Abstract
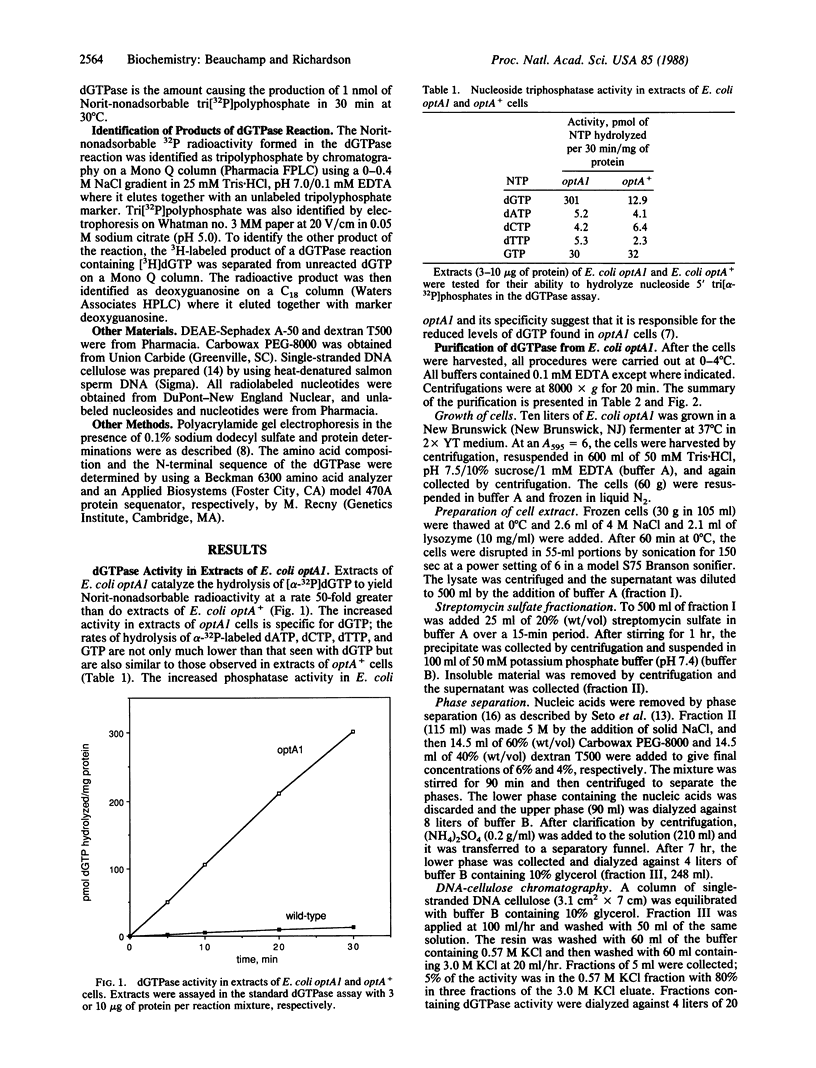
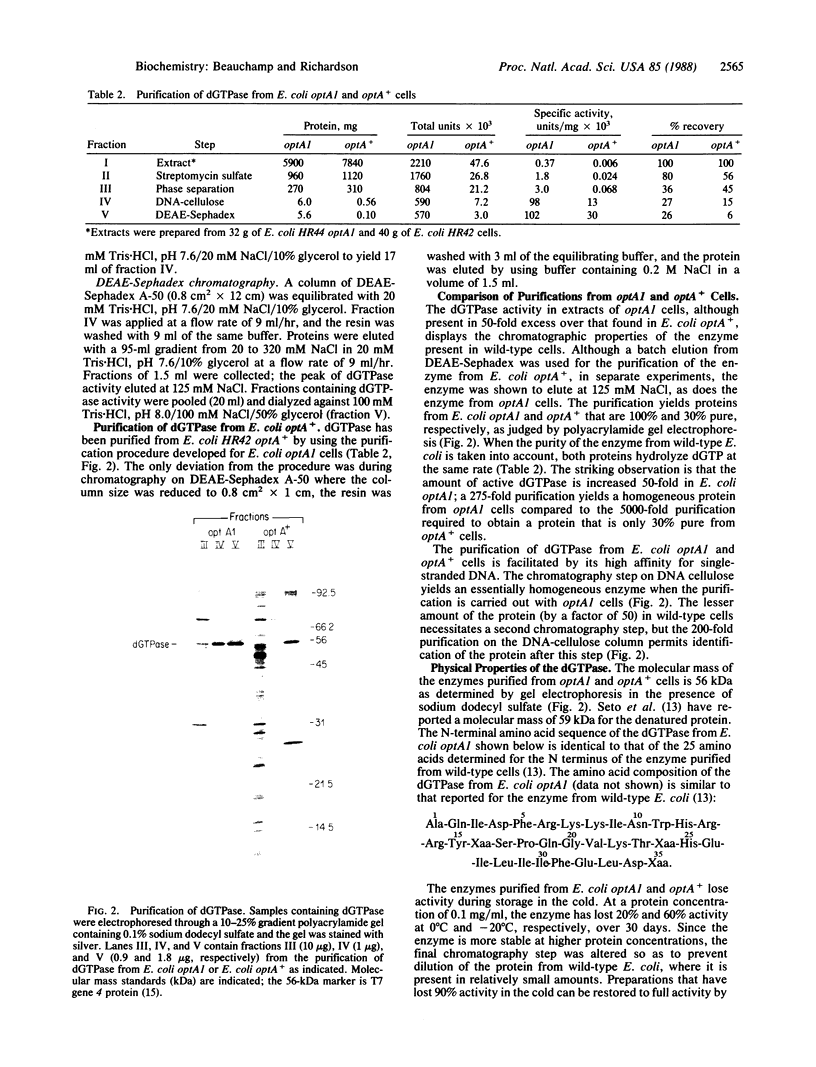
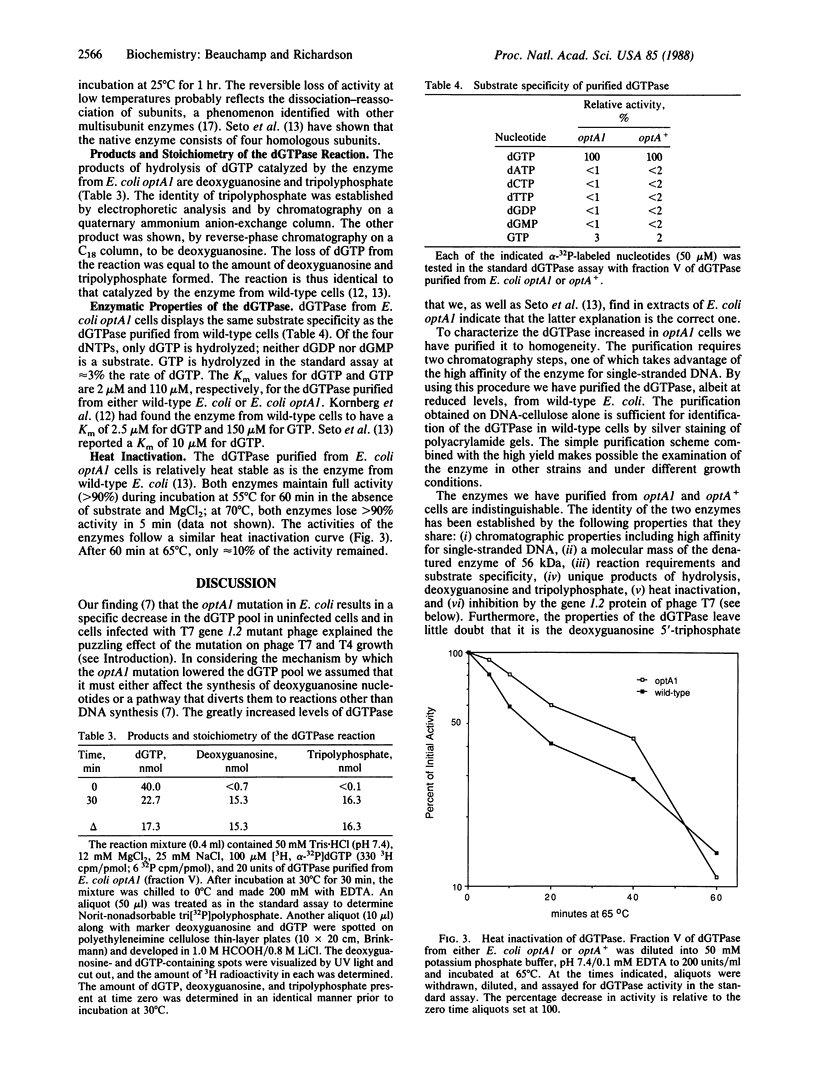
Escherichia coli optA1, a mutant unable to support the growth of T7 phage containing mutations in gene 1.2, contains reduced amounts of dGTP. Extracts of E. coli optA1 catalyze the hydrolysis of dGTP at a rate 50-fold greater than do extracts of E. coli optA+. The dGTPase responsible for the increased hydrolysis has been purified to apparent homogeneity. Purification of the protein is facilitated by its high affinity for single-stranded DNA. By using this purification scheme an identical dGTPase has been purified from E. coli optA+. The purified proteins catalyze the hydrolysis of dGTP to yield deoxyguanosine and tripolyphosphate. The products of hydrolysis, chromatographic properties, denatured molecular mass of 56 kDa, N-terminal amino acid sequence, substrate specificity, and heat inactivation indicate that the proteins purified from optA1 and from optA+ cells are identical and identify the enzyme as the deoxyguanosine 5'-triphosphate triphosphohydrolase purified to homogeneity from wild-type E. coli [Seto, D., Bhatnagar, S. K. & Bessman, M. J. (1988) J. Biol. Chem. 263, 1494-1499]. OptA1 cells contain approximately equal to 50-fold more active molecules of the 56-kDa dGTPase than do E. coli optA+ cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein J. A., Richardson C. C. A 7-kDa region of the bacteriophage T7 gene 4 protein is required for primase but not for helicase activity. Proc Natl Acad Sci U S A. 1988 Jan;85(2):396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., Greening E. O. Suppression of chemical mutagenesis in bacteriophage T4 by genetically modified DNA polymerases. Proc Natl Acad Sci U S A. 1970 Jul;66(3):823–829. doi: 10.1073/pnas.66.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Frieden C. Protein-protein interaction and enzymatic activity. Annu Rev Biochem. 1971;40:653–696. doi: 10.1146/annurev.bi.40.070171.003253. [DOI] [PubMed] [Google Scholar]
- Gauss P., Doherty D. H., Gold L. Bacterial and phage mutations that reveal helix-unwinding activities required for bacteriophage T4 DNA replication. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1669–1673. doi: 10.1073/pnas.80.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss P., Gayle M., Winter R. B., Gold L. The bacteriophage T4 dexA gene: sequence and analysis of a gene conditionally required for DNA replication. Mol Gen Genet. 1987 Jan;206(1):24–34. doi: 10.1007/BF00326532. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. Control of mutation frequency by bacteriophage T4 DNA polymerase. I. The CB120 antimutator DNA polymerase is defective in strand displacement. J Biol Chem. 1976 Sep 10;251(17):5219–5224. [PubMed] [Google Scholar]
- KORNBERG S. R., LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic cleavage of deoxyguanosine triphosphate to deoxyguanosine and tripolyphosphate. J Biol Chem. 1958 Jul;233(1):159–162. [PubMed] [Google Scholar]
- Myers J. A., Beauchamp B. B., Richardson C. C. Gene 1.2 protein of bacteriophage T7. Effect on deoxyribonucleotide pools. J Biol Chem. 1987 Apr 15;262(11):5288–5292. [PubMed] [Google Scholar]
- Myers J. A., Beauchamp B. B., White J. H., Richardson C. C. Purification and characterization of the gene 1.2 protein of bacteriophage T7. J Biol Chem. 1987 Apr 15;262(11):5280–5287. [PubMed] [Google Scholar]
- OLESON A. E., KOERNER J. F. A DEOXYRIBONUCLEASE INDUCED BY INFECTION WITH BACTERIOPHAGE T2. J Biol Chem. 1964 Sep;239:2935–2943. [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Genetic analysis of gene 1.2 of bacteriophage T7: isolation of a mutant of Escherichia coli unable to support the growth of T7 gene 1.2 mutants. J Virol. 1981 Jan;37(1):343–351. doi: 10.1128/jvi.37.1.343-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Processing of mRNA by ribonuclease III regulates expression of gene 1.2 of bacteriophage T7. Cell. 1981 Dec;27(3 Pt 2):533–542. doi: 10.1016/0092-8674(81)90395-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Tabor S., Tamanoi F., Richardson C. C. Nucleotide sequence of the primary origin of bacteriophage T7 DNA replication: relationship to adjacent genes and regulatory elements. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3917–3921. doi: 10.1073/pnas.77.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto D., Bhatnagar S. K., Bessman M. J. The purification and properties of deoxyguanosine triphosphate triphosphohydrolase from Escherichia coli. J Biol Chem. 1988 Jan 25;263(3):1494–1499. [PubMed] [Google Scholar]