Abstract
OBJECTIVE
Activated protein C (APC) is neuroprotective in stroke models and promotes post-ischemic neovascularization and neurogenesis. We utilized a controlled cortical impact (CCI) in mice to determine APC's effects on neuroprotection and angiogenesis and neurogenesis following traumatic brain injury (TBI).
METHODS
Mice were given: (1) single-dose APC (0.8 mg/kg i.p.) 15 minutes post-injury, (2) multi-dose APC (0.8 mg/kg i.p.): 15 minutes, and 6–48 hours post-injury, or (3) vehicle. We then assessed APC's effects on post-traumatic motor function with the rotarod and wire grip and beam balance tasks, and determined the lesion volumes and studied the formation of new blood vessels and markers of neurogenesis.
RESULTS
Mice treated with single-dose or multi-dose APC, compared to vehicle, showed significantly improved motor function on all tests. In the single-dose and multi-dose APC treatment groups, at 7 days post-treatment, lesion volume was significantly decreased by 30% and 50%, respectively. Multi-dose APC, but not single-dose, increased new blood vessel formation as shown by CD105+/Ki-67+ double immunostaining by nearly 2-fold at 7 days. Multi-dose APC also promoted post-traumatic proliferation of neuroblasts in the subventricular zone (SVZ) and their migration from the SVZ to the peri-lesional area.
CONCLUSIONS
APC improves functional outcome and is neuroprotective after TBI. It also promotes angiogenesis and survival and migration of neuroblasts from the SVZ to the peri-lesional area, but the exact role of these brain repair mechanisms remains to be determined. The present findings suggest that APC therapy may hold a significant therapeutic potential for TBI.
Keywords: Activated protein C, Angiogenesis, Controlled cortical impact, Neurogenesis, Neuroprotection, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a common cause of death and disability in the United States, often resulting in permanent functional deficits (20). Neurologic impairment is due to both immediate CNS tissue disruption (primary injury) and post-traumatic cellular and molecular events that worsen the original neurological insult. Ischemia, neuronal death cascades, cerebral swelling, and inflammation may cause secondary injury. Numerous pharmacologic agents have been explored as potential therapies aimed at ameliorating damage after TBI, but without much success (12,18,23). Several unsuccessful clinical trials have targeted a single injury mechanism, thus it is likely that successful therapy may require favorable effects on multiple deleterious cascades, rather than targeting a single pathophysiologic pathway.
Activated protein C (APC) is an endogenous serine protease that is part of a systemic anticoagulant and anti-inflammatory surveillance system (8,19). APC anticoagulant activity is mediated by highly specific, irreversible proteolytic inactivation of factors Va and VIIIa, with contributions from various non-enzymatic plasma cofactors (19). Independent of its systemic anticoagulant and anti-inflammatory activities, APC has direct cytoprotective effects that include anti-apoptotic activities and alterations in gene expression profiles (19) and stabilization of endothelial barriers (5,6). Cellular effects of APC are mediated primarily by the protease-activated receptor 1 (PAR1) as well as endothelial protein C receptor (EPCR) on endothelial cells (19). Despite APC's anticoagulant properties, APC decreases tissue plasminogen activator (tPA)-mediated hemorrhage by inhibiting a pro-hemorrhagic tPA-induced nuclear factor κB-dependent matrix metalloproteinase-9 pathway, through PAR1 and EPCR, which prevents BBB breakdown, thus decreasing hemorrhage (3,13).
APC reduces the severity of both ischemia/reperfusion-induced brain injury (2,3,13,21,22,33) and compression-induced spinal cord injury in rats (11). Additional studies show that APC, when given in a multi-dose regimen following onset of cerebral ischemia, enhances cerebral perfusion, blocks blood brain barrier (BBB) breakdown and increases the number of endothelial replicating cells (22). In contrast to other pro-angiogenic agents, APC promotes new blood vessel formation while simultaneously protecting the BBB and preventing endothelial barrier leakage (22). APC also significantly increases post-ischemic proliferation of neuronal progenitor cells in the subventricular zone (SVZ), and migration of newly formed neuroblasts from the SVZ toward the ischemic border (22). It has been suggested that angiogenic and neurogenic properties of APC are independent of its direct neuroprotective effects and reductions in the infarct size (22).
With significant evidence supporting neuroprotection and brain repair properties of APC in post-ischemic brains of mice (2,3,13,21,22,23), we studied whether these beneficial effects can extend to a controlled cortical impact (CCI) model of TBI in mice. We hypothesized APC following TBI would: (1) improve motor function after injury, (2) be neuroprotective, causing decreased lesion volume, and (3) increase brain repair through formation of new blood vessels and neurogenesis.
MATERIALS AND METHODS
Mouse Controlled Cortical Impact Model
Procedures were approved by the Animal Care Committee at the University of Rochester using National Institutes of Health (NIH) guidelines. Eleven week old male C57Bl6 mice were subjected to CCI as previously described (27). Briefly, mice were anesthetized with 2% isoflurane, N2O, and oxygen (2:1) using a nose cone, and were then positioned in the stereotaxic frame. A 5-mm craniotomy was made using a portable drill, and a 5-mm trephine over the left parietotemporal cortex. The resultant bone flap was removed. Mice were then subjected to CCI using a pneumatic cylinder with a 3-mm flat-tip impounder, at a velocity of 6 m/s, 100 milliseconds duration and a depth of 1.0 mm. Sham-injured animals (n=9) underwent the identical procedure as the trauma group, however, no injury was delivered. Recombinant wild-type murine APC was used for all experiments. Single-dose APC (0.8 mg/kg, n=10), or saline (n=15) was administered 15 minutes after impact via i.p. injection. From prior APC dose studies, this i.p. dose produced blood levels equivalent to those seen after 0.2 mg/kg injections via the femoral vein (22). The multi-dose group (n=14) received additional i.p. APC injections at 6, 12, 24, and 48 hours post-injury. The mice were then assessed during the next 7 days using various behavioral tests (described later), and subsequently sacrificed on post-injury day 7 for further analysis as described below.
Volumetric Data
Morphometric image analysis (Image-Pro Plus 4.5.1, Media Cybernetics Inc., Bethesda, MD) was used to determine lesion volume at 24 hours and 7 days after CCI, as reported (25). Mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine, and then transcardially perfused with heparin in saline. Brains were subsequently removed and frozen in O.C.T. embedding media (Fisher Scientific, Houston, TX) with powdered dry ice for cryostat sectioning (10 μm). At each 0.5-mm interval, sections were obtained and stained with Cresyl violet solution (FD NeuroTechnologies Inc., Baltimore, MD). The areas of the lesions were determined using image analysis (Image-Pro Plus 4.5.1, Media Cybernetics Inc., Bethesda, MD), and were then summed to obtain the corresponding lesion volumes.
Evaluation of Motor Function
Rotarod
To assess behavioral deficits after TBI, the standard rotarod test (10,33) was performed at 1–7 days after CCI. All mice were trained on the rotarod for 5 days before the trauma. An initial velocity of 5 rpm was used for the first 60 seconds, the velocity then increased from 5 to 10 rpm over the time frame of 60–160 seconds, followed by an increase in velocity from 10 to 25 rpm over the time frame of 160–240 seconds. Pre-injury baseline rotarod tests were performed 24 hours before TBI for each animal. Two trials were performed for each mouse and the average taken. Latencies for each group of mice were averaged and expressed as a percentage of their respective baseline values for days 1–7 following injury.
Wire Grip Test
Vestibulomotor function was assessed by wire grip testing on post-injury days 1–7. Briefly, mice were picked up by the tail and placed on a taut metal wire suspended between two upright bars 50 cm above a padded table. The time and manner in which the mouse could hold onto the wire were noted and scored on a scale of 0–5 (33). Each mouse was tested 3 consecutive times a day. The daily score and latency reported is the average of these individual trials. A composite group score was calculated as the mean of these daily scores and used for analysis.
Beam Balance
The beam balance task was used to assess the more complex components of vestibulomotor function and coordination for mice (33). Briefly, mice were placed on a narrow (1.0-cm) wooden beam, and the ability to maintain equilibrium was scored from 0–6. Mice were pre-trained 24 hours before injury so that they could balance on the beam with a steady posture for 1 minute.
Immunocytochemistry
Coronal cryostat sections (25 μm) were mounted on slides and postfixed in either acetone or 4% paraformaldehyde in phosphate buffered saline for 10 minutes, then stored at −20°C until immunostaining.
Initially, sections were hydrated 3 times with tris-buffered saline (TBS) for 5 minutes each rinse. Then, sections were washed with TBS containing 0.1% Triton X-100 (TBS-T).
For the analysis of blood vessels, brain sections were incubated with an antibody to CD105 or Ki-67 overnight at 4°C followed by incubation with the secondary antibodies (e.g., anti-goat IgG-Cy3 to detect CD105+ endothelial structures or an anti-mouse IgG-FITC conjugate to detect Ki-67+ cells). All secondary antibodies were incubated for 1 hour at 37°C.
For the analysis of neurogenesis, brain sections were initially prepared for Dcx and Ki-67 staining as explained above, and incubated with primary antibodies to Dcx and Ki-67 followed by incubation with their respective secondary antibodies (e.g., anti-rabbit IgG-Cy3 to detect Dcx and anti-mouse IgG-FITC to detect Ki-67).
Following immunostaining, TBS-T was used to rinse sections for 15 minutes following incubation. Slides were immediately mounted using Vectashield® HardSet Mounting Medium with Fluorescence (Vector Labs., Burlingame, CA).
Image analysis and quantification
Confocal laser-scanning three-color microscopy (LSM510 META; Carl Zeiss MicroImaging) was used to acquire images from immunostained sections. Argon laser (excitation, 488 nm; emission, 500–550 nm) and helium-neon laser (excitation, 543 nm; emission, 560–615 nm) were used to excite FITC and Cy3, respectively. In angiogenesis analysis, the following pseudocolors were used: CD105-Cy3, red; Ki-67-FITC, green. For images of neuroblasts we used the following pseudocolors: Dcx-Cy3, green; Ki-67-FITC, red.
Quantitative image analysis was performed at original magnification X20 on a light microscope. The number of CD105+/Ki-67+ cells were counted in the injured area and averaged. Sections were selected at 250-μm separations over the entire lesion depth. Dcx+/Ki-67+ cells corresponding to migrating neuroblasts (4) were counted in several sections spanning the SVZ in addition to those that were migrating from the SVZ towards the injury site in the corpus callosum.
Statistical analysis
All data is presented as the means ± standard error of the mean (SEM). Analysis of variance (ANOVA) for repeated measures was used to determine statistically significant differences for all behavioral tests. 24-hour lesion volumes were compared by Student's t-test. ANOVA followed by Tukey's post-hoc test was used for all other comparisons. Differences were considered statistically significant if P<0.05.
RESULTS
APC Improves Motor Function after Single and Multi-Dose Regimens
Rotarod
Mice treated with single-dose APC showed early improvement on the rotarod starting at post-injury day 1 and continuing until post-injury day 7, when compared to vehicle-treated mice. Mice treated with single-dose APC were able to perform at 60% of their pre-injury baseline on post-injury day 1 compared to vehicle-treated controls who performed at 20% of their pre-injury baseline on post-injury day 1 (P<0.001; ANOVA) (Fig. 1A). Mice treated with the multi-dose APC regimen were able to perform at 80% of their pre-injury baseline on post-injury day 1, demonstrating significant improvement in motor performance compared to the single-dose APC group (P<0.05; ANOVA) and vehicle-treated controls (P<0.001; ANOVA). Of note, mice in the multi-dose APC group returned to their pre-injury baseline by post-injury day 2, while mice in the single-dose APC group did not return to pre-injury baseline until post-injury day 5. Vehicle-treated mice did not fully return to their pre-injury baseline during the 7-day post-injury study period. Un-injured control mice treated with APC showed no change in the rotarod performance (not shown).
Figure 1. APC Improves Motor Neurologic Function after Single and Multi-Dose Regimens.
(A) Rotarod latency, (B) beam balance score, (C) wire grip score, (D) wire grip latency, one week following TBI, in sham-operated controls (n=9), vehicle-treated controls (n=15), single-dose APC (n=10) and multi-dose APC (n=14) groups. Values are mean ± SEM.
Beam Balance Task
Mice treated with either single-dose or multi-dose APC performed significantly better at the beam balance task compared to vehicle-treated mice (Fig. 1B). Mice treated with single-dose APC had significantly improved beam balance score (P<0.001; ANOVA), as did mice treated with the multi-dose APC regimen (P<0.001; ANOVA), when compared with vehicle-treated mice. In addition, mice in both APC treatment groups returned to their pre-injury baseline performance by post-injury day 7, while vehicle-treated mice did not fully return to their pre-injury baseline within the 7-day post-injury study period. Un-injured control mice treated with APC showed no significant improvement in beam balance performance when compared to their pre-treatment baseline performance (not shown).
Wire Grip Test
Mice treated with either single-dose APC (P<0.01; ANOVA) or the multi-dose regimen (P<0.001; ANOVA) had significantly improved wire grip scores compared to vehicle-treated mice (Fig. 1C,D). Mice in both APC treatment groups also had increased wire grip latency times compared to vehicle-treated controls (single-dose P<0.01, multi-dose P<0.001; ANOVA). Both treatment groups demonstrated significant improvement over the vehicle-treated mice. Mice in the multi-dose group had slightly better performance in the first 3 days post-injury (NS). While vehicle-treated mice had a 70% reduction in their motor score at post-injury day 1, reflecting significant deficits, APC treated mice (both groups) had only a 30% reduction in their score at post-injury day 1. Un-injured control mice treated with APC showed no significant improvement in wire grip performance (not shown).
APC Neuroprotection after Single and Multi-Dose Regimens
Lesion Volume
Mice were killed for lesion volume analysis at 2 time points post-injury (24 hours and 7 days). Brains were removed for sectioning and Cresyl violet staining to analyze lesion volume (Fig. 2C–H). At 24 hours (Fig. 2A) mice treated with single-dose APC had a 50% decrease in lesion volume compared to vehicle-treated controls (P<0.05; Student's t-test). At 7 days (Fig. 2B), compared to vehicle-treated controls, single-dose APC reduced lesion volume by 30% (P<0.001; Tukey's post-hoc test) and in the multi-dose treatment group by approximately 50% (P<0.001; Tukey's post-hoc test). In addition, mice in the multi-dose treatment group had significantly decreased lesion volume compared to the single-dose treatment group at post-injury day 7 (P<0.001; Tukey's post-hoc test).
Figure 2. APC Reduces Lesion Volume after Single and Multi-Dose Regimens.
(A) Lesion volumes 24 hours after TBI (n=4–5 mice per group) and (B) lesion volumes 7 days after TBI (n=4–5 mice per group). All lesion volumes are mean ± SEM. (C,D,E) Representative Cresyl violet staining of brain sections 24 hours after TBI, in vehicle, single-dose and sham-operated mice, respectively. (F,G,H) Cresyl violet staining of brain sections 7 days after TBI, in vehicle, single-dose and multi-dose APC treated mice, respectively.
APC Mediates New Blood Vessel Formation after Controlled Cortical Impact
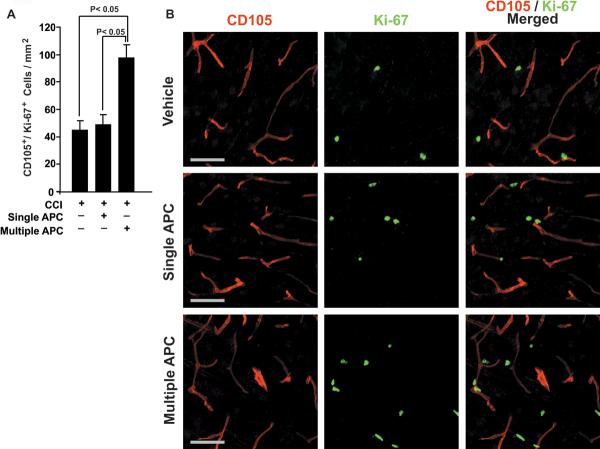
Compared to vehicle, single-dose APC treatment did not significantly increase the number of CD105+/Ki-67+ cells – a marker for newly generated endothelial cells, at 7 days post-CCI. In contrast, multi-dose APC therapy resulted in an increase in CD105+/Ki-67+ immunoreactivity by nearly 2-fold over both single-dose APC and vehicle groups (P<0.05; Tukey's post-hoc test) (Fig. 3A,B).
Figure 3. APC Mediates Formation of New Blood Vessels after CCI.
(A) CD105+/Ki-67+ cells are counted per mm2. Values are mean ± SEM (n=4–5 mice per group). (B) CD105 (left column) and Ki-67 (middle column) staining as well as their overlay (right column); Vehicle (top row), single-dose APC (middle row) and multi-dose APC (bottom row) are placed horizontally. Scale bar = 50 μm.
APC Promotes Neurogenesis after Controlled Cortical Impact
Single-dose APC treatment did not result in a significant increase in Dcx+/Ki-67+ cells in the ipsilateral SVZ or peri-lesional area at 7 days post-CCI, when compared to vehicle. However, as shown by 3-color confocal laser-scanning microscopy, APC multi-dose treatment, compared to vehicle, increased the number of Dcx+/Ki-67+ cells in the ipsilateral SVZ by 75% within 7 days after CCI injury (P<0.001; Tukey's post-hoc test) (Fig. 4A,B). Multi-dose APC treatment also increased the number of Dcx+/Ki-67+ neuroblasts migrating towards the peri-lesional area along the corpus callosum more than 2-fold, compared to vehicle-treated controls (P<0.001; Tukey's post-hoc test) (Fig. 4C,D).
Figure 4. APC Promotes Neurogenesis after CCI.
(A) Neuroblasts in the subventricular zone (SVZ) were identified using Dcx/Ki-67 double immunostaining. All Dcx+/Ki-67+ cells in the SVZ were counted on each section. Values are mean ± SEM (n=4–5 mice per group). (B) Dcx+/Ki-67+ migrating neuroblasts were quantified in the corpus callosum traveling towards the injury site. Scale bar = 50 μm.
DISCUSSION
The major findings of our study are as follows: 1) mice treated with single or multi-dose APC had significantly improved functional recovery reflected in rapid improvement on the rotarod, wire grip task, and balance beam when compared with vehicle-treated groups; 2) mice treated with single or multi-dose APC post-TBI showed significantly reduced lesion volume at 7 days post-injury compared to vehicle-treated groups, with multi-dose APC treatment reducing lesion volume significantly more than single-dose; 3) post-traumatic brain repair was enhanced in the multi-dose APC group, by increased formation of new blood vessels and neurogenesis when compared to the single-dose and vehicle-treated groups.
APC exerts direct neuronal and vascular protective effects in vitro and in vivo against divergent inducers of apoptosis and blocks both the intrinsic, mitochondria-mediated and the extrinsic, death receptors-mediated apoptotic pathways (2,9,13). An important point to address is whether neuroprotection and/or brain repair is responsible for the presently observed improvement in functional recovery as seen in the APC treated mice, when compared to vehicle. From prior studies examining the effects of APC and other agents on angiogenesis and neurogenesis following ischemic stroke in rodents, it has been shown that these brain repair processes are not seen until several days after injury (1,22,26,28,29,31,32). Similarly, angiogenic or neurogenic responses were not found 24 h after injury in brain trauma models in rodents (see for example refs. 7, 14–17). Therefore, the early functional improvements seen in the mice after APC treatment is likely attributed to neuroprotection only. To precisely determine the specific role of brain repair, as one of APC's beneficial effects, future studies should examine whether APC given at the time when it no longer reduces lesion volume, but promotes angiogenesis and neurogenesis, for example beginning at 48 hours after injury, may still improve the functional outcome after TBI.
In the current study, with a 1 mm CCI injury, most mice in the study are at, or close to, their pre-injury functional levels at the 7 day endpoint. Therefore, the present model may not be sensitive enough to study the role of brain repair after APC treatment because the functional outcome corrects itself without any treatment within 7 days due to high brain plasticity in young mice. But, a more significant injury model, possibly with a deeper injury, or studies in older mice, which would not allow a rapid spontaneous functional recovery, may offer better opportunities to study the true benefits of neurogenesis and angiogenesis on functional recovery vs. neuroprotective effects of APC.
Another important aspect of APC treatment in TBI remaining to be clarified is the timing of treatment necessary to achieve treatment benefits. First, as seen with tPA treatment of stroke, timing of treatment can be critical, as delayed treatment can actually increase injury severity (30). From a previous study in an ischemic stroke model, APC given at 6 or 24 hours after ischemia significantly improved functional outcome, reduced cerebral infarction volume, and enhanced repair of damaged brain tissue by promoting cerebral angiogenesis and neurogenesis (22). In future studies, APC treatment in a TBI model administered at time points further out from injury will help clarify this important issue.
Previous studies have looked at other treatments such as recombinant human erythropoietin, statins, and inducible nitric oxide as possible ways to promote brain repair through angiogenesis and neurogenesis following TBI (7, 14–17). While some benefits from these treatments have been shown, presently no definitive treatment has been established. This study demonstrates APC is a neuroprotective agent after TBI and promotes brain repair by stimulating post-TBI angiogenesis and neurogenesis. Until recently, APC has been studied mostly in the treatment of stroke, sepsis, and multiple sclerosis. Our data, however, show that APC may have a promising future in the treatment of TBI, and warrants further investigations to more clearly elucidate the true benefits of APC in the treatment of patients after TBI.
Article Summary.
Traumatic brain injury (TBI) is a common cause of death and disability in the United States, often resulting in permanent functional deficits. Numerous pharmacologic agents have been explored as potential therapeutic interventions aimed at ameliorating damage after TBI, but without much success. This study shows a significant decrease in lesion volume, and improvement in functional recovery in mice treated with activated protein C (APC) following TBI. In addition, we found that APC enhances brain repair in mice treated with multiple doses of APC. APC augmented new blood vessel formation, and may play an important role in tissue recovery and functional outcome after head injury. In addition, APC enhanced proliferation and migration of neuroblasts which may also contribute to functional recovery after TBI. Previous studies have looked at other possible treatments to promote brain repair, however, to this point no definitive treatment has been established. This study establishes APC as a protective agent in TBI in addition to its newly reported cerebral repair effects. Our data show that APC has a promising future in the treatment of TBI, and warrants further investigations into the efficacy of APC in the treatment of TBI.
Grant Information/ Other Acknowledgements
This work was supported in part by the National Institutes of Health Grants HL63290, HL081528 (to B.V.Z), and TL1 RR024135 (to A.H.M.). We are thankful to Dr. Michael Whalen at MGH for his advice and guidance.
Footnotes
Financial Disclosure: B.V.Z. is a scientific founder of Socratech L.L.C., a startup biotech company with a mission to develop new treatments for the aging brain, stroke, and Alzheimer's disease. B.V.Z is an inventor on pending patents related to APC.
REFERENCES
- 1.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 2.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 3.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, Larue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 4.Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36:1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- 5.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 6.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 7.Foley LM, Hitchens K, Melick JA, Bayir H, Ho C, Kochanek PM. Effect of inducible nitric oxide synthase on cerebral blood flow after experimental traumatic brain injury in mice. J Neurotrauma. 2008;25:299–310. doi: 10.1089/neu.2007.0471. [DOI] [PubMed] [Google Scholar]
- 8.Griffin JH, Zlokovic B, Fernandez JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 10.Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 11.Hirose K, Okajima K, Toaka Y, Uchiba M, Tagami H, Nakano K, Utoh J, Okabe H, Kitamura N. APC reduces the ischemia/reperfusion-induced spinal cord injury in rats by inhibiting neutrophil activation. Ann Surg. 2000;232:272–280. doi: 10.1097/00000658-200008000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 14.Lu D, Mahmood A, Qu C, Goussev A, Schalert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 15.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in the rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, Chopp M. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 18.McKee JA, Brewer RP, Macy GE, Borel CO, Reynolds JD, Warner DS. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit Care. 2005;2:342–351. doi: 10.1385/NCC:2:3:342. [DOI] [PubMed] [Google Scholar]
- 19.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 20.Rutland-Brown W, Langlois J, Thomas K, Yongli Y. Incidence of traumatic brain injury in the United States, 2003. J Head Traum Reh. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 22.Thiyagarajan M, Fernandez J, Lane S, Griffin J, Zlokovic B. Activated protein C promotes neovascularization and neurogenesis in postischemic brain via protease-activated receptor 1. J Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–79. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, Suda T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes microvascular tissue-plasminogen activator in rats. J Cereb Bloof Flow Metab. 1997;17:136–146. doi: 10.1097/00004647-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 27.Whalen MJ, Carlos TM, Dixon CE, Schiding JK, Clark RS, Baum E, Yan HQ, Marion DW, Kochanek PM. Effect of traumatic brain injury in mice deficient in intercellular adhesion molecule-1: assessment of histopathologic and functional outcome. J Neurotrauma. 1999;16:299–309. doi: 10.1089/neu.1999.16.299. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita T, Ninomiya M, Acosta PH, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Zhang L, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 32.Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin A, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: A study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zlokovic BV, Zhang CL, Liu D, Fernandez J, Griffin JH, Chopp M. Functional recovery after embolic stroke in rodents by activated protein C. Ann Neurol. 2005;58:474–477. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]