Abstract
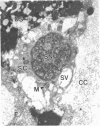
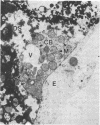
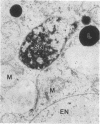
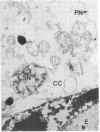
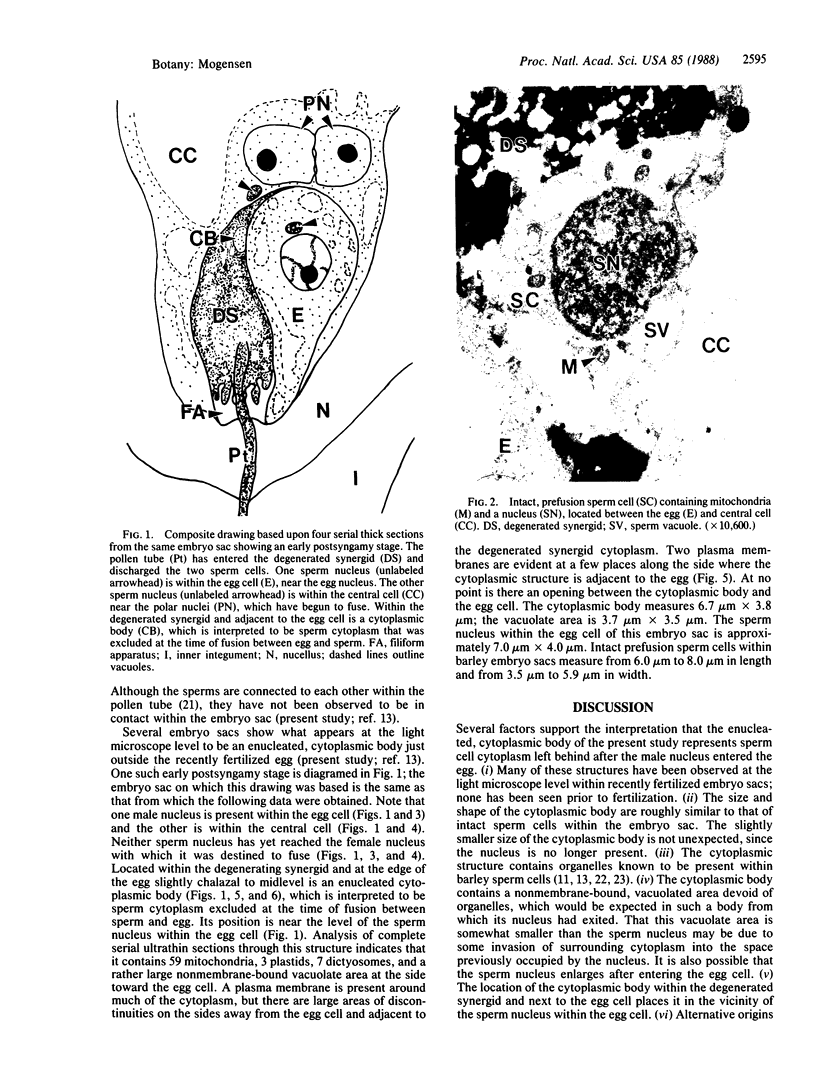
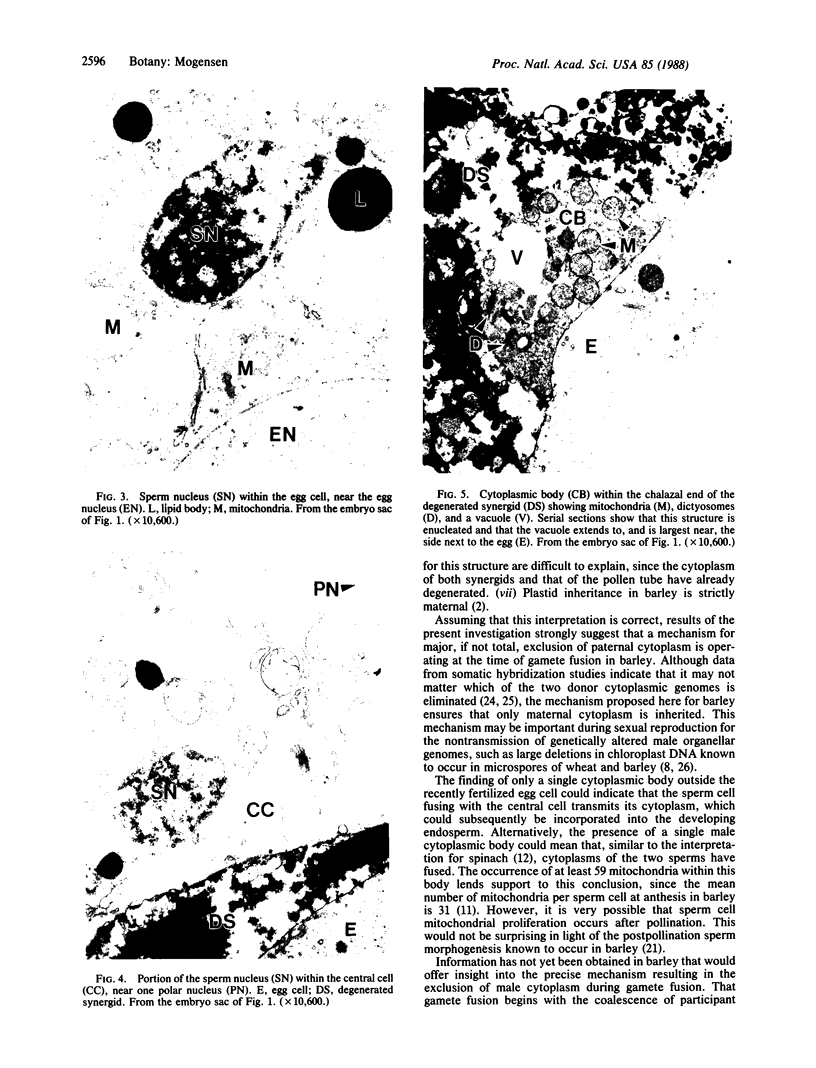
It is known from genetic analyses that maternal inheritance of cytoplasmic organelles is the rule among plants and animals. Although recognized as one of several possible mechanisms for strictly maternal cytoplasmic inheritance, exclusion of sperm cytoplasm at the time of gametic fusion has remained poorly documented for the flowering plants. In the present investigation, enucleated, cytoplasmic bodies approximately the size of intact, prefusion sperm cells have been observed within degenerated synergids and adjacent to recently fertilized egg cells. A complete series of ultrathin sections (68 sections) through such a cytoplasmic body revealed 59 mitochondria, 3 plastids, 7 dictyosomes, and a large vacuole with no limiting membrane. This structure is interpreted as the entire male cytoplasm that was left outside the egg during fusion between egg and sperm. The observation of only one cytoplasmic body per embryo sac may indicate a preliminary fusion between sperm cells or, more likely, the existence of a fundamentally different mechanism of fertilization between the second sperm and the central cell.
Keywords: embryo sac, fertilization, quantitative ultrastructure, sperm cells
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLWIN L. H., COLWIN A. L. ROLE OF THE GAMETE MEMBRANES IN FERTILIZATION IN SACCOGLOSSUS KOWALEVSKII (ENTEROPNEUSTA). II. ZYGOTE FORMATION BY GAMETE MEMBRANE FUSION. J Cell Biol. 1963 Dec;19:501–518. doi: 10.1083/jcb.19.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A., Ellis T. H. Chloroplast DNA deletions associated with wheat plants regenerated from pollen: possible basis for maternal inheritance of chloroplasts. Cell. 1984 Dec;39(2 Pt 1):359–368. doi: 10.1016/0092-8674(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Friedmann I. Cell Membrane Fusion and the Fertilization Mechanism in Plants and Animals. Science. 1962 May 25;136(3517):711–712. doi: 10.1126/science.136.3517.711. [DOI] [PubMed] [Google Scholar]
- Gyllensten U., Wharton D., Wilson A. C. Maternal inheritance of mitochondrial DNA during backcrossing of two species of mice. J Hered. 1985 Sep-Oct;76(5):321–324. doi: 10.1093/oxfordjournals.jhered.a110103. [DOI] [PubMed] [Google Scholar]
- Russell S. D. Participation of Male Cytoplasm During Gamete Fusion in an Angiosperm, Plumbago zeylanica. Science. 1980 Oct 10;210(4466):200–201. doi: 10.1126/science.210.4466.200. [DOI] [PubMed] [Google Scholar]
- Russell S. D. Preferential fertilization in Plumbago: Ultrastructural evidence for gamete-level recognition in an angiosperm. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6129–6132. doi: 10.1073/pnas.82.18.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M. L., Davidson R. L. Elimination of mitochondrial elements and improved viability in hybrid cells. Somatic Cell Genet. 1981 Jan;7(1):73–88. doi: 10.1007/BF01544749. [DOI] [PubMed] [Google Scholar]