Abstract
Focal cerebral ischemia is associated with expression of both inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), enzymes whose reaction products contribute to the evolution of ischemic brain injury. We tested the hypothesis that, after cerebral ischemia, nitric oxide (NO) produced by iNOS enhances COX-2 activity, thereby increasing the toxic potential of this enzyme. Cerebral ischemia was produced by middle cerebral artery occlusion in rats or mice. Twenty-four hours after ischemia in rats, iNOS-immunoreactive neutrophils were observed in close proximity (<20 μm) to COX-2-positive cells at the periphery of the infarct. In the olfactory bulb, only COX-2 positive cells were observed. Cerebral ischemia increased the concentration of the COX-2 reaction product prostaglandin E2 (PGE2) in the ischemic area and in the ipsilateral olfactory bulb. The iNOS inhibitor aminoguanidine reduced PGE2 concentration in the infarct, where both iNOS and COX-2 were expressed, but not in the olfactory bulb, where only COX-2 was expressed. Postischemic PGE2 accumulation was reduced significantly in iNOS null mice compared with wild-type controls (C57BL/6 or SV129). The data provide evidence that NO produced by iNOS influences COX-2 activity after focal cerebral ischemia. Pro-inflammatory prostanoids and reactive oxygen species produced by COX-2 may be a previously unrecognized factor by which NO contributes to ischemic brain injury. The pathogenic effect of the interaction between NO, or a derived specie, and COX-2 is likely to play a role also in other brain diseases associated with inflammation.
Keywords: middle cerebral artery occlusion/aminoguanidine/NS-398/gene expression/inducible nitric oxide synthase null mice
Focal cerebral ischemia is associated with a local inflammatory response that participates in the progression of ischemic brain injury (e.g., refs. 1, 2, 3, and 4; for a review, see ref. 5). Recent evidence indicates that nitric oxide (NO), produced by the “immunologic” isoform of NO synthase (iNOS), is one of the mechanisms by which postischemic inflammation contributes to cerebral ischemic damage (see ref. 6 for a review). Occlusion of the rat middle cerebral artery (MCA) leads to iNOS induction in the ischemic brain (7). iNOS expression peaks 24–48 hr after ischemia and occurs in neutrophils infiltrating the ischemic brain and in cerebral vascular cells (7). Delayed administration of the relatively selective iNOS inhibitor aminoguanidine (AG) reduces the size of the infarct produced by MCA occlusion (8). Furthermore, iNOS null mice have smaller infarcts and a better neurological outcome after focal ischemia (9). These observations suggest that large amounts of NO produced by iNOS are toxic to the injured brain and contribute to the late stages of cerebral ischemia.
Cerebral ischemia also enhances the expression of the prostaglandin-synthesizing enzyme cyclooxygenase-2 (COX-2) (10, 11, 12, 13). Although the time course of the up-regulation is similar to that of iNOS, COX-2 is expressed predominantly in neurons located at the border between normal and ischemic brain (10). The COX-2 inhibitor NS-398 reduces the size of the infarct produced by MCA occlusion, suggesting that COX-2 activity is deleterious to the ischemic brain (10). Therefore, both COX-2 and iNOS are expressed after cerebral ischemia and contribute to ischemic brain injury. Recent data indicate that NO has a profound effect on COX-2 catalytic activity (see ref. 14 for a review). In several systems, NO increases COX-2 activity (15, 16, 17, 18). The finding that, in cerebral ischemia, COX-2 and iNOS are induced at the same time raises the possibility that NO produced by iNOS activates COX-2 in the ischemic brain. Therefore, COX-2-derived pro-inflammatory prostanoids and reactive oxygen species (14, 19) could contribute to the toxic effect of NO.
In this study, therefore, we used rodent models of focal cerebral ischemia to test the hypothesis that NO produced by iNOS enhances COX-2 activity in the ischemic brain. We found that, at the infarct’s periphery, iNOS-positive neutrophils are in close proximity to COX-2-positive neurons. The iNOS inhibitor AG attenuates postischemic accumulation of the COX-2 reaction product prostaglandin E2 (PGE2) only in regions of the ischemic hemisphere wherein both iNOS and COX-2 are expressed. Furthermore, PGE2 accumulation is reduced in iNOS null mice. These observations suggest that NO produced by iNOS influences COX-2 activity after cerebral ischemia. Toxic products of COX-2 activity may contribute to the deleterious effect of NO in the late stages of ischemic brain injury and in other brain diseases associated with inflammation as well.
METHODS
Animals.
Experiments were performed either in Sprague–Dawley rats (250–350 g; Harlan Breeders, Indianapolis) or in mice. Homozygous iNOS null mice were obtained from an in-house colony (9) developed from breeding pairs provided by C. Nathan and J. Mudgett (20). C57BL/6 and SV129 mice were obtained from The Jackson Laboratory or Taconic Farms. Mice were 8–10 weeks old at the time of study.
Focal Cerebral Ischemia in Rats.
The MCA was occluded transiently in rats by using an intravascular occlusion model (21) that has been described in detail (7). Under halothane anesthesia (induction, 5%; maintenance, 1%) a 3–0 nylon monofilament with a rounded tip was inserted centripetally into the external carotid artery and was advanced into the internal carotid until it reached the circle of Willis. Throughout the procedure body temperature was maintained at 37 ± 0.5°C by a thermostatically controlled lamp. Two hr after induction of ischemia, rats were re-anesthetized, and the filament was withdrawn (7). Animals then were returned to their cages and were monitored closely until recovery from anesthesia. In sham-operated rats, the external carotid artery was prepared surgically for insertion of the filament, but the filament was not inserted (7). Rats were killed at different time points after transient ischemia for immunocytochemistry or for measurement of PGE2 (see below).
Focal Cerebral Ischemia in Mice.
Mice were anesthetized with 2% halothane in 100% oxygen, and their rectal temperature was controlled. A 2 mm-hole was drilled in the inferior portion of the temporal bone to expose the left MCA. The MCA was cauterized distal to the origin of the lenticulostriate branches (9). Wounds were sutured, and mice were allowed to recover and returned to their cages. Rectal temperature was controlled until mice regained full consciousness. Mice were killed at different time points after MCA occlusion for determination of mRNA, for immunocytochemistry, or for measurement of PGE2 (see below).
Immunohistochemistry.
Immunocytochemical procedures were identical to those described (9, 10). At different time points (6, 12, 24, 48, and 96 hr after ischemia), rats (n = 3–6 per time point) were anesthetized (pentobarbital, 100 mg/kg i.p.) and were perfused through the heart with 4% paraformaldehyde. Brains were removed, postfixed, and embedded in paraffin. Coronal sections (4 μm) were cut by using a microtome and were mounted on microscope slides. After removing paraffin, sections were treated with 0.01% Triton-X and were incubated with 1.5% normal goat serum for 30 min. Adjacent sections were incubated overnight (4°C) with polyclonal antibodies to, respectively, COX-2 (Cayman Chemicals, Ann Arbor, MI; Oxford Biomedical Research, Oxford, MI) or iNOS (Upstate Biotechnology, Lake Placid, NY). After incubation with the appropriate secondary antibody and quenching with 0.3% H2O2, the immunocomplex was visualized by using diaminobenzidine as a chromogen in a peroxidase reaction (Kirkegaard & Perry Laboratories). In some experiments, the distribution of iNOS and COX-2 was studied in the same section by using a double staining technique. Sections first were processed for COX-2 immunocytochemistry by using diaminobenzidine as a chromogen. After treatment with 0.03 N HCl, sections were washed and processed for iNOS immunocytochemistry by using TrueBlue (Kirkegaard and Perry Laboratories) as a chromogen. Slides were viewed and photographed by using a Nikon Optiphot microscope.
PGE2 Enzyme Immunoassay.
Tissue concentration of PGE2, a COX reaction product, was determined by using an enzyme immunoassay kit (Cayman Chemicals) as described (10). The cerebral cortex and olfactory bulb ipsilateral and contralateral to the occluded MCA were removed 24 hr after ischemia in rat and 48 hr after ischemia in mice. Samples were homogenized in 0.05 M Tris⋅HCl (pH 7.4; 4–40 ml/g) and were extracted with 100% methanol according to the method of Powell (10). After centrifugation, the supernatant was diluted with acidified 0.1 M phosphate buffer (pH 4) and was applied to activated ODS-silica reverse phase columns (Sep-Pak C18, Waters). The columns were rinsed with 5 ml of distilled water followed by 5 ml of hexane, and PGE2 was eluted twice with 2 ml of ethyl acetate containing 1% methanol. The ethyl acetate fraction was evaporated and resuspended in 1 ml of buffer. Data on the recovery rate of this extraction procedure have been published (10). PGE2 concentration was determined spectrophotometrically in duplicate samples according to the instructions provided with the kit.
Reverse Transcription (RT)—PCR.
mRNA for COX-2 and iNOS was detected by the RT-PCR as described (22, 10). Mice were killed 12, 24, 48, and 96 hr after ischemia (n = 4–7 per time point), and their brains were removed. A 4-mm-thick coronal brain slice was cut at the level of the optic chiasm, and a sample including the infarcted cortex was collected and frozen in liquid nitrogen. The corresponding region of the contralateral cortex also was sampled. Total RNA was extracted from the tissue according to the method of Chomczynski and Sacchi (23). Aliquots of total RNA (0.25 μg) were used in the RT reaction mixed with 0.5 μg of oligo (dT) primer as directed (18-mer; New England Biolabs). First strand cDNA synthesis then was carried out by using M-MuLV reverse transcriptase (New England Biolabs) according to manufacturer’s instructions. After heating at 95°C for 10 min, 5 μl from each RT reaction mixture were used for PCR amplification. Primers (0.2 μM each) for the sequence of interest and for porphobilinogen deaminase, a ubiquitously expressed sequence, were used in a final volume of 50 μl. The COX-2 primers were as follows: forward, 5′-GCAAATCCTTGCTGTTCCAATC-3′; reverse: 5′-GGAGAAGGCTTCCCAGCTTTTG-3′, which results in a PCR product of 336 bp. The iNOS primers were as follows: forward, 5′-ACAACGTGAAGAAAACCCCTTGTG-3′; reverse, 5′-ACAGTTCCGAGCGTCAAAGACC-3′, which results in a PCR product of 557 bp (7). The porphobilinogen deaminase primers were as follows: forward, 5′-GCCACCACAGTCTCGGTCTGTATGCGAGC-3′; reverse, TGTCCCGGTAACGGCGGCGCGGCCACAAC-3′. The “hot start” method was used with the following cycle parameters: 94°C, 15 sec; 68°C, 30 sec; and 73°C, 20 sec for 5 cycles, then 94°C, 15 sec; 64°C, 30 sec; and 73°C, 20 sec for 35 cycles, and 73°C, 15 min. Reaction products then were separated on a 8% polyacrylamide gel, were stained with ethidium-bromide, and were photographed. Each set of PCR reactions included control samples run without RNA or in which the RT step was omitted to ensure that PCR products resulted from amplification from the COX-2 mRNA rather than genomic DNA. The optical density of the bands was determined by an gel image analysis system (Molecular Analyst, Bio-Rad). Measurements were normalized to the optical density of the porphobilinogen deaminase band used as an internal standard (10, 7).
Effect of NS-398 or Aminoguanidine on Postischemic PGE2 Elevation.
The MCA was occluded for 2 hr in rats as described above. In one group of rats (n = 7), the COX-2 inhibitor NS-398 (Cayman Chemical; 20 mg/kg; i.p.) was administered 6, 14, and 22 hr after induction of ischemia. NS-398 inhibits COX-2 with an IC50 that is >1,000 times smaller than that of COX-1 (24). This dose of NS-398 decreases PGE2 accumulation in the postischemic brain (10). In other rats, the iNOS inhibitor AG (Sigma) was administered i.p. at doses of 100 mg/kg (n = 6) or 200 mg/kg (n = 7) 6, 14, and 22 hr after ischemia. In this model, AG reduces calcium-independent, but not calcium-dependent, NOS activity in the postischemic brain, attesting to the selectivity of iNOS inhibition (8). In other rats (n = 8), vehicle (saline) was administered (1 ml; i.p.) 6, 14, and 22 hr after ischemia. Sham-operated rats (n = 7) served as controls. Animals were killed 2 hr after the last administration of NS-398, AG, or vehicle for PGE2 determination in brain and stomach.
Data Analysis.
Data in text and figures are expressed as mean ± SE. Two group comparisons were evaluated by the paired or unpaired t test, as appropriate. Multiple comparisons were analyzed by the ANOVA and Tukey’s test. Differences were considered statistically significant for P < 0.05.
RESULTS
Time Course and Spatial Distribution of COX-2 and iNOS Immunoreactivity in the Ischemic Brain.
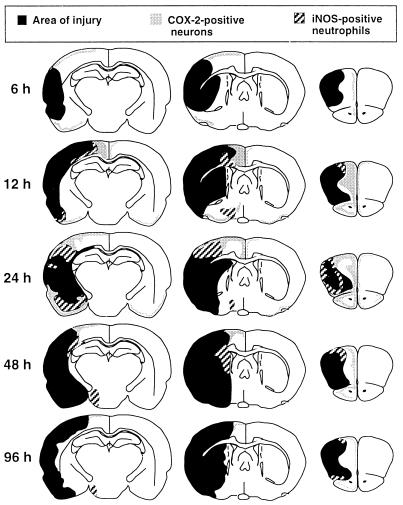
In these experiments, we used immunocytochemistry to determine the spatial and temporal features of COX-2 and iNOS expression in the postischemic brain. In sham-operated rats, few COX-2 immunoreactive neurons were observed in selected brain regions, including the cerebral cortex and olfactory bulb (cf. ref. 25). iNOS immunoreactivity was not observed in sham-operated rats. The time course of COX-2 and iNOS expression after transient MCA occlusion is presented in Fig. 1. COX-2 immunoreactivity was observed starting 6 hr after ischemia (Fig. 1). At this time, COX-2-positive neurons were located in the superficial layers of the cerebral cortex adjacent to the ischemic area and in the olfactory bulb (Fig. 1). iNOS immunoreactivity was not present in the brain at this time. Twelve hours after ischemia, both COX-2-immunoreactive neurons and iNOS-positive neutrophils were observed at the edges of the ischemic lesion (Fig. 1). COX-2 and iNOS immunoreactivity reached the greatest expression at 24 hr and began to decline at 48 hr. Little COX-2 and iNOS immunoreactivity was present 96 hr after ischemia.
Figure 1.
Time course of the expression of iNOS and COX-2 immunoreactivity in rats after transient occlusion of the MCA. Changes in infarct area over time—e.g., 48 vs. 96 hr—reflect interanimal variability in the size of the infarct. COX-2-positive neurons were first observed 6 hr after MCA occlusion in the superficial layers of the cerebral cortex medial and inferior to the ischemic area and in the olfactory bulb. iNOS immunoreactivity was not present in the brain at this time. Twelve hours after ischemia, both COX-2 immunoreactive neurons and iNOS-positive neutrophils were observed at the medial and inferior edges of the ischemic lesion. COX-2 and iNOS immunoreactivity reached the greatest expression at 24 hr and began to decline 48 hr after ischemia.
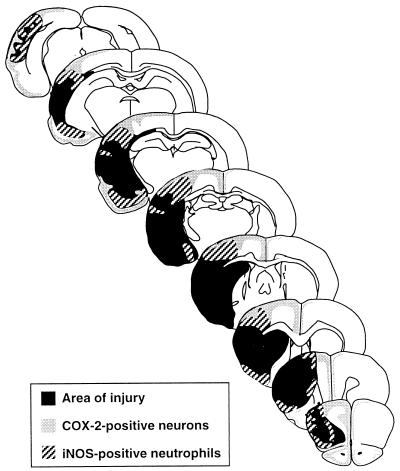
The spatial distribution of the immunoreactivity throughout the rostro-caudal extent of the infarct 24 hr after ischemia is presented in Fig. 2. COX-2 immunoreactivity was observed at the periphery of the infarct, wherein it was seen in shrunken cells with the morphological characteristics of ischemic neurons (Figs. 2, 3). In this region, iNOS-positive neutrophils were intermingled with COX-2-positive neurons (Fig. 3). The proximity between COX-2-immunoreactive neurons and iNOS positive neutrophils was well appreciated in sections double-labeled for COX-2 and iNOS (Fig. 3). In double-labeled sections (n = 12 from 4 rats), the average distance between iNOS-positive neutrophils (n = 100) and the nearest COX-2-positive soma was 16 ± 1 μm. In addition, 10 ± 1 COX-2-positive cell bodies were located within a 50-μm radius from an iNOS-positive neutrophil (n = 100). iNOS-positive neutrophils were also present deeper into the infarct, where COX-2 positive neurons were not observed. Conversely, COX-2 immunoreactivity was seen in regions remote from the ischemic lesion, e.g., cingulate and entorhinal cortices and olfactory bulb, in which iNOS immunoreactivity was not observed (Figs. 2, 3). Therefore, at the time of maximal expression, COX-2-positive neurons reside in close proximity to iNOS-immunoreactive neutrophils at the periphery of the infarct. In areas remote from the ischemic lesion, such as the olfactory bulb and cingulate cortex, only COX-2 neurons are present.
Figure 2.
Spatial distribution of iNOS and COX-2 immunoreactivity throughout the rostro-caudal extent of the ischemic lesion, 24 hr after MCA occlusion. At the periphery of the infarct, iNOS-positive neutrophils overlap with COX-2-positive neurons. In cingulate cortex and olfactory bulb, only COX-2 positive neurons are observed.
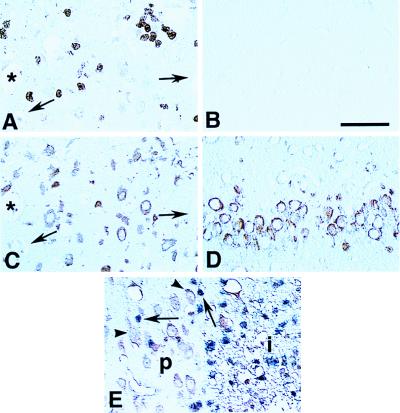
Figure 3.
Localization of COX-2-positive neurons and iNOS-positive neutrophils in the postischemic brain 24 hr after cerebral ischemia in paraffin-embedded sections. iNOS-immunoreactive neutrophils are observed at the periphery of the ischemic lesion in neocortex (A) but not in the olfactory bulb (B). In adjacent sections processed for COX-2 immunocytochemistry, COX-2 neurons are observed in the same regions of the ischemic lesion (C). Asterisk and arrows indicate blood vessels used as landmarks. In the olfactory bulb, only COX-2-immunoreactive neurons are observed (D). In double-labeled sections for iNOS (blue chromogen) and COX-2 (brown chromogen), the close spatial proximity between COX-2 positive neurons (arrow heads) and iNOS positive neutrophils (arrows) can be appreciated. p, periphery of the ischemic lesion; i, ischemic lesion. (Bar = 50 μm.)
Effect of COX-2 or iNOS Inhibition on PGE2 Elevation in the Ischemic Brain.
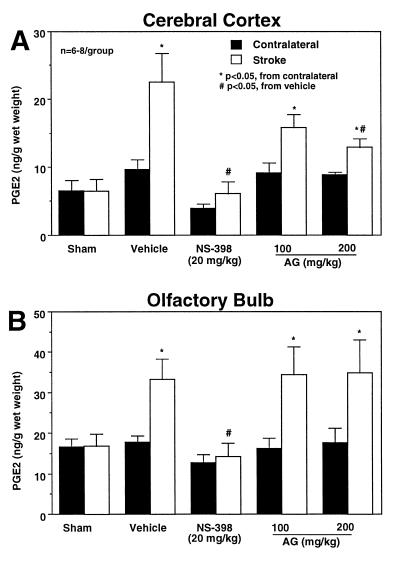
In these experiments, we studied the effect of COX-2 and iNOS inhibition on the COX-2 reaction product PGE2 24 hr after cerebral ischemia. In vehicle-treated rats (n = 8), PGE2 concentration increased in the ischemic cortex (P < 0.05; paired t test) and olfactory bulb (P < 0.05) (Fig. 4). In both regions, treatment with the relatively selective COX-2 inhibitor NS-398 (20 mg/kg) attenuated significantly the elevation in PGE2 induced by ischemia (Fig. 4) (P < 0.05 from vehicle, ANOVA and Tukey’s test; n = 7). NS-398 tended to reduce resting PGE2 concentration in the brain contralateral to the occluded MCA (Fig. 4). However, the effect did not reach statistical significance (P > 0.05). The iNOS inhibitor AG (100–200 mg/kg) attenuated the elevation in PGE2 in cerebral cortex (−43% at 200 mg/kg; P < 0.05 from vehicle; n = 7), but not in the olfactory bulb (P > 0.05 from vehicle) (Fig. 4). AG (200 mg/kg) did not attenuate PGE2 concentration in the stomach (sham-operated: 67.5 ± 5.5; MCA occlusion-vehicle: 67.5 ± 5.3; MCA occlusion-AG: 66.9 ± 5.0 ng/g wet weight).
Figure 4.
Effect of the COX-2 inhibitor NS-398 or the iNOS inhibitor AG on the elevation in PGE2 after cerebral ischemia. PGE2 concentration was measured in the ischemic hemisphere and contralaterally 24 hr after MCA occlusion in rats. In vehicle-treated rats, focal ischemia increases PGE2 concentration in both the cerebral cortex (A) and the olfactory bulb (B) (P < 0.05, paired t test). The COX-2 inhibitor NS-398 attenuates the elevation in PGE2 both in the cerebral cortex (A) and in the olfactory bulb (B) (P < 0.05 from vehicle stroke size; ANOVA and Tukey’s test). The iNOS inhibitor AG attenuates PGE2 accumulation in the cerebral cortex (A) but not in the olfactory bulb (B).
PGE2 Concentration in iNOS Null Mice After MCA Occlusion.
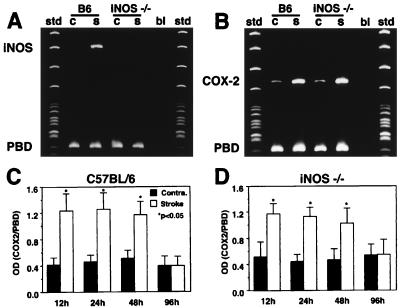
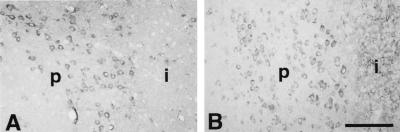
We used mice with deletion of the iNOS gene to provide additional evidence for an effect of iNOS-derived NO on COX-2 activity. First, we sought to determine whether COX-2 message and protein are expressed in iNOS null mice. Baseline COX-2 mRNA expression was present in the contralateral nonischemic brain both in C57BL/6 and iNOS null mice (Fig. 5)(cf. ref. 10). Although null mice did not express iNOS mRNA in the ischemic brain, the COX-2 mRNA up-regulation did not differ in magnitude and time-course from that of C57BL/6 (Fig. 5). Similarly, COX-2 immunoreactivity in the postischemic brain was comparable between iNOS null mice and wild-type controls (Fig. 6). Therefore, COX-2 expression is not impaired in iNOS null mice.
Figure 5.
Expression of iNOS and COX-2 mRNA in iNOS null mice and C57BL/6 controls after occlusion of the MCA. iNOS and COX-2 mRNA was determined in the ischemic brain by RT-PCR. Porphobilinogen deaminase mRNA, a ubiquitously expressed sequence, was coamplified and used as internal standard. MCA occlusion induces iNOS mRNA in the ischemic brain in C57BL/6 mice but not in iNOS null mice (A). MCA occlusion up-regulates COX-2 mRNA expression in the ischemic brain of iNOS null mice and C57BL/6 mice (B). The time course of COX-2 up-regulation is comparable in C57BL/6 mice (C) and iNOS null mice (D). B6, C57BL/6; iNOS −/−, iNOS null mice; OD, optical density; bl, blank (sample without the reverse transcriptase step); c, contralateral; s, stroke; std, standards; PBD, porphobilinogen deaminase.
Figure 6.
Expression of COX-2 immunoreactivity after middle cerebral artery occlusion in C57BL/6 mice (A) and in iNOS null mice (B). COX-2-positive cells with the morphological characteristics of neurons are observed at the periphery of the infarct. p, periphery of the infarct; i, infarct. (Bar = 100 μm.)
We then studied PGE2 concentration in iNOS null mice (n = 12) 48 hr after MCA occlusion. Both SV129 (n = 12) and C57BL/6 (n = 12) mice were used as wild-type controls. In the hemisphere contralateral to the stroke, PGE2 concentration did not differ between iNOS null mice (11 ± 1 ng/g wet weight) and wild-type controls (SV129: 14 ± 1; C57BL/6: 12 ± 1; P > 0.05). However, in the ischemic hemisphere, the increase in PGE2 was attenuated by 40–50% in the null mice (15 ± 2) compared with C57BL/6 (22 ± 3) or SV129 mice (23 ± 3) (P < 0.05 from iNOS nulls).
DISCUSSION
We sought to test the hypothesis that NO produced by iNOS influences COX-2 function in the postischemic brain. The rationale for this hypothesis is that, in other systems, NO produced by iNOS activates COX-2 and increases the cytotoxic effects of this enzyme (e.g., ref. 17). NO is thought to be highly diffusible but short-lived (26). Consequently, in order for NO, or related chemical species, to interact with COX-2, iNOS and COX-2 must be present in the brain at the same time and in close spatial proximity. We, therefore, investigated the time-course and spatial distribution of COX-2 and iNOS expression in the postischemic brain. We found that COX-2 and iNOS immunoreactivity is expressed during the same time period. Furthermore, we found that both COX-2-positive neurons and iNOS-positive neutrophils are located at the border of the ischemic territory and in close proximity. The distance between iNOS-positive neutrophils and COX-2-positive neurons is well within the diffusional capacity of NO (26). In regions remote from the infarct—e.g., the olfactory bulb—only COX-2 immunoreactive neurons were observed. The close spatial and temporal association between iNOS and COX-2 expression supports the possibility of an interaction between NO and COX-2 in regions of the ischemic territory at risk for infarction.
To provide functional evidence of an interaction between NO produced by iNOS and COX-2, we studied the effect of iNOS inhibition on the production of PGE2. We anticipated that, if NO stimulates COX-2 activity, iNOS inhibition would reduce the production of PGE2, a COX-2 reaction product. Such inhibition would be expected to occur only at the infarct border, wherein both iNOS and COX-2 are expressed, and not in the olfactory bulb, where only COX-2 is up-regulated. Consistent with this prediction, it was found that the iNOS inhibitor AG attenuates PGE2 accumulation in the infarcted cortex but not in the olfactory bulb.
The reduction in PGE2 production by AG cannot be attributed to direct effects of this inhibitor on COX-2 activity or expression, because AG treatment does not affect the elevation in the COX-2 reaction product PGE2 in the olfactory bulb after ischemia. Furthermore, AG does not affect activity of purified COX-2 in vitro (27). It could be argued that the inhibition of PGE2 production in the infarct but not in the olfactory bulb is a consequence of blood–brain barrier disruption in the region of the infarct, allowing the penetration of AG only in the lesioned brain. However, the observation that AG does not inhibit PGE2 production in the stomach, an organ that is readily permeable to AG (28), indicates that poor penetration of AG does not contribute to its lack of effectiveness in the olfactory bulb. The effect of AG on PGE2 cannot be a consequence of the reduction in infarct size associated with AG treatment (7), because rats were killed 24 hr after MCA occlusion. The reduction in infarct size produced by early treatment with AG develops slowly and is not present 24 hr after ischemia (F.Z. and C.I., unpublished observations). In addition, the effect of AG on PGE2 cannot be attributed to inhibition of COX-1, because: (i) Cerebral ischemia does not up-regulate COX-1 in this model (10); and (ii) the postischemic increase in PGE2 is blocked by the highly selective COX-2 inhibitor NS-398, indicating that COX-2, and not COX-1, is responsible for PGE2 elevation. Therefore, the most likely explanation for the effect of AG on PGE2 production is that AG inhibits iNOS and that the associated reduction in NO production decreases COX-2 activity. Consistent with this idea, AG is effective only in regions in which COX-2 and iNOS are expressed in close proximity.
To obtain additional evidence for an interaction between iNOS and COX-2, mice with deletion of the iNOS gene were used. These mice do not express iNOS after focal cerebral ischemia and have a reduced susceptibility to cerebral ischemic damage (9). We reasoned that, if NO produced by iNOS influences COX-2 catalytic activity, then postischemic PGE2 accumulation should be reduced in iNOS null mice. First, we demonstrated that COX-2 mRNA and protein expression is comparable in iNOS null mice and wild-type controls. Then, PGE2 concentration in the ischemic hemisphere was measured. We found that the PGE2 concentration was reduced in the ischemic hemisphere of iNOS null mice when compared with either SV129 or C57BL/6 wild-type controls. Of interest, PGE2 concentration was not affected in the nonischemic hemisphere, providing further evidence that baseline COX activity is comparable in the brain of null mice and wild-type controls. One drawback of using null mice relates to the fact that their genetic background is mixed, including both the SV129 strain, from which the embryonic stem cells are derived, and the C57BL/6 strain, which provides blastocysts in which the stem cells are injected (29). The observation that the PGE2 up-regulation in iNOS-deficient mice is reduced in comparison to both parental strains (C57BL/6 and SV129) minimizes concerns that this finding results from strain-related genetic differences rather than from deletion of the gene of interest (29). Two additional observations support this conclusion. First, permanent MCA occlusion in C57BL/6 or SV129 mice produces infarcts that are identical in size (9). Therefore, the reduction in PGE2 production in iNOS null mice cannot be attributed to differences in the expression of ischemic brain damage between C57BL/6 and SV129. Second, postischemic COX-2 mRNA and protein expression in null mice is not different from that of C57BL/6. Therefore, reduced COX-2 expression in iNOS nulls is unlikely to contribute to the findings. The data in iNOS null mice, in concert with the data with AG, provide strong evidence that NO produced by iNOS enhances the catalytic activity of COX-2 in the postischemic brain.
Although there is extensive evidence that NO increases COX-2 activity in several systems (15, 16, 17, 18), the mechanisms of this effect have not been elucidated. One possibility is that NO, or a related chemical specie, binds to the heme group of COX-2 and activates the enzyme in a manner analogous to the NO-mediated activation of soluble guanylyl cyclase (17). However, this possibility has been questioned because the affinity of NO for ferrous heme, the most common heme form in COX-2, is low (30). Another possibility is that NO increases COX-2 half-life by generating free radicals and inhibiting COX-2 autoinactivation (18, 31). A third possibility is that lipid peroxidation initiated by peroxynitrite, the product of the reaction of NO with superoxide, liberates arachidonic acid from the cell membrane, which in turn activates COX-2 (32). Whatever the mechanisms of the interaction, the present study provides evidence that iNOS-derived NO modulates COX-2 enzymatic output in brain. To our knowledge, this may be the first demonstration of such interaction in the central nervous system in vivo. Further studies will be required to understand fully the mechanisms of the apparent activation of COX-2 by NO.
It is well established that relatively high concentrations of NO, such as those produced by iNOS, are neurotoxic (33, 34, 35). The toxicity of NO has been attributed to multiple mechanisms, including DNA damage, peroxynitrite-mediated oxidative damage, and energy failure (see ref. 6 for a review). The results of the present study suggest that, in the postischemic brain, NO also can exert its toxic effects by influencing COX-2 activity. COX-2, a rate-limiting enzyme for prostanoid synthesis, also generates reactive oxygen species (14, 19). The resulting oxidative damage is thought to be the main mechanism of the toxicity associated with COX-2 up-regulation during inflammation (36). Therefore, after cerebral ischemia, NO produced by iNOS may activate COX-2 and increase the production of reactive oxygen species, thereby contributing to the toxic effects of NO. NO-mediated COX-2 activation may, therefore, be a previously unrecognized factor in the evolution of ischemic brain damage. In addition, the interaction between NO and COX-2 is likely to play a role also in other brain diseases associated with inflammation, such as AIDS dementia, multiple sclerosis, brain neoplasms and Alzheimer disease (37, 38, 39, 40).
In conclusion, we have investigated the interaction between iNOS and COX-2 in the postischemic brain. After cerebral ischemia, iNOS and COX-2 are expressed at the same time and in close proximity in regions at risk for infarction. Pharmacological inhibition of iNOS attenuates accumulation of PGE2 in the ischemic brain whereas mice with deletion of the iNOS gene have reduced postischemic levels of PGE2. The data is consistent with the hypothesis that, in the ischemic brain as in other organs during inflammation, NO produced by iNOS activates COX-2 and increases production of reactive oxygen species and toxic prostanoids. NO-mediated COX-2 activation may be a heretofore unrecognized mechanism by which NO exerts its deleterious effect in the late stages of ischemic brain injury and in other brain diseases associated with inflammation as well.
Acknowledgments
We thank Ms. Karen MacEwan for the excellent editorial assistance. This work was supported by National Institute of Health Grants NS34179 and NS35806.
ABBREVIATIONS
- AG
aminoguanidine
- COX-2
cyclooxygenase-2
- iNOS
inducible nitric oxide synthase
- MCA
middle cerebral artery
- NO
nitric oxide
- PGE2
prostaglandin E2, RT, reverse transcription
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Pozzilli C, Lenzi G L, Argentino C, Carolei A, Rasura M, Signore A, Bozzao L, Pozzilli P. Stroke. 1985;16:251–255. doi: 10.1161/01.str.16.2.251. [DOI] [PubMed] [Google Scholar]
- 2.Clark W M, Madden K P, Rothlein R, Zivin J A. Stroke. 1991;22:877–883. doi: 10.1161/01.str.22.7.877. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Chopp M, Bodzin G. Neurosci Res Commun. 1992;11:93–99. [Google Scholar]
- 4.Chen H, Chopp M, Zhang R L, Bodzin G, Chen Q, Rusche J R, Todd R F., 3rd Ann Neurol. 1994;35:458–463. doi: 10.1002/ana.410350414. [DOI] [PubMed] [Google Scholar]
- 5.Feuerstein G Z, Wang X, Barone F C. In: Cerebrovascular Diseases. Ginsberg M D, Bogousslavsky J, editors. Cambridge, MA: Blackwell Science; 1998. pp. 507–531. [Google Scholar]
- 6.Iadecola C. Trends Neurosci. 1997;20:132–138. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Zhang F, Casey R, Clark H B, Ross M E. Stroke. 1996;27:1373–1380. doi: 10.1161/01.str.27.8.1373. [DOI] [PubMed] [Google Scholar]
- 8.Iadecola C, Zhang F, Xu X. Am J Physiol. 1995;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C, Zhang F, Casey R, Nagayama M, Ross M E. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogawa S, Zhang F, Ross M E, Iadecola C. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miettinen S, Fusco F R, Yrjanheikki J, Keinanen R, Hirvonen T, Roivainen R, Narhi M, Hokfelt T, Koistinaho J. Proc Natl Acad Sci USA. 1997;94:6500–6505. doi: 10.1073/pnas.94.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planas A M, Soriano M A, Rodriguez-Farre E, Ferrer I. Neurosci Lett. 1995;200:187–190. doi: 10.1016/0304-3940(95)12108-g. [DOI] [PubMed] [Google Scholar]
- 13.Collaco-Moraes Y, Aspey B, Harrison M, de Belleroche J. J Cereb Blood Flow Metab. 1996;16:1366–1372. doi: 10.1097/00004647-199611000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Wu K K. Adv Pharmacol. 1995;33:179–207. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]
- 15.Mollace V, Muscoli C, Rotiroti D, Nistico G. Biochem Biophys Res Commun. 1997;238:916–919. doi: 10.1006/bbrc.1997.7155. [DOI] [PubMed] [Google Scholar]
- 16.Kanematsu M, Ikeda K, Yamada Y. J Bone Miner Res. 1997;12:1789–1796. doi: 10.1359/jbmr.1997.12.11.1789. [DOI] [PubMed] [Google Scholar]
- 17.Salvemini D, Misko T P, Masferrer J L, Seibert K, Currie M G, Needleman P. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvemini D, Settle S L, Masferrer J L, Seibert K, Currie M G, Needleman P. Br J Pharmacol. 1995;114:1171–1178. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontos H A, Wei E P, Povlishock J T, Dietrich W D, Magiera C J, Ellis E F. Science. 1980;209:1242–1245. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- 20.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, et al. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 21.Longa E Z, Weinstein P R, Carlson S, Cummins R. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Ross M E, Iadecola C. In: Methods in Enzymology. Packer L, editor. Orlando, FL: Academic; 1996. pp. 408–426. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Reitz D B, Li J J, Norton M B, Reinhard E J, Collins J T, Anderson G D, Gregory S A, Koboldt C M, Perkins W E, Seibert K, et al. J Med Chem. 1994;37:3878–3881. doi: 10.1021/jm00049a005. [DOI] [PubMed] [Google Scholar]
- 25.Breder C D, Dewitt D, Kraig R P. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood J, Garthwaite J. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 27.Zingarelli B, Southan G J, Gilad E, O’Connor M, Salzman A L, Szabo C. Br J Pharmacol. 1997;120:357–366. doi: 10.1038/sj.bjp.0700892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaven M A, Gordon J W, Jacobsen S, Severs W B. J Pharmacol Exp Therap. 1969;165:14–22. [PubMed] [Google Scholar]
- 29.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 30.Tsai A L, Wei C, Kulmacz R J. Arch Biochem Biophys. 1994;313:367–372. doi: 10.1006/abbi.1994.1400. [DOI] [PubMed] [Google Scholar]
- 31.Egan R W, Paxton J, Kuehl F J. J Biol Chem. 1976;251:7329–7335. [PubMed] [Google Scholar]
- 32.Davidge S T, Baker P N, Laughlin M K, Roberts J M. Circ Res. 1995;77:274–283. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- 33.Chao C C, Hu S, Molitor T W, Shaskan E G, Peterson P K. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 34.Hewett S J, Csernansky C A, Choi D W. Neuron. 1994;13:487–494. doi: 10.1016/0896-6273(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 35.Dawson V L, Brahmbhatt H P, Mong J A, Dawson T M. Neuropharmacology. 1994;33:1425–1430. doi: 10.1016/0028-3908(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 36.Seibert, K., Masferrer, J., Zhang, Y., Gregory, S., Olson, G., Hauser, S., Leahy, K., Perkins, W. & Isakson, P. (1995) Agents Actions 46, Suppl., 41–50. [DOI] [PubMed]
- 37.Bo L, Dawson T M, Wesselingh S, Mork S, Choi S, Kong P A, Hanley D, Trapp B D. Ann Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- 38.Vodovotz Y, Lucia M S, Flanders K C, Chesler L, Xie Q W, Smith T W, Weidner J, Mumford R, Webber R, Nathan C, et al. J Exp Med. 1996;184:1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J C, Christov V I, Dawson T M, Dawson V L. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 40.Cobbs C S, Brenman J E, Aldape K D, Bredt D S, Israel M A. Cancer Res. 1995;55:727–730. [PubMed] [Google Scholar]