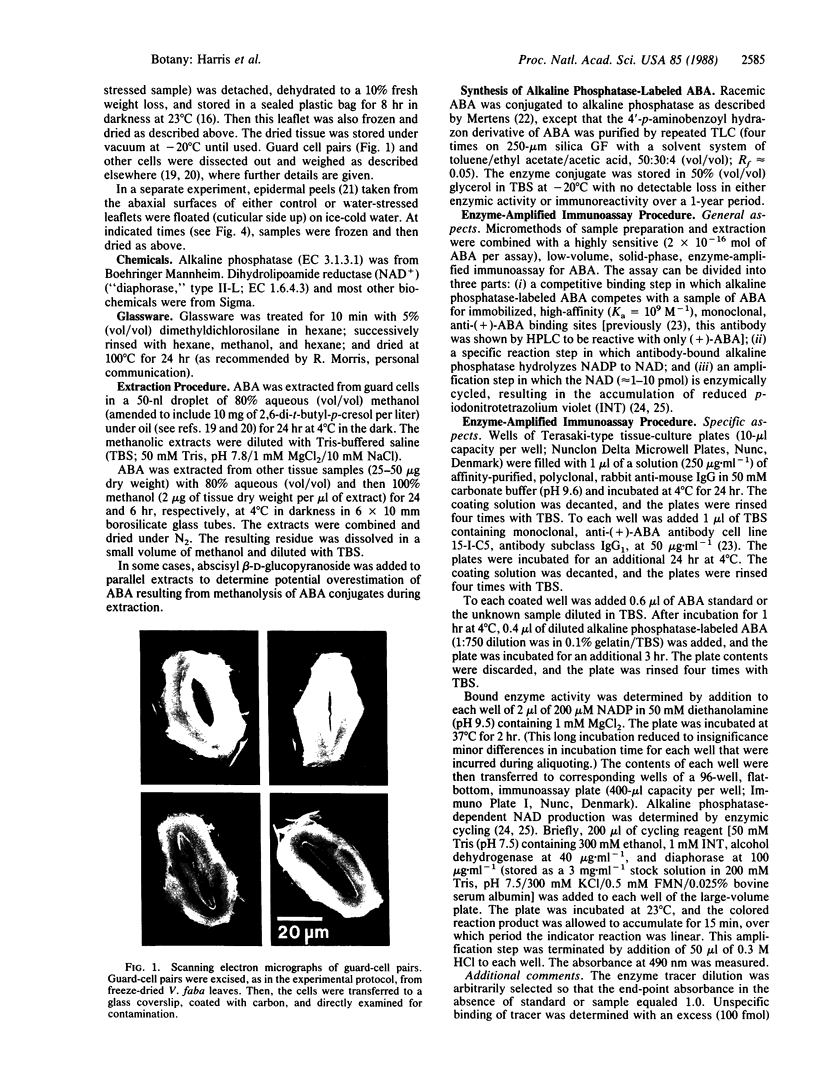
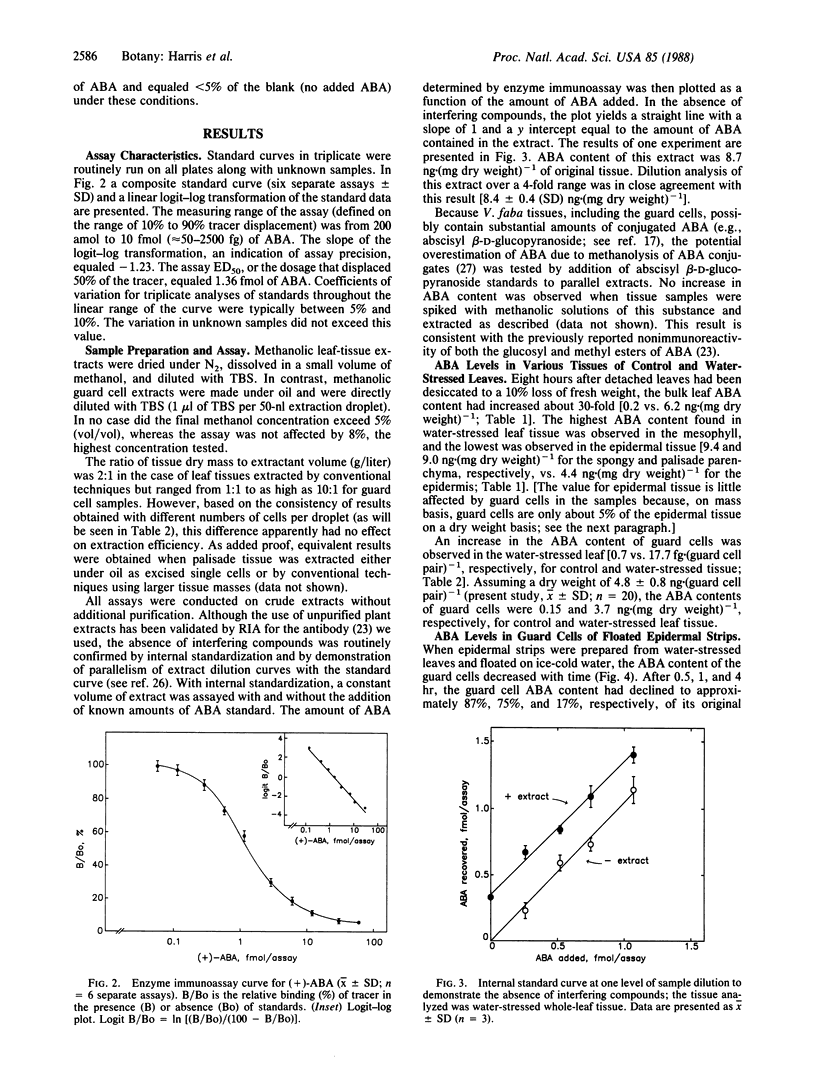
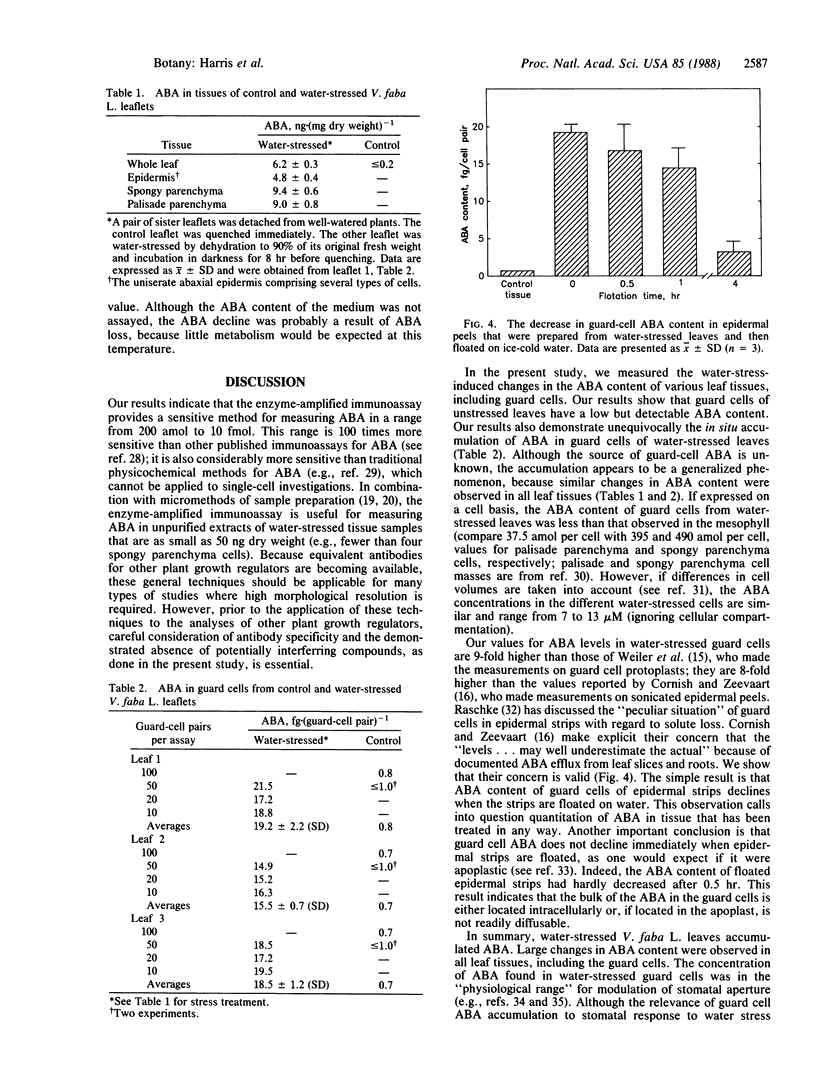
Abstract
A highly sensitive, solid-phase, enzyme-amplified immunoassay for the plant growth regulator (+)-abscisic acid (ABA) was developed. The assay sensitivity (0.2-10 fmol) was sufficient for analyzing free ABA in homogeneous tissue samples dissected from Vicia faba L. leaves. Eight hours after detached leaves had been desiccated to 10% loss of fresh weight, the bulk leaf ABA content increased from ≤0.2 to 6.2 ng·(mg dry weight)-1. Epidermal tissue, spongy parenchyma cells, and palisade parenchyma cells from this water-stressed leaf had the following ABA contents, respectively: 4.8, 9.4, and 9.0 ng·(mg dry weight)-1. Guard cells, which respond to exogenous ABA by losing solutes and volume, were also assayed. When they were dissected from control (fully hydrated) leaves, their ABA content was ≈0.7 fg·(cell pair)-1 [[unk]0.2 ng·(mg dry weight)-1]. In contrast, the ABA content of guard cells of water-stressed leaves was ≈17.7 fg·(cell pair)-1. These results indicate that ABA accumulation in a highly stressed V. faba leaflet is generalized; guard cells contain only 0.15% of bulk leaf ABA. The time course for loss of ABA from guard cells of a floating epidermal peel was studied. There was little loss within 30 min, but after 4 hr, the ABA content was only 17% of the original value. These results indicate that the bulk of guard cell ABA is not readily diffusible (i.e., probably not apoplastic). The results also indicate that common laboratory procedures results in lowered guard cell ABA content.
Keywords: stomata, gas exchange, plant growth regulator, transpiration, photosynthesis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardsell M. F., Cohen D. Relationships between Leaf Water Status, Abscisic Acid Levels, and Stomatal Resistance in Maize and Sorghum. Plant Physiol. 1975 Aug;56(2):207–212. doi: 10.1104/pp.56.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Accumulation by in Situ and Isolated Guard Cells of Pisum sativum L. and Vicia faba L. in Relation to Water Stress. Plant Physiol. 1986 Aug;81(4):1017–1021. doi: 10.1104/pp.81.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantz D. A., Ho T. H., Uknes S. J., Cheeseman J. M., Boyer J. S. Metabolism of Abscisic Acid in Guard Cells of Vicia faba L. and Commelina communis L. Plant Physiol. 1985 May;78(1):51–56. doi: 10.1104/pp.78.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson A., Ellis D. H., Bates D. L., Plumb A. M., Stanley C. J. Enzyme amplification for immunoassays. Detection limit of one hundredth of an attomole. J Immunol Methods. 1986 Feb 27;87(1):7–11. doi: 10.1016/0022-1759(86)90337-6. [DOI] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self C. H. Enzyme amplification--a general method applied to provide an immunoassisted assay for placental alkaline phosphatase. J Immunol Methods. 1985 Feb 11;76(2):389–393. doi: 10.1016/0022-1759(85)90316-3. [DOI] [PubMed] [Google Scholar]