Abstract
Cranial neural crest cells migrate into the periocular region and later contribute to various ocular tissues including the cornea, ciliary body and iris. After reaching the eye, they initially pause before migrating over the lens to form the cornea. Interestingly, removal of the lens leads to premature invasion and abnormal differentiation of the cornea. In exploring the molecular mechanisms underlying this effect, we find that semaphorin3A (Sema3A) is expressed in the lens placode and epithelium continuously throughout eye development. Interestingly, neuropilin-1 (Npn-1) is expressed by periocular neural crest but down-regulated, in a manner independent of the lens, by the subpopulation that migrates into the eye and gives rise to the cornea endothelium and stroma. In contrast, Npn-1 expressing neural crest remain in the periocular region and contribute to the anterior uvea and ocular blood vessels. Introduction of a peptide that inhibits Sema3A/Npn-1 signaling results in premature entry of neural crest cells over the lens that phenocopies lens ablation. Furthermore, Sema3A inhibits periocular neural crest migration in vitro. Taken together, our data reveal a novel and essential role of Sema3A/Npn-1 signaling in coordinating periocular neural crest migration that is vital for proper ocular development.
Keywords: semaphorin3A, neuropilin-1, neural crest, cornea, lens
Introduction
Vertebrate eye development is an intricate process that involves the amalgamation of tissues and cells from different embryonic origins, including the optic cup, ectoderm, and neural crest. The optic cup gives rise to the neural and pigmented retina, whereas the lens and cornea epithelium are derived from the surface ectoderm. Neural crest that migrate toward the eye contribute to numerous ocular tissues including the cornea endothelium and stroma, as well as the anterior uvea comprised of the ciliary body, iris, trabecular meshwork, and Schlemm’s canal. However, the molecular signals regulating neural crest cell migration during eye development remain unknown.
Neural crest cells originate at the junction of the closing neural tube and ectoderm, and migrate to various regions of the developing embryo where they give rise to numerous derivatives (Le Douarin, 1982). In the cranial region, a subpopulation of neural crest cells migrates along the optic vesicles into the presumptive eye region. Shortly thereafter, neural crest cells populate the periocular region surrounding the rudimentary eye, which at this time consists of the optic cup, lens, and overlying surface ectoderm. Following a pause in the periocular region, neural crest cells migrate into the region between the lens and presumptive corneal epithelium and give rise to the endothelium and stroma layers of the cornea (Hay, 1980; Hay and Revel, 1969; Lwigale et al., 2005). Neural crest cells that remain in the periocular region contribute to the anterior uvea, ocular blood vessels, connective tissues and extraocular muscles (Johnston et al., 1979; Creuzet et al., 2005).
Developmental eye disorders including Axenfeld-Rieger and Peter’s anomalies, have been attributed to defects in neural crest development. There is growing evidence that several genes play critical roles during patterning and differentiation of neural crest derived ocular tissues, the mutation of which causes ocular abnormalities with phenotypes similar to human birth defects (see review by Cvekl and Tamm, 2004). For example, deletion of the transcription factor Pax6 that is expressed in the lens, iris, ciliary body and corneal epithelium, leads to increased accumulation of neural crest cells in the intraocular cavities of the eye during development (Kanakubo et al., 2006). The homeobox transcription factors Pitx2 (Evans and Gage, 2005) and Six3 (Hsieh et al., 2002) are expressed in the periocular neural crest and their mutation leads to eye defects with phenotypes such as; small eye size, decreased cornea thickness and transparency, and reduced vasculature. In addition, extraocular mesenchyme comprised mostly of neural crest cells, is necessary for proper patterning of the optic vesicle (Fuhrmann et al., 2000) and inhibits lens formation outside the ocular region (Bailey et al., 2006). Therefore, neural crest cells and/or genes associated with them play major roles during eye development.
To receive and send inductive signals to other ocular tissues, neural crest cells have to be in the right place at the right time. This is manifested in the migratory behavior of neural crest cells in the periocular region, where they remain for a relatively long period of time before entering the rudimentary eye (Hay and Revel, 1969; Hay, 1979; Lwigale et al., 2005). It has been hypothesized that transforming growth factor-β (TGFβ) signaling from the lens regulates periocular neural crest migration by acting as chemoattractant (Saika et al., 2001), since loss of TGFβ signaling results in diminished accumulation of neural crest cells in the cornea. Whether TGFβ is an attractive cue, or if other factors are also involved in regulating periocular neural crest cell migration during eye development remains unknown.
The chemorepellent Sema3A, a well-known axon guidance molecule, is expressed in the lens vesicle at a critical time during eye development (Chilton and Guthrie, 2003), prior to the onset of periocular neural crest migration. Previous studies have shown that Sema3A/Npn-1 signaling mediates neural crest migration in the hindbrain region (Eickholt et al., 1999; McLennan and Kulesa, 2007; Osborn et al., 2005) during craniofacial development, in the midbrain (Schwarz et al., 2008) and trunk (Eickholt et al., 1999) regions during cranial and dorsal root gangliogenesis. Nonetheless, the role of Sema3a/Npn-1 signaling during neural crest migration into the eye is yet to be studied. During eye development, Sema3A signaling from the lens regulates corneal innervation by Npn-1 expressing trigeminal sensory axons (Lwigale and Bronner-Fraser, 2007). This raises the intriguing possibility that Sema3A/Npn-1 signaling may also function at earlier times, such as during periocular neural crest migration and ocular development.
In this study we show that inhibition of Sema3A, either biochemically or by lens ablation, results in premature neural crest migration and malformation of the cornea. Sema3A is expressed very early during eye development in the lens placode and is maintained in the lens epithelium throughout development. Npn-1 is expressed by periocular neural crest, but interestingly, is down-regulated as cells migrate between the ectoderm and lens to form the cornea. We show that the pause in periocular neural crest migration is critical to eye development and that Npn-1 acts as a switch that regulates this process, thus segregating the cells into various tissues of the anterior segment. Thus, our findings suggest a novel role of Sema3A/Npn-1 signaling during ocular development.
Materials and methods
Embryos
Fertile White Leghorn chick (Gallus gallus domesticus) and Japanese quail (Coturnix coturnix japonica) eggs were obtained from a commercial supplier (Chino Valley, CA.). Eggs were incubated at 38°C under humidified conditions for various periods of time.
Chimeras
Quail-chick chimeras used to track periocular neural crest cells were created as previously described (Lwigale et al., 2005). Briefly, eggs were incubated at 38°C for 27–30 hours to obtain HH stage 9 (Hamburger and Hamilton, 1951) embryos. Dorsal neural tubes (containing premigratory neural crest cells) encompassing the region between caudal diencephalon and rhombomere 1 were dissected from quail embryos and grafted into stage-matched chick embryos from which similar tissue had been extirpated. Embryos were reincubated until various stages of development (E3, E4, E5, and E6). Neural crest contribution to the eye was assessed in the chimeras using a quail-specific marker as described below.
Lens ablation
Lens ablations were performed as previously described (Lwigale and Bronner-Fraser, 2007). Briefly, lens vesicles were carefully removed through an incision made in the surface ectoderm overlaying the right eye of each E3 quail-chick chimera. The left eye was left unoperated and served as control. Operated embryos were reincubated and collected at E4 and E5.
Peptide injection
Peptide injections were performed as described (Lwigale and Bronner-Fraser, 2007). The Sema3A-blocking peptide (N-Ac-HAVEHGFMQTLLKVTLE-NH2, Williams et al., 2005) and as control, a VEGF-blocking peptide (Ac-NTDSRCKARQLENER-NH2) were pressure injected at 10 mM concentration into the lumen of the right lens of E3 chick embryos. Injected embryos were reincubated and collected at E4 and E5.
Immunohistochemistry
Immunofluorescence staining of cryo and paraffin embedded sections (8–12μm in thickness) was performed as previously described (Lwigale et al., 2005). The following antibodies were used: the quail nucleus-specific marker (mouse anti-QCPN, DHSB, 1:1) was used to label quail neural crest cells in quail-chick chimeras; rat anti-N-cadherin (DSHB, MNCD2 clone; 1:1) was used as a marker for the cornea endothelial layer but also labels the lens and optic cup; and anti-HNK-1 (American Type Culture; 1:3) was used to label migratory neural crest cells. Fluorescent secondary antibodies (Molecular Probes) Alexa Fluor 594 goat anti-mouse IgG1, Alexa Fluor 488 goat anti-rat, and Alexa Fluor 488 goat anti-mouse IgM were used at 1:200 dilution. Phalloidin 488 (Molecular Probes) was used at 1: 200 to label periocular neural crest in vitro. Fluorescent-labeled sections were counterstained with DAPI to visualize all nuclei.
In situ hybridization
Whole-mount (Henrique et al., 1995) and section (Etchevers et al., 2001) in situ hybridization were performed at 65°C. Antisense riboprobes were made from Sema3A cDNA (Lwigale and Bronner-Fraser, 2007) and full-length Npn-1 cDNA (Kind gift from J. A. Raper) templates. Paraffin embedded embryos were sectioned between 8–12μm.
Periocular mesenchyme cultures
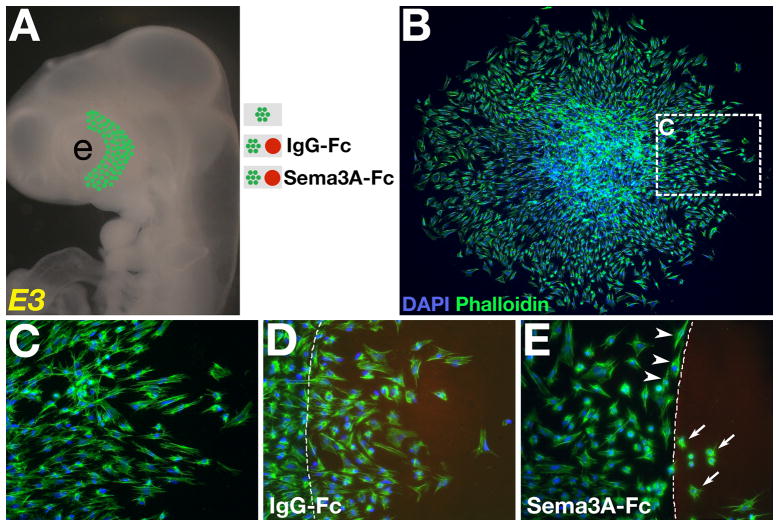
Heads from E3 chick embryos were collected in Ringer’s solution, transferred into DMEM (GIBCO, supplemented with 10% fetal bovine serum and antibiotics [100 units Penicillin; 100 μg Streptomycin/ml]) containing 1.5 mg/ml dispase, and digested at room temperature for 5 minutes. Digested embryos were rinsed extensively in Ringer’s and the surface ectoderm overlying the periocular region was removed along the temporal half of the eye (Fig. 5A). Underlying periocular mesenchyme was dissected out and trimmed into small cubes with approximately 1mm edges that were incubated under various conditions. All cultures were on fibronectin-coated (10μg/ml, BD Biosciences) four-well chamber slides (Lab-TekII, NUNC). For co-cultures, a drop of recombinant human Sema3A-Fc (80μg/ml, Eickholt et al., 1999) or control recombinant human IgG1-Fc (80μg/ml, R and D Systems) protein containing Alexa Fluor 594 (1:1000) was manually placed in each well and incubated at 37°C for 1 hour. Slides were rinsed 3 times with sterile water to remove any unbound -Fc protein then coated with fibronectin for 2 hours at 37°C. After aspirating the fibronectin solution, a drop of DMEM containing one cube of periocular mesenchyme was placed adjacent to each -Fc spot (Fig. 5A) and pre-incubated for up to 1 hour in a humidified CO2 incubator at 37°C to allow the cells to attach. Upon observation of cell attachment, O.5 ml of DMEM was added to each chamber then reincubated for 12–16 hours. Cultures were fixed in 4% paraformaldehyde, counterstained with Phalloidin 488 and DAPI, and then imaged.
Fig. 5. Periocular neural crest cells are migratory in culture and responsive to Sema3A.
(A) Periocular neural crest mesenchyme was dissected from the temporal region of the eye of E3 chick embryos and cultured under various conditions. (B–C) Mesenchyme cultured on fibronectin-coated slide showing numerous cells migrating independently away from the explant. Co-culture of explanted mesenchyme adjacent to (D) IgG-Fc protein spot showing no effect on periocular neural crest migration, and (E) Sema3A-Fc protein spot showing avoidance or morphological changes. Cultures were stained with DAPI (blue) and phalloidin-488 (green). Fc protein was stained with Alexafluor 594 (red). Dotted lines indicate margin of the spot. e, eye.
Results
Lens regulates periocular neural crest migration
In avian embryos, the quail-chick chimeric technique has served as a valuable tool for identifying and following neural crest cell lineage and contributions to diverse derivatives. Studies using this technique have revealed that a subset of cranial neural crest cells makes extensive contributions to the eye (Hays, 1980; Johnston et al., 1979; Creuzet et al., 2005; Lwigale et al., 2005). Here, we used quail-chick chimeras to track neural crest cells at various stages of eye development. Neural crest cells migrate from the grafted quail dorsal neural tube (see methods section) and populate the ocular, trigeminal, and maxillo-mandibular regions of the host (data not shown). Sections through the eye region encompassing the periocular mesenchyme, optic cup, lens, and cornea were immunostained using a quail nuclear-specific marker (QCPN) to label all quail-derived neural crest cells.
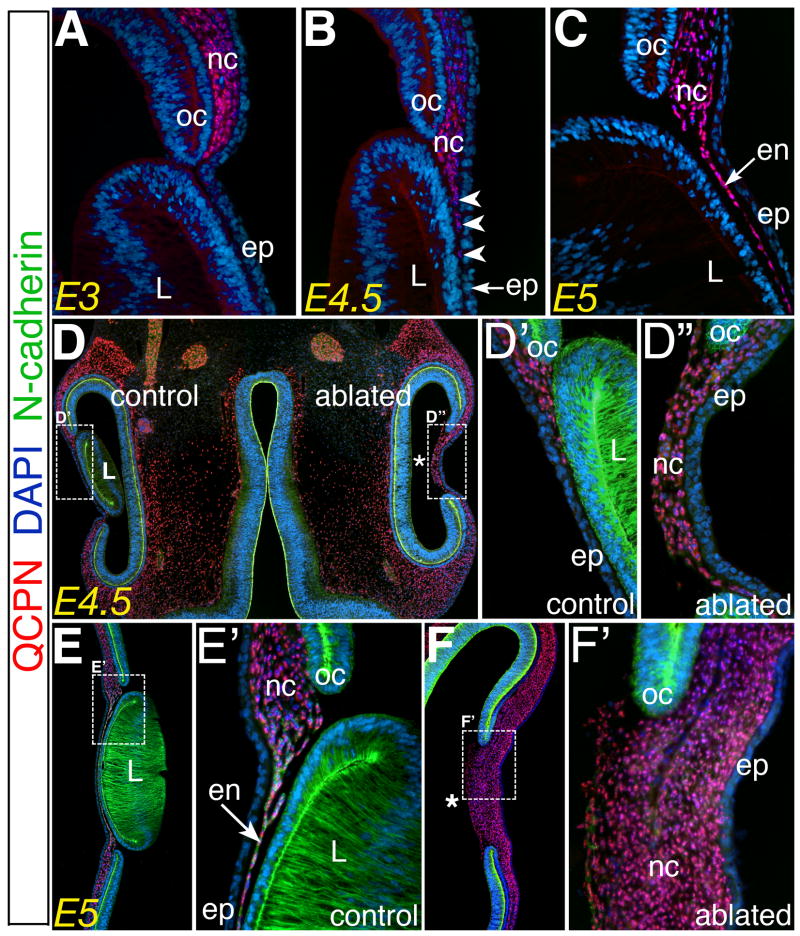
To investigate the role of the lens in the migration of neural crest cells, lens vesicles were ablated from the right eyes of chimeric embryos at E3, keeping the left eyes as an unoperated control. As expected, immunostaining of sections through the control eyes revealed QCPN positive quail-derived neural crest cells had accumulated in the periocular region at E3 (Fig. 1A). They remained in this location until about E4.5 and then commenced migration into the region between the presumptive cornea epithelium and lens (Fig. 1B, arrowheads), subsequently forming a monolayer of cells (Fig. 1C, arrow) or the cornea endothelium by E5.
Fig. 1. Periocular neural crest migration is regulated by the lens.
(A–C) Sections through the eye showing the location of QCPN positive (red) neural crest cells at E3, E4.5, and E5, respectively. (D–D″) Section through E4.5 and (E–F′) E5 showing neural crest location after migrating and differentiating in the presence and absence of the lens. L, lens; ep, cornea epithelium; nc, periocular neural crest; oc, optic cup; en, cornea endothelium; * region of the eye after lens ablation.
A different scenario was observed in the absence of the lens, where numerous neural crest cells prematurely migrated into the eye by E4.5 (n=5, Fig. 1D,D″) compared to the left control (Fig. 1D,D′). By E5 a thick mass of neural crest cells had migrated into the region of the presumptive cornea (n=6, Fig. 1F,F′) but failed to form the N-cadherin positive corneal endothelium evident in the control eye (Fig. 1E,E′, arrow).
Altogether, these results suggest that the lens plays a critical role during periocular neural crest migration into the eye, raising two possible explanations: 1) the lens acts as a physical barrier to migration, or 2) the lens is a source of a chemorepellent that hinders neural crest migration into the developing eye. However, the former possibility seems less likely at E4.5 given that the lens remains adjacent to the epithelium at the onset of normal neural crest migration into the eye (Fig. 1B,D).
Expression pattern of Sema3A during eye development
To explore the possibility that the lens may produce a chemorepellent, we examined candidate inhibitory ligands expressed by the lens. Sema3A was a good candidate since we previously showed that at later stages, lens-derived Sema3A functions as an inhibitor of trigeminal sensory axons (Lwigale and Bronner-Fraser, 2007). Sema3A is expressed in the lens vesicle at E3 (Chilton and Guthrie, 2003) as well as in the lens epithelium later at E5, persisting throughout development (Lwigale and Bronner-Fraser, 2007).
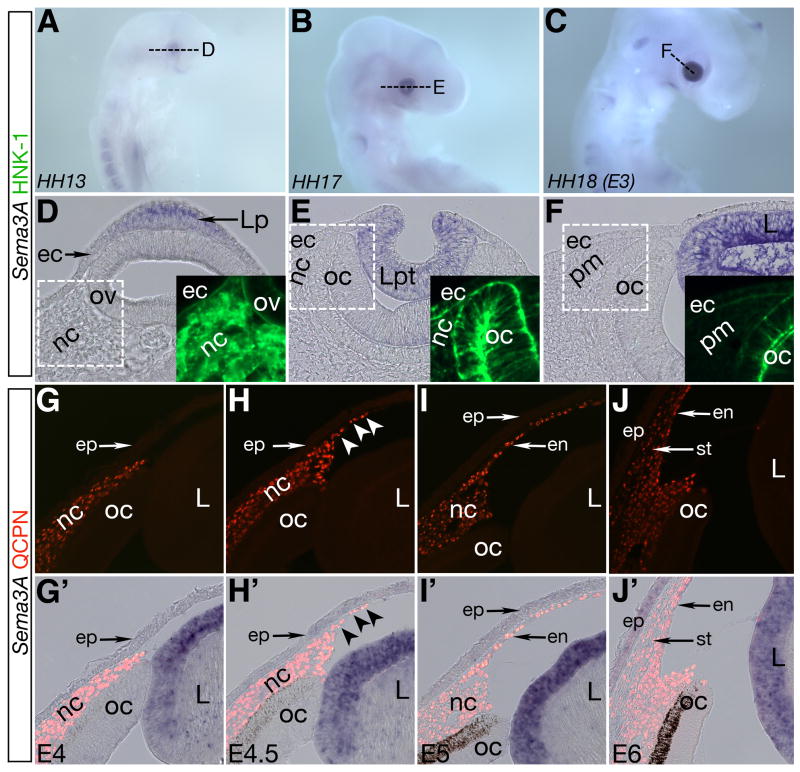
We examined the temporal expression of Sema3A in the lens from E2 to E3 (stage 13–18), from lens placode to vesicle stage, and during the time of periocular neural crest migration into the vicinity of the lens. In situ hybridization for Sema3A on whole-mount embryos revealed weak expression restricted to the presumptive eye region at stage 13 (Fig. 2A). At stage 17 and 18, strong expression of Sema3A was evident in the developing lens (Fig. 2B,C) but absent in surrounding ocular tissues. Cross sections through the eye region revealed that Sema3A expression is restricted to the lens placode shortly after its induction (Fig. 2D), and persists throughout the formation of the lens pit and vesicle (Fig. 2E and F). Concomitantly, HNK-1 positive migratory neural crest cells invade the presumptive eye region but avoid the Sema3A expressing lens placode and vesicle during this time period (Fig. 2D–E and Fig. S1A). Since periocular mesenchyme down-regulates HNK-1 by E3 (Fig. 2F and Fig. S1A), we tracked neural crest cells at this (Fig. S1B, Lwigale et al., 2005) and subsequent stages using quail-chick chimeric technique and QCPN staining.
Fig. 2. The lens expresses Sema3A during early eye development.
(A–C) Sema3A is expressed in the lens placode and vesicle during lens induction. (D–F) Cross sections through A–C showing restricted expression of Sema3A in the lens placode, pit, and vesicle surrounded by periocular neural crest. (G–J) QCPN staining showing localization of neural crest cells at different migration stages during eye development. (G′–J′) Co-localization of QCPN with Sema3A showing that expression is maintained in the lens epithelium. nc, periocular neural crest; pm, periocular mesenchyme; ov, optic vesicle; oc, optic cup; Lp, lens placode; Lpt, lens pit; L, lens; ec, ectoderm; ep, cornea epithelium; en, cornea endothelium; st, cornea stroma.
At E4, expression of Sema3A by the lens is restricted to the epithelial layer and absent from lenticular cells. Consistent with a possible inhibitory role, neural crest cells remained in the periocular region at this time (Fig. 2G, G′). Interestingly, Sema3A expression remained unchanged in the lens during the onset of QCPN positive neural crest migration between the cornea epithelium and lens at E4.5 (Fig. 2H, H′, arrowheads). In fact, Sema3A expression persisted throughout and beyond the time during which the cornea endothelium and stroma formed (Fig. 2I, I′ and J,J′).
Npn-1 expression is transient and acts cell autonomously to regulate periocular neural crest migration into the rudimentary eye
Our observation that Sema3A expression remains constant in the lens raises the intriguing question of why neural crest cells pause prior to entering the rudimentary eye. One possibility is that the receptor for Sema3A may be dynamically regulated. To address this issue, we examined the pattern of Npn-1 expression during eye development.
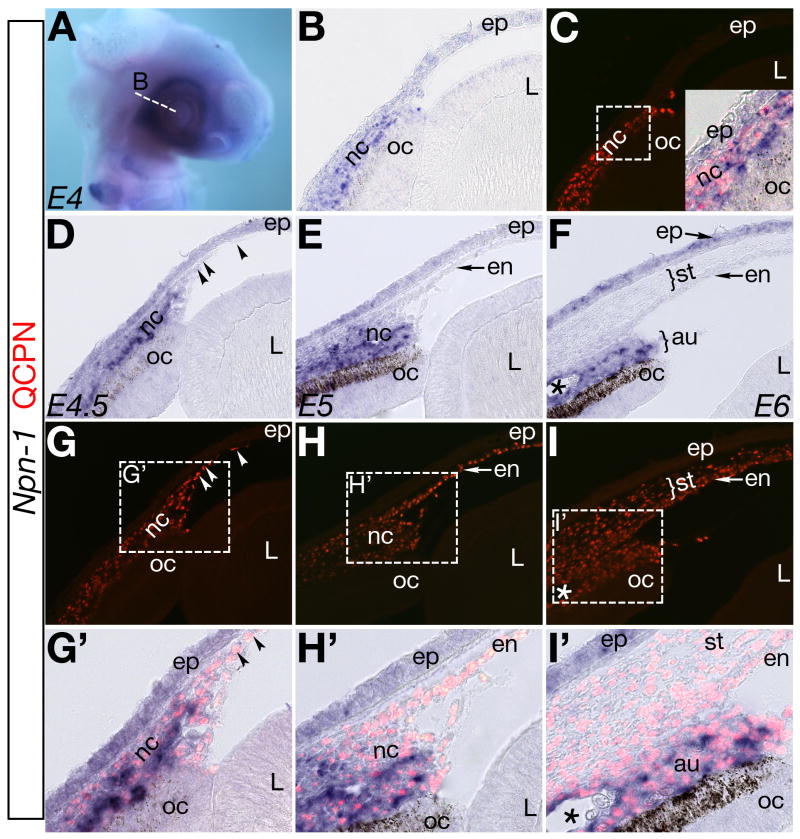
In cranial neural crest, Npn-1 expression is restricted to the hindbrain at stage 12 (Eickholt et al., 1999). Npn-1 expression is not detected in the periocular region until stage 19 (Chilton and Guthrie, 2003), corresponding to a period when neural crest pause before migrating into the eye (Hay, 1980; Hay and Revel, 1969; Lwigale et al., 2005). Because Sema3A is expressed in the lens vesicle at the same time periocular neural crest express Npn-1, we carefully examined Npn-1 expression during neural crest migration. Using a combination of quail-chick chimeras and in situ hybridization, we found that at E4, Npn-1 is expressed in the periocular region (Fig. 3A) in a pattern similar to E3 (Chilton and Guthrie, 2003; and data not shown). Cross sections through the eye region at E4 revealed expression of Npn-1 in the periocular region between the ectoderm and optic cup (Fig. 3B), overlapping with the QCPN staining of quail derived neural crest (Fig. 3C). Shortly afterwards at E4.5, Npn-1 expression remained in the periocular region (Fig. 3D). Interestingly, a few QCPN positive cells that had down-regulated Npn-1 expression, had migrated between the lens and epithelium (Fig. 3D,G,G′ arrowheads). At E5, more QCPN positive cells had down-regulated Npn-1 expression and migrated into the eye forming the cornea endothelium (Fig. 3E,H,H′). Between E4.5 and E5, Npn-1 negative neural crest cells migrated adjacent to the Sema3A expressing lens epithelium.
Fig. 3. Expression of Npn-1 during neural crest migration into the developing eye.
(A–B) Npn-1 is expressed in the periocular region at E4. (C) Where QCPN positive neural crest cells reside. (D,G,G′) At E4.5 Npn-1 expression is down-regulated in a subset of neural crest that have migrated between the epithelium and lens (arrowheads). Npn-1 is down-regulated by the subpopulation of QCPN positive neural crest cells that form the cornea endothelium at E5 (E,H,H′) and stroma at E6 (F,I,I′). Npn-1 expressing neural crest cells remain in the periocular region and later form tissues of the anterior uvea (au). Asterisk in (F,I,I′) indicates ocular blood vessel. nc, periocular neural crest; oc, optic cup; L, lens; ep, cornea epithelium; en, endothelium; st, stroma; au, presumptive anterior uvea.
At E6 the anterior chamber forms and separates the cornea from the lens. Analysis of cross sections of eyes at this stage revealed a clear difference in Npn-1 expression between periocular neural crest destined to form the cornea (endothelium and stroma) and tissues of the anterior uvea (iris, ciliary body, and blood vessels). QCPN positive neural crest cells that migrated into the eye to form the cornea had down-regulated Npn-1, whereas those that remained in the periocular region expressed Npn-1 (Fig. 3F,I,I′) and formed the anterior uvea. By this time the cornea epithelium expressed Npn-1.
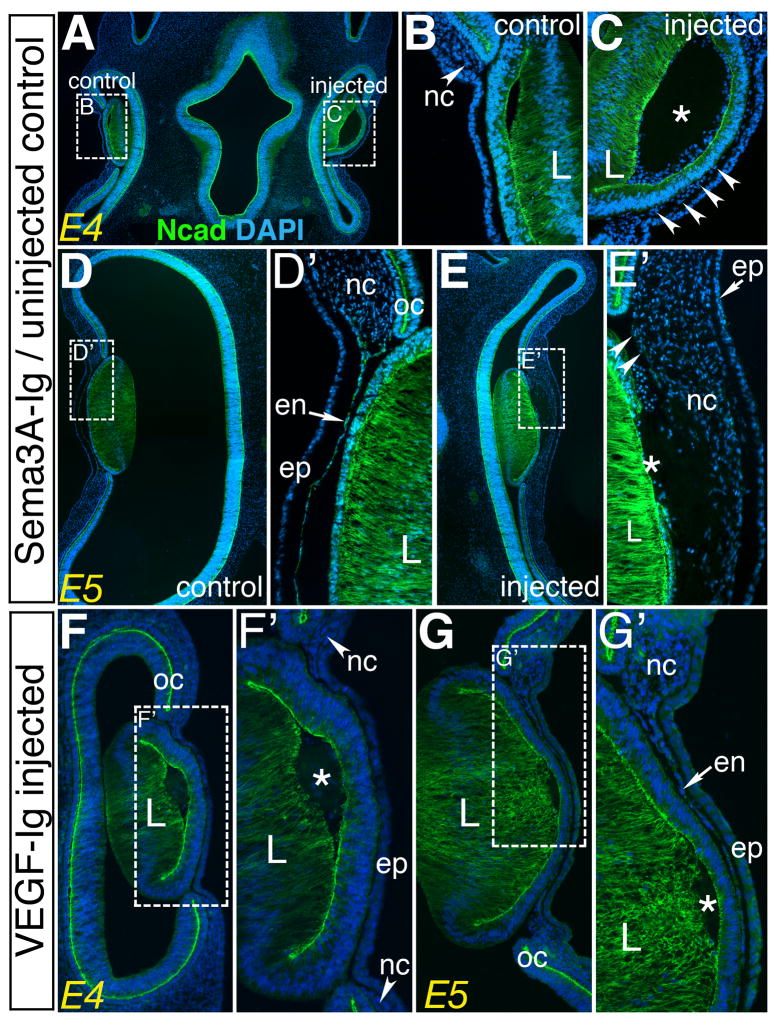
Inhibition of Sema3A/Npn-1 signaling mimics lens ablation
Previously, we showed that a peptide inhibitor that specifically blocks the Sema3A-Ig domain from interacting with Npn-1 (Williams et al., 2005) abrogates trigeminal axon repulsion by the lens both in vitro and in vivo (Lwigale and Bronner-Fraser, 2007). To assess whether Sema3A/Npn-1 signaling between the lens and periocular neural crest plays a role in their migration, we injected the Sema3A-Ig peptide inhibitor in the lens vesicle of right eye of each embryo at E3. As control, similar injections were performed with a VEGF peptide inhibitor that blocks VEGF-Ig domain from interacting with Npn-1 (Barr et al., 2005) and has no effect on Sema3A/Npn-1 signaling (Lwigale and Bronner-Fraser, 2007). In both cases the uninjected left eye serving as an internal control. Embryos were collected 1–2 days after injection of the inhibitor and assessed for neural crest migration and differentiation into cornea endothelium. Having established that QCPN positive neural crest cells occupy the periocular region and give rise to the cornea endothelium (Fig. 1 and Fig. 2), we analyzed their behavior in chick embryos using DAPI and N-cadherin staining. The results show that unlike the left control eye where neural crest remained in the periocular region at E4 (Fig. 4A,B; arrowhead), the right Sema3A-Ig injected eye had premature migration of neural crest between the epithelium and the lens vesicle (Fig. 4A,C; arrowheads, n=7). At E5, neural crest cells in the uninjected eye had migrated and formed the cornea endothelium, shown here as an N-cadherin-positive monolayer of cells between the epithelium and lens (Fig. 4D,D′). In contrast, the experimental eye had large masses of multilayered neural crest cells in the same region (Fig. 4E,E′, n=6). Furthermore, these cells did not form the N-cadherin-positive endothelial layer of the cornea; only a few cells stained weakly for N-cadherin (Fig. 4E′; arrowheads). In contrast to the Sema3A-Ig, eyes injected with the VEGF-Ig peptide did not show premature neural crest migration at E4 (Fig. 4F, F′, n=6) and E5 (Fig. 4G,G′, n=4) and resembled the uninjected controls (Fig. 4B,D, and data not shown). Thus, the lens serves as a source of Sema3A that regulates neural crest migration from the periocular region into the eye, through Npn-1. Premature neural crest migration due to disruption of Sema3A/Npn-1 signaling appears to result in abnormal differentiation, indicating that the spatiotemporal regulation of neural crest migration is necessary for proper formation of the cornea.
Fig. 4. Sema3A signaling by the lens is required for proper neural crest migration during eye development.
(A–C) Section through an E4 embryo showing localization of neural crest cells in: (B) the periocular region (arrowhead) of the uninjected control eye, and (C) premature migration between the lens and epithelium (arrowheads) in the presence of the Sema3A-Ig peptide inhibitor. (D–E′) section through E5 eyes: (D,D′) left uninjected eye showing controlled neural crest migration resulting in the formation of an N-cadherin positive monolayer of cells between the lens and epithelium, and (E,E′) injected eye, showing unguided migration of numerous neural crest cells from the periocular region and absence of the N-cadherin monolayer. (F–G) Sections through E4 and E5 eyes injected with the VEGF-Ig peptide inhibitor: (F,F′) E4 eye, and (G,G′) E5 eye, showing no effect on neural crest migration and differentiation. Asterisks indicate sight of peptide injection. nc, neural crest; ep, cornea epithelium; oc, optic cup; en, cornea endothelium; L, lens.
Sema3A signaling contributes to the prolonged pause of neural crest cells in the periocular region
During eye development in chick, neural crest cells pause in the periocular region for approximately 2 days before migrating between the ectoderm and lens to form the cornea (Hay and Revel, 1969; Hay, 1980; Lwigale et al., 2005). At this time, periocular neural crest remains interposed between the ectoderm and optic cup (Fig. 1A and Fig. S1B) with a small opening towards a Sema3A expressing lens vesicle (Fig. 2F). To address whether periocular neural crest migration is directly affected by Sema3A, we first investigated its migratory potential in the absence of other ocular tissues. Periocular neural crest mesenchyme was dissected from the temporal region of the eye (Fig. 5A) of E3 embryos that strongly expresses Npn-1 (Fig. 3A). Neural crest mesenchyme was sectioned into smaller pieces of equal sizes that were cultured in various conditions. To determine whether neural crest cells maintained their migratory potential while pausing in the periocular region and prior to invasion of the eye, mesenchyme was cultured on fibronectin-coated slides for 12–16 hours. We found that numerous cells migrated away from the explant (n=5, Fig. 5B) forming a halo around it. Higher magnification revealed that the cells migrated individually from the explant (Fig. 5C).
We next examined the response of cultured periocular neural crest to Sema3A. To this end, mesenchyme explants were cultured adjacent to Sema3A-Fc or as control, to IgG-Fc spots (Fig. 5A). In control cultures, we found that numerous periocular neural crest cells migrated onto the IgG-Fc spot (n=4, Fig. 5D and Fig. S2A). In contrast, when co-cultured adjacent to Sema3A-Fc, majority of the periocular neural crest cells avoided the spot and, instead, migrated along its border (n=4, Fig. 5E, arrowheads and Fig. S2B). The few individual cells that migrated onto the Sema3A-Fc spot, lost their outspread lamellipodia, and rounded up (Fig. 5E, arrows). Taken together, our results indicate that periocular neural crest are migratory and their avoidance of the rudimentary eye is cell autonomous and due to negative response to Sema3A.
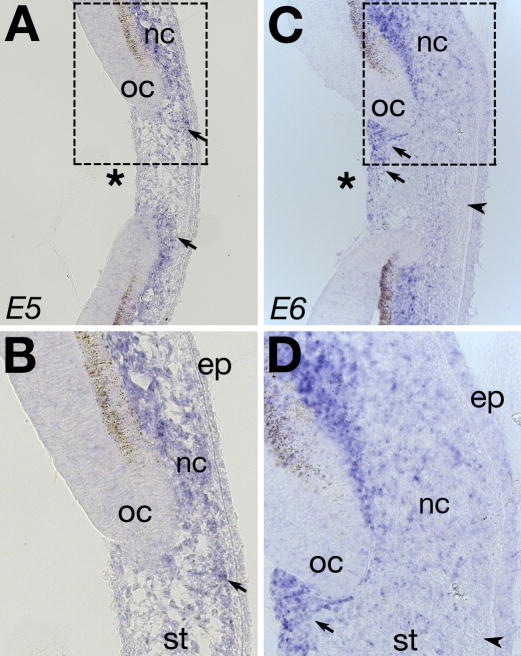
Ectopic neural crest cells in the cornea after lens ablation express Npn-1
Because lens ablation results in premature neural crest migration into the eye (Fig. 1), we investigated whether signals from the lens regulate Npn-1 expression by periocular neural crest. To this end, we performed in situ hybridization on cornea sections of E5 and E6 lens ablated eyes, encompassing a period of premature migration of numerous periocular neural crest cells into the eye. In the unoperated eyes at E5 and E6, all Npn-1 expressing mesenchyme is restricted to the periocular region (Fig. 3). In the absence of the lens, however, Npn-1 is expressed by neural crest cells within the malformed cornea, from which it is normally absent at E5, in addition to the periocular region (Fig. 6A and B). The level of Npn-1 expression varies in the cornea, with the strongest expression observed adjacent to the optic cup (Fig. 6A,B; arrows). By E6, there is strong expression of Npn-1 in the periocular region and throughout the cornea after lens ablation, particularly in the posterior region adjacent to the optic cup (Fig. 6C,D; arrows). Despite the influx of numerous Npn-1 expressing cells, some cells in the cornea remain Npn-1-negative and are located adjacent to the epithelium (Fig. 6C, arrowhead). Taken together, our results indicate that Npn-1 expression by periocular neural crest cells is independent of the lens. These results further suggest that the lens regulates periocular crest migration by inhibiting Npn-1 expressing cells from entering the eye.
Fig. 6. Npn-1 expression in lens ablated eyes.
Cross section through (A–B) E5, and (C–D) E6 lens ablated eyes showing ectopic expression of Npn-1 in the cornea after premature migration of periocular neural crest mesenchyme. Strong expression is seen in regions adjacent to the optic cup (arrows), and also in few individual cells with in the stroma. Asterisk indicate region of ablated lens. nc, neural crest; oc, optic cup; ep, cornea epithelium; st, cornea stroma.
Discussion
Proper development of the eye relies on coordinated interactions between the surface ectoderm, lens, optic cup, and periocular neural crest mesenchyme. Signals from the lens have been shown to be critical for early eye development (Coulombre and Coulombre, 1964; Genis-Galvez et al., 1966; Yamamoto and Jeffery, 2000; Beebe and Coats, 2000). In addition, the lens plays a major role during innervation of the cornea (Lwigale and Bronner-Fraser, 2007). Here, we show that ablation of the lens allows premature entry of periocular neural crest into the eye, which in turn leads to corneal malformation (Fig. 1). Earlier studies on eye development have revealed an inductive interaction between the lens and ectoderm that leads to the secretion of extracellular matrix by the presumptive cornea epithelium and acts as a scaffold for migratory periocular neural crest (Bard and Hay, 1975; Bard et al., 1975). Moreover, lens ablation or transposition in the eye results in malformation of the cornea (Genis-Galvez, 1966; Genis-Galvez et al., 1967; Zinn, 1970; Zak and Linsenmayer, 1985; Beebe and Coats, 2000), suggesting that signals from the lens epithelium are necessary for proper development of the cornea. In all these studies, the lens was considered to act as a physical barrier to periocular neural crest migration and later as a factor mediating induction of corneal differentiation. Recently, its been shown that TGFβ signaling by the lens is essential for proper cornea development and thus implicated as a chemoattractant to periocular neural crest (Saika et al., 2001). We show that in the absence of the lens, there is premature migration of numerous periocular neural crest cells into the eye (Fig. 1D″ and F′). Our results raise two possibilities: (1) that the lens acts as a physical barrier as earlier suggested; or (2) the lens secretes inhibitory signals that prevent periocular neural crest from migrating into the eye. Since the lens is still in close contact with the surface ectoderm at E4.5 when periocular neural crest begin to migrate into the eye (Fig. 1B), it is unlikely that it acts merely as a physical barrier. We therefore investigated the possibility that inhibitory signals from the lens regulate neural crest migration within the eye.
The lens vesicle expresses Sema3A at the time when neural crest cells accumulate in the periocular region (Chilton and Guthrie, 2003), making it a good candidate for an inhibitory source. Since migratory neural crest cells arrive in the periocular region as early as E2, we investigated the expression of Sema3A during this time, which corresponds to the period of lens induction. Our results show that Sema3A is expressed in the lens placode shortly after its induction and persists throughout formation of the lens vesicle (Fig. 2D–F). Interestingly, during this time, neural crest cells migrate into the periocular region but avoid the region adjacent to the lens placode and forming vesicle. Neural crest cells accumulate in the periocular region between E2-E4. Since Sema3A expression persists in the lens epithelium at the onset of periocular neural crest migration and during formation of the cornea (Fig. 2G–J), we investigated the expression of Npn-1 by the periocular neural crest.
In the cranial region, Npn-1 is expressed by migratory neural crest cells adjacent to the hindbrain (Eickholt et al., 1999). It therefore follows that perturbation of Sema3A/Npn-1 signaling affects the pattern of neural crest migration in this region resulting in malformation of cranial ganglia (Eickholt et al., 1999; McLennan and Kulesa, 2007; Osborn et al., 2005; Schwarz et al., 2008). Our analysis of Sema3A expression pattern shows that the periocular neural crest avoids the Sema3A-expressing lens placode as early as E2. However, Npn-1 is not expressed in this region until later (Eickholt et al., 1999; Chilton and Guthrie, 2003). Therefore it is more likely that the tight association between the optic vesicle and ectoderm acts as a physical barrier at this early stage. By E3 the lens vesicle induces the overlying ectoderm to secrete extracellular matrix (Toole and Trelstad, 1971; Meier and Hay, 1973; Bard et al., 1975; Coulombre and Coulombre, 1975) that is conducive to neural crest migration. However, neural crest cells remain in the periocular region at this time, likely because they express Npn-1 whereas the lens vesicle expresses Sema3A. An intriguing possibility is that Npn-1 expression is developmentally regulated during the time when periocular neural crest actively migrate into the rudimentary eye to form the cornea. In agreement with this, our results reveal an interesting pattern of Npn-1 expression that segregates periocular neural crest into two groups. Starting at about E4.5, a subpopulation of cells down-regulates Npn-1 and migrates into the eye, such that by E6 all neural crest forming the endothelium and stroma of the cornea are Npn-1 negative. Periocular neural crest adjacent to the optic cup remains Npn-1 positive and aggregates to form the anterior uvea. These results reveal a fundamental role of Npn-1, in response to Sema3A signaling, in regulating the fate of periocular neural crest by acting as a switch that segregates periocular neural crest into two populations.
The reciprocal expression of Sema3A in the lens and its Npn-1 ligand by periocular neural crest, coupled with the lens ablation results suggest that lens-derived Sema3A may inhibit periocular neural crest migration into the eye. We confirmed this requirement by blocking Sema3A signaling between the lens and Npn-1 expressing periocular neural crest cells using a peptide inhibitor (Williams et al., 2005; Lwigale and Bronner-Fraser, 2007). Our results show that in the presence of the Sema3A-Ig inhibitor, periocular neural crest cells prematurely migrate into the eye and as a result, fail to differentiate properly to form the cornea. These effects mimic the results of lens ablation. However, injection of a VEGF-Ig peptide inhibitor has no effect on periocular neural crest migration and differentiation. These observations suggest that Sema3A signaling by the lens plays a major role by inhibiting migration of periocular neural crest cells during the time that they express Npn-1. Thus continuous Sema3A signaling by the lens coupled with transient expression of Npn-1 by periocular neural crest cells account for their spatiotemporal behavior that is critical to proper eye development.
The developing eye is a complex mixture of tissues and cell types. Therefore neural crest may receive signals from the overlying surface ectoderm, optic vesicle, or the lens that affect their migratory behavior as they accumulate in the periocular region. Neural crest cells are highly migratory in culture, express Npn-1, and bind Sema3A (Eickholt et al., 1999). We have shown that periocular neural crest cells are highly migratory when isolated from other ocular tissues. In addition, we show that periocular neural crest cells migrate individually with outspread lammelipodia and either avoid or round up when they contact the Sema3A-Fc spot. Similar morphological changes were observed by electron microscopy and in vivo as mesenchymal cells migrate to form the cornea endothelium or stay in the periocular region (Bard and Hay, 1975; Bard et al., 1975). Thus, periocular neural crest migration is regulated by lens-derived Sema3A and is crucial for proper eye development.
Periocular neural crest cells do not express Npn-1 until about E3 (Chilton and Guthrie, 2003). Our results show that Npn-1 expression is transient in a subpopulation of periocular neural crest cells that migrate in close proximity to the lens. We analyzed Npn-1 expression by periocular neural crest in the absence of the lens to determine whether it plays a role in regulating Npn-1 expression. In the absence of the lens, both Npn-1-expressing and non-expressing cells migrate into the eye with no apparent effect on the levels of gene expression, suggesting that the lens has no effect on Npn-1 expression by periocular neural crest. However, absence of the lens’ Sema3A inhibitory signal permits Npn-1 positive periocular neural crest cells to migrate and ectopically occupy the malformed cornea.
The molecular mechanisms involved in regulating Npn-1 expression by neural crest or sensory neurons are not well understood. Because Npn-1 expression is transient in periocular neural crest, it is possible that signals from the optic cup are responsible for inducing and maintaining its expression in adjacent cells. Thus continuous proliferation of periocular neural crest coupled with ocular growth places some cells beyond the boundary of the signals from the optic cup, resulting in down-regulation of Npn-1 followed by migration into the eye. In the absence of the lens there is no selective migration of cells from the periocular region into the malformed cornea, but strong Npn-1 staining persists in the cells adjacent to the optic cup (Fig. 6).
In summary, our study reveals a novel role for Sema3A/Npn-1 signaling during eye development. The results show that the lens plays an essential role in regulating periocular neural crest migration, since its ablation results in premature entry into the eye. At a molecular level, this role is mediated by the lens’ expression of the guidance molecule Sema3A, which appears shortly after lens induction and is maintained throughout eye development. Conversely, Npn-1 is expressed by periocular neural crest, but down-regulated in a lens-independent fashion, by all cells that migrate into the presumptive eye to form the cornea. Inhibition of Sema3A/Npn-1 signaling between the lens and periocular neural crest cells mimics the effects of lens ablation. Consistent with this observation, periocular neural crest migration in vitro is inhibited by Sema3A-Fc. Taken together, these results show that Sema3A/Npn-1 signaling between the lens and periocular neural crest is crucial for the spatiotemporal behavior of neural crest cells during eye development and determines their fate in the anterior segment.
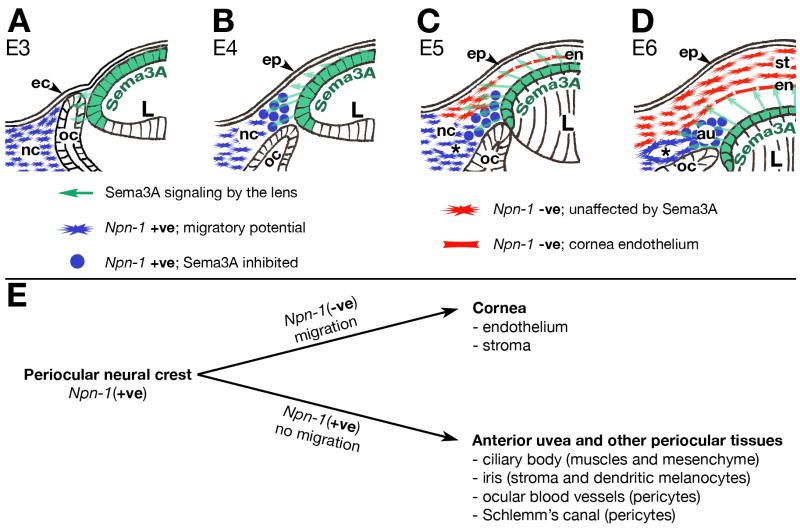
Based on our findings, we propose that Npn-1 expressing neural crest occupy the periocular region by E3, but are initially inhibited from migrating into the rudimentary eye by a physical barrier created by the optic cup and ectoderm (Fig. 7A). By E4, extracellular matrix secreted by the newly induced epithelium separates the two tissues allowing the Sema3A secreted by the lens vesicle to diffuse into the periocular region (Fig. 7B, arrows), where it inhibits Npn-1 expressing neural crest cells from migrating into the presumptive eye. Later, a subset of periocular neural crest cells down-regulate Npn-1, thus becoming unresponsive to the inhibitory Sema3A signal. They undergo a morphological change, migrate into the eye, and form the cornea endothelium at E5 (Fig. 7C), and stroma at E6 (Fig. 7D). In contrast, Npn-1 expressing neural crest cells respond to the secreted Sema3A, round up, and remain in the periocular region where they eventually contribute to the anterior uvea and ocular blood vessels. Therefore, Sema3A signaling from the lens segregates periocular neural crest into two lineages; Npn-1 negative cells that form the cornea from Npn-1-expressing cells that form the anterior uvea and other periocular tissues (Fig. 7E). Disruption of Sema3A/Npn-1 signaling results in ocular malformations, suggesting that this event plays an essential role in regulating the spatiotemporal behavior of neural crest in the periocular region that is critical to ocular development.
Fig. 7. Model of Sema3A/Npn-1 signaling regulating periocular neural crest migration during eye development.
(A) Migration of Npn-1(+ve) neural crest is inhibited by physical barrier between the ectoderm and optic cup. (B) Optic cup separates from the induced epithelium, but migration of Npn-1(+ve) neural crest is inhibited by Sema3A secreted by the lens. (C–D) Npn-1(−ve) neural crest are not inhibited by Sema3A, migrate between epithelium and lens to form the cornea endothelium and stroma. Npn-1(+ve) neural crest remain inhibited in the periocular region and form the anterior uvea and ocular blood vessels. (E) Sema3A/Npn-1 signaling segregates periocular neural crest into two lineages: Npn-1(−ve) cells that migrate and form the cornea, and Npn-1(+ve) cells that are non-migratory and form the anterior uvea and other periocular tissues.
Supplementary Material
(A) Sema3A is expressed early in the lens placode and maintained throughout the formation of the lens vesicle. HNK-1 is expressed by the surrounding periocular neural crest cells but down-regulated by E3. (B) QCPN staining showing tremendous neural crest contribution to the periocular mesenchyme. nc, periocular neural crest; ov, optic vesicle; oc, optic cup; Lp, lens placode; Lpt, lens pit; L, lens.
(A) Isolated periocular neural crest cells migrate on to a control IgG-Fc protein spot but (B), avoid the Sema3A-Fc protein spot.
Acknowledgments
The authors would like to thank Jonathan A. Raper for the full length Npn-1 cDNA and Anitha Rao for technical assistance. This work was supported in part by NIH grants, EY018050 (to PYL) and DE016459 (to MBF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey A, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bard J, Hay E. The behavior of fibroblasts from the developing avian cornea. Morphology and movement in situ and in vitro. J Cell Biol. 1975;67:400–418. doi: 10.1083/jcb.67.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard J, Hay E, Meller S. Formation of the endothelium of the avian cornea: a study of cell movement in vivo. Dev Biol. 1975;42:334–361. doi: 10.1016/0012-1606(75)90339-5. [DOI] [PubMed] [Google Scholar]
- Barr M, Byrne A, Duffy A, Condron C, Devocelle M, Harriott P, Bouchier-Hayes D, Harmey J. A peptide corresponding to the neuropilin-1-binding site on VEGF induces apoptosis of neuropilin-1-expressing breast tumour cells. British Journal of Cancer. 2005;92:328–333. doi: 10.1038/sj.bjc.6602308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D, Coats J. The Lens Organizes the Anterior Segment: Specification of Neural Crest Cell Differentiation in the Avian Eye. Dev Biol. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Chilton J, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev Dyn. 2003;228:726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Vincent C, Couly G. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol. 2005;49:161–71. doi: 10.1387/ijdb.041937sc. [DOI] [PubMed] [Google Scholar]
- Coulombre A, Coulombre J. Lens development. I Role of the lens in eye growth. J Exp Zool. 1964;156:39–47. doi: 10.1002/jez.1401560104. [DOI] [PubMed] [Google Scholar]
- Coulombre A, Coulombre J. Mechanisms of ocular development. International ophthalmology clinics. 1975;15:7–18. doi: 10.1097/00004397-197501510-00003. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm E. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt B, Mackenzie S, Graham A, Walsh F, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- Etchevers H, Vincent C, Le Douran N, Couly G. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and fore brain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Evans A, Gage P. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Human Molecular Genetics. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Levine E, Reh T. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Genis-Galvez J. Role of the lens in the morphogenesis of the iris and cornea. Nature. 1966;210:209–210. doi: 10.1038/210209a0. [DOI] [PubMed] [Google Scholar]
- Genis-Galvez J, Santos-Gutierrez L, Rios-Gonzalez A. Causal factors in Corneal Development: An experimental analysis in the chick embryo. Exptl Eye Res. 1967;6:48–56. doi: 10.1016/s0014-4835(67)80053-8. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. Dev Dyn. 1951;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hay E, Revel J. Fine structure of the developing cornea. Karger; Basel: 1969. [PubMed] [Google Scholar]
- Hay E. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hsieh Y, Zhang X, Lin E, Oliver G, Yang X. The homeobox gene Six3 is a potential regulator of anterior segment formation in the chick eye. Dev Biol. 2002;24:265–280. doi: 10.1006/dbio.2002.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre A. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29:27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells. 2006;11:919–933. doi: 10.1111/j.1365-2443.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. The Neural Crest. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- Lwigale P, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007;306:750–759. doi: 10.1016/j.ydbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Cressy PA, Bronner-Fraser M. Corneal keratocytes retain neural crest progenitor cell properties. Dev Biol. 2005;288:284–293. doi: 10.1016/j.ydbio.2005.09.046. [DOI] [PubMed] [Google Scholar]
- McLennan R, Kulesa P. In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick. Dev Biol. 2007;301:227–239. doi: 10.1016/j.ydbio.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Meier S, Hay E. Synthesis of sulfated glycosaminoglycans by embryonic corneal epithelium. Dev Biol. 1973;35:318–331. doi: 10.1016/0012-1606(73)90027-4. [DOI] [PubMed] [Google Scholar]
- Osborne N, Begbie J, Chilton J, Schmidt H, Eickholt B. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev Dyn. 2005;232:939–949. doi: 10.1002/dvdy.20258. [DOI] [PubMed] [Google Scholar]
- Saika S, Liu C, Azhar M, Sanford L, Doetschman T, Gendron R, Kao C, Kao W. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Schwarz Q, Vieira J, Howard B, Eickholt B, Ruhrberg C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Development. 2008;135:1605–1613. doi: 10.1242/dev.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B, Trelstad R. Hyaluronate production and removal during corneal development in the chick. Dev Biol. 1971;26:28. doi: 10.1016/0012-1606(71)90104-7. [DOI] [PubMed] [Google Scholar]
- Williams G, Eickholt BJ, Maison P, Prinjha R, Walsh FS, Doherty P. A complementary peptide approach applied to the design of novel Semaphorin/neuropilin antagonists. J Neurochem. 2005;92:1180–1190. doi: 10.1111/j.1471-4159.2004.02950.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Jeffery B. Probing teleost eye development by lens transplantation. Methods. 2002;28:420–426. doi: 10.1016/s1046-2023(02)00261-x. [DOI] [PubMed] [Google Scholar]
- Zak N, Linsenmayer T. Analysis of corneal development with monoclonal antibodies. I Differentiation in isolated corneas. Dev Biol. 1985;108:443–54. doi: 10.1016/0012-1606(85)90047-8. [DOI] [PubMed] [Google Scholar]
- Zinn K. Changes in cornea1 ultrastructure resulting from early lens removal in the developing chick embryo. Invest Ophthalmol. 1970;9:165–182. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Sema3A is expressed early in the lens placode and maintained throughout the formation of the lens vesicle. HNK-1 is expressed by the surrounding periocular neural crest cells but down-regulated by E3. (B) QCPN staining showing tremendous neural crest contribution to the periocular mesenchyme. nc, periocular neural crest; ov, optic vesicle; oc, optic cup; Lp, lens placode; Lpt, lens pit; L, lens.
(A) Isolated periocular neural crest cells migrate on to a control IgG-Fc protein spot but (B), avoid the Sema3A-Fc protein spot.