.1158/1078-0432.CCR-07-0955. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell A, Faivre S, Burris HA, III, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka C, O'Reilly T, Kovarik JM, Shand N, Hazell K, Judson I, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 18.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–10. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 20.Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–8. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 22.Pascual J. Everolimus in clinical practice—renal transplantation. Nephrol Dial Transplant. 2006;21(Suppl 3):iii18–23. doi: 10.1093/ndt/gfl300. [DOI] [PubMed] [Google Scholar]
- 23.Kovarik JM, Hsu CH, McMahon L, Berthier S, Rordorf C. Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clin Pharmacol Ther. 2001;70:247–54. doi: 10.1067/mcp.2001.118022. [DOI] [PubMed] [Google Scholar]
- 24.Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803–10. doi: 10.1002/ijc.22442. [DOI] [PubMed] [Google Scholar]
- 25.Feng W, Brown RE, Trung CD, Li W, Wang L, Khoury T, et al. Morphoproteomic profile of mTOR, Ras/Raf kinase/ERK, and NF-kappaB pathways in human gastric adenocarcinoma. Ann Clin Lab Sci. 2008;38:195–209. [PubMed] [Google Scholar]
- 26.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA. 2008;105:13544–9. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto I, Koizumi K, Tatematsu M, Minami T, Cho S, Takeno N, et al. Blocking on the CXCR4/mTOR signalling pathway induces the anti-metastatic properties and autophagic cell death in peritoneal disseminated gastric cancer cells. Eur J Cancer. 2008;44:1022–9. doi: 10.1016/j.ejca.2008.02.043. [DOI] [PubMed] [Google Scholar]